Abstract
Hippocampus (HPC) receives dopaminergic (DA) projections from the ventral tegmental area (VTA) and substantia nigra. These inputs appear to provide a modulatory signal that influences HPC dependent behaviors and place fields. We examined how efferent projections from VTA to HPC influence spatial working memory and place fields when the reward context changes. CA1 and CA3 process environmental context changes differently and VTA preferentially innervates CA1. Given these anatomical data and electrophysiological evidence that implicates DA in reward processing, we predicted that CA1 place fields would respond more strongly to both VTA disruption and changes in the reward context than CA3 place fields.
Rats (N=9) were implanted with infusion cannula targeting VTA and recording tetrodes aimed at HPC. Then they were tested on a differential reward, win-shift working memory task. One recording session consisted of 5 baseline and 5 manipulation trials during which place cells in CA1/CA2 (N=167) and CA3 (N=94) were recorded. Prior to manipulation trials rats were infused with either baclofen or saline and then subjected to control or reward conditions during which the learned locations of large and small reward quantities were reversed. VTA disruption resulted in an increase in errors, and in CA1/CA2 place field reorganization. There were no changes in any measures of CA3 place field stability during VTA disruption. Reward manipulations did not affect performance or place field stability in CA1/CA2 or CA3; however, changes in the reward locations “rescued” performance and place field stability in CA1/CA2 when VTA activity was compromised, perhaps by trigging compensatory mechanisms. These data support the hypothesis that VTA contributes to spatial working memory performance perhaps specifically by maintaining place field stability selectively in CA1/CA2.
Keywords: hippocampus, place cell, dopamine, context, navigation
Introduction
Dopamine (DA) is well known for its involvement in learning and memory functions that are mediated by several different brain structures. DA levels in ventral striatum are positively correlated with learning appetitive classical conditioning tasks (Stuber et al., 2008) and DA levels in prefrontal cortex correlate with performance on delayed win-shift spatial working memory tasks (Phillips et al., 2004). DA also has modulatory effects on hippocampal (HPC) dependent functions, including spatial learning and memory, novelty detection, and context processing. For example, DA agonists and antagonists infused into HPC improve and impair performance on spatial tasks, respectively (Packard and White, 1991; Gasbarri et al., 1996). HPC receives the majority of its DA input from the ventral tegmental area (VTA) (Gasbarri et al., 1994). Consistent with the anatomy, temporary inactivation of VTA impairs performance on a HPC-dependent win-shift spatial working memory task and induces context dependent impairments in spatial working memory performance (Martig et al., 2009). DA agonists facilitate induction of long-term potentiation (a prominent model for the cellular mechanism for learning and memory) and DA antagonists attenuate novelty-induced enhancements in LTP in HPC (Li et al., 2003). It has been proposed that DA affects mnemonic functions by modulating neural plasticity in target brain areas (for review see, El-Ghundi et al., 2007).
Initial single unit evidence provides further support for the view that DA regulates HPC-dependent learning and neuroplasticity mechanisms. In navigating animals, a predominant behavioral correlate of the principle cells in HPC is location specific firing (O’Keefe and Dostrovsky, 1971). Place cells in HPC exhibit learning, memory, and context related changes in activity (e.g. Smith and Mizumori, 2006b) and DA has been shown to affect place field stability. For example, D1 receptor knock out mice do not have stable place fields (Kentros et al., 2004). Furthermore, DA antagonism via D1 receptor knock out or subcutaneous injection of D1 receptor antagonists has been found to influence place field stability in context dependent ways (Gill and Mizumori, 2006; Tran et al., 2008). All of these studies focused on place cells in the CA1 subregion of HPC. Place cells in different subregions of HPC, however, respond to changes in context in different ways. For example, CA3 place fields are known to reorganize to a greater extent than CA1 place fields during large changes in context whereas CA1 place fields are known to reorganize to a greater extent than CA3 place fields during more subtle changes in context (Guzowski et al., 2006). In addition, VTA DA neurons project differentially to subregions of HPC: Dorsal and ventral CA1 and ventral subiculum receive more DA input than CA3 or dentate gyrus (Gasbarri et al. 1994). Thus it remains unclear how VTA influences HPC-based, behaviorally-relevant neurocircuitry.
To provide a more detailed view of how VTA impacts HPC processing, we investigated the effects of temporary disruption of VTA activity on place fields in CA1 and CA3 of HPC while rats were performing a differential reward win-shift spatial working memory task. Also, given the known context dependent effects of DA manipulations on place field stability (Gill and Mizumori, 2006; Tran et al., 2008), we investigated context dependent effects of VTA disruption on place fields. Previous work has investigated context changes in terms of changes in the visual environmental context such as new or sparse extramaze cues, or changes in the shape of the environment. Given the extensive electrophysiological evidence that DA neurons provide a reward based prediction signal to enhance learning (Schultz et al., 1997), we manipulated the context by changing the magnitude of expected reward found in different locations of the maze. In addition, HPC place fields respond to changes in expected reward locations (Smith et al., 2006a), indicating that manipulating reward expectations serve as sufficient context changes to induce place field reorganization.
We predict that VTA disruption will be detrimental to asymptotic spatial working memory performance and place field stability. Changes in the reward context (without VTA disruption) may normally enhance place field stability and performance by facilitating DA release from VTA. On the other hand, since changes in reward locations cause place fields to reorganize (Smith et al., 2006a), changes in expected reward magnitude may compromise place field stability. In either case, any changes in place field stability and performance as a result of alterations in the reward context should be blocked by VTA disruption. Given the regionally-specific patterns of innervation by VTA neurons, we expect that VTA disruption will compromise place field stability in CA1 more so than CA3, and changes in the reward context should be reflected more strongly by changes in CA1 neural activity than by changes in CA3 activity.
Methods
Subjects
Nine male Long-Evans rats between 4 and 7 months old were housed individually in a temperature and humidity controlled environment with a 12-hour light/dark cycle. All subjects were given ad libitum access to food and water and were handled daily for 5 minutes for at least 5 days before behavioral testing began. From the start of behavioral training, rats were maintained at approximately 85% of their free-feeding body weight.
Apparatus
Behavioral training took place on an elevated (79 cm from the floor) eight arm radial maze. The maze arms (58×5.5 cm) were constructed out of black Plexiglas and radiated from a center platform (19.5 cm diameter) also constructed out of black Plexiglas. An experimenter in an adjacent testing room controlled access to the rewards on the maze arms via a remote system. A black curtain surrounded the maze, and extramaze cues were fixed to the curtain.
Behavioral Training
First rats were habituated to the maze environment and trained to retrieve chocolate milk that was available in food cups located at the ends of the maze arms. Chocolate milk quantities varied on every other maze arm between volumes of .1ml and .5ml throughout training. Arms containing large and small quantities of chocolate milk were counterbalanced between rats in that half of the rats received large reward on odd numbered arms of the maze and half of the rats received large reward on even numbered arms of the maze. After rats were running down all maze arms and drinking chocolate milk from the food cups consistently, spatial working memory training began. One trial of a spatial working memory test consisted of two phases. First the rats were given a forced choice phase during which the experimenter presented four randomly selected maze arms (two of which contained large rewards, two small rewards) individually to the rat. After the rat consumed the reward from all four of the presented arms, the test phase began. During the test phase all eight arms were simultaneously available and the rat had to go to the arms that it had not visited to obtain the remaining chocolate milk. Each training session consisted of ten trials separated by a 1.5-minute inter-trial interval. Errors were recorded when the rat placed all four paws on the erroneous arm (Fig. 1).
Figure 1.
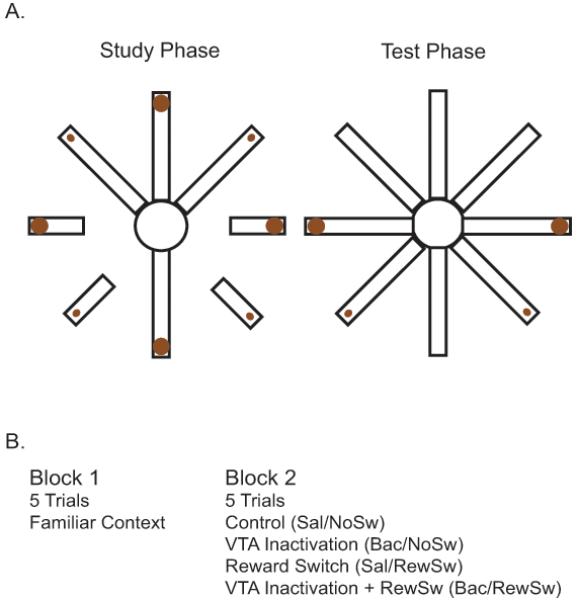
A. Schematic of the differential reward spatial working memory task. During the study phase, four pseudo randomly chosen arms (two with large reward and two with small reward quantities) are presented individually to the rat. During the test phase, all eight arms are available to the rat and it must visit the arms with the remaining chocolate milk to obtain reward. B. Table of experimental conditions. During Block 1, all rats perform 5 baseline trials. Rats are then removed from the maze, receive an infusion of saline or baclofen and are tested for 5 trials either in the same context or when the locations of the differential reward quantities are reversed.
Surgical Procedures
After rats reached asymptotic performance of less than one error per trial they were given ad libitum access to food and water and prepared for surgery. Rats were anesthetized with an isoflurane/oxygen mixture and given subcutaneous injections of an antibiotic (Baytril 5mg/kg) and an analgesic (Ketophen 5mg/kg). Each rat was chronically implanted bilaterally with custom-made microdrives targeting HPC (−3.3 mm AP relative to Bregma, ± 2.5 mm ML, and 1.7 mm ventral to dura) and custom-made guide cannula targeting VTA (−5.3 mm AP relative to Bregma, ± .08 ML, and 6.5 mm ventral to dura). Recording tetrodes consisted of four 25 μm lacquer coated tungsten wires twisted together. The tips of each of the four wires were gold plated to reach impedances of 100-500 kΩ. Each hemisphere was implanted with a single microdrive that contained two tetrodes. A reference electrode (114 μm stainless steel) was implanted near corpus callosum, and a ground wire was attached to the skull. Guide cannulas were constructed out of 25 GA stainless steel tubing and implanted 1 mm above the center of VTA. Infusion cannulas were constructed out of 33 GA stainless steel tubing, and these extended 1 mm beyond the guide cannula tips. All rats were allowed to recover for at least seven days before recording and infusion habituation began.
Post-surgical Procedures
Rats were retrained to asymptotic performance before unit recording began. During this re-training period, tetrodes were lowered until stable units were found, and rats were habituated to the tether and to infusions. Rats first performed 5 baseline trials (Block 1), then were removed from the maze and given bilateral infusions of .5 uL of saline over the course of 1 minute. Next the rats performed 5 more trials (Block 2). Reward and pharmacological manipulations began when rats were making less than one error per trial in both Block 1 and Block 2 of training. The pharmacological manipulation consisted of a .5 uL infusion of saline mixed with 2.5 ng of baclofen to disrupt VTA neural activity. Baclofen is a GABAB receptor agonist that, when applied locally, preferentially targets DA neurons in VTA (Xi and Stein, 1998). Previous studies determined 2.5 ng of baclofen to be a dose that allows animals to continue to navigate the maze (Martig et al. 2009) and 2.5 ng has been shown to be a sufficient dose to influence unit activity in striatum without affecting the ability of animals to complete an operant conditioning task (Yun et al., 2004). The reward manipulation consisted of reversing the large and small locations of chocolate milk reward. The permutations of the pharmacological and reward manipulations led to four conditions: 1) Saline infusion without a reward switch (Sal/NoSw), 2) Saline infusion with a reward switch (Sal/RewSw), 3) Baclofen infusion without a reward switch (Bac/NoSw), and 4) Baclofen infusion with a reward switch (Bac/RewSw) (Fig. 1). Originally, the experiment included another context manipulation where all of the lights were extinguished. However animals could not reliably navigate the maze in this condition so it was excluded from analysis.
Data Analysis
Position and Unit Activity Data Collection
An infrared light emitting diode array was used to monitor the position of each rat as they were tested on the spatial working memory task. Position information was sampled at 30 Hz, at a 2.5 cm/pixel resolution. Position data were viewed offline and event markers were inserted to identify the beginning and end of each trial, errors, and blocks. Analog waveform traces were digitized, then recorded at a sampling rate of 32 kHz, amplified 1,000 to 10,000 times, and filtered between 600Hz and 6 kHz. Single units were considered to be well isolated and suitable for recording if the waveform amplitude exceeded background noise levels by at least 3 times. In addition, since the animals were being unplugged from the recording apparatus for the infusion and plugged back in, only units that exhibited stable waveforms and cluster positions for the duration of the recording sessions were considered for analysis. All position and unit data were acquired by Cheetah data acquisition software (Neuralynx, Boseman, MT). Single unit activity was isolated from other units and background activity using Mclust sorting software (A. Redish, University of Minnesota, Minneapolis). Chris Higginson provided additional template matching software.
Behavioral Analysis
Working memory performance was compared across Block 1 and Block 2 by calculating an error difference score (EDS) of the average number of errors per trial in Block 2 minus the average number of errors per trial in Block 1. One-way ANOVA was used to determine if there were significant differences in performance relative to the Sal/NoSw condition. Tukey’s Honestly Significant Difference (HSD) post-hoc tests were conducted for individual group comparisons. Large vs. small reward arm preference was assessed by calculating the proportion of trials the rat visited a large reward arm first, second, third, and fourth during the test phase of a trial; error entries were excluded from the calculation. MANOVA was used to determine if there were significant differences in reward arm preferences between groups during Block 2. Bonferonni corrected pairwise comparisons were used to determine individual group differences. Given the involvement of VTA in movement and motivation, a movement analysis was also conducted on the rat’s velocity (2.24 cm/s bin size, determined by video data collected through Cheetah software) by calculating a difference score of the average velocity during Block 2 minus the average velocity during Block 1. One-way ANOVA was used to determine if there were significant differences in performance across conditions. Tukey’s HSD post-hoc tests were conducted for individual group comparisons. Descriptive statistics are presented as the mean ± standard error.
Place Field Classification and Analysis
To determine the spatial correlates of hippocampal cell firing, the maze area was divided into equal size pixels (2.5 cm × 2.5 cm) and the firing rate of the cell in each visited pixel was calculated. A cell was determined to be a place cell if it passed several criteria within at least one Block of five trials. First the maze area with the highest firing rate (primary firing field) must have occupied at least four adjacent pixels. The firing rate within the primary field must have exceeded the firing rate outside of the field by at least 2 fold. Two reliability measures were used as classification criteria for fields located on the maze arms. The cell must have fired at least 25% of the time the rat passed through the primary field. The cell must also have fired at least 50% of the time the rat traversed the maze arm that contained the field. Using these criteria, cells with fields that exhibited firing only when the rat was travelling in a particular direction on individual arms could be classified as place cells. If the primary field was located on the center of the maze the cell must have fired at least 50% of the time the rat passed through the field. In addition, Pearson’s correlation values were calculated based on pixel-by-pixel firing rate comparisons of commonly visited pixels across trials. To meet place field criteria, a cell must have had a spatial correlation value of at least .70 across 5 trials.
Once a cell was determined to exhibit a place field, the field was subject to further analysis. Reliability, specificity, spatial correlation, and in-field firing rate measures were calculated for each cell to determine if there were changes in place field properties in response to experimental manipulations. Reliability was calculated as the number of times a cell fired given that the rat was in the place field. Specificity was calculated as the number of times the rat was in the place field location given that the cell was firing. Spatial correlation values (calculated as described above) compared firing rate distributions across blocks of trials. Spatial correlation was used as a measure of global re-mapping whereas in-field firing rates were examined to investigate rate re-mapping. In-field firing rates reflected the average firing rate of a cell’s primary place field. One-way ANOVAs were used to determine if there were significant differences in each of these measures across different HPC subregions. Reliability, specificity, and in-field firing rates are reported in terms of differences between the measures between Block 1 and Block 2. One-way ANOVAs were used to determine if there were significant changes in each of these measures across conditions. If significant changes were detected, Tukey’s HSD post-hoc tests were conducted for individual group comparisons. Descriptive statistics are presented as the mean ± standard error.
Histology
Once tetrodes were lowered past the region of interest, the final positions of the tetrodes were marked by passing a 25 μA current through the electrodes for 25 seconds to create lesions while the rats were under isoflurane anesthesia. Rats were immediately transcardially perfused with 9% saline followed by a 10% formalin/saline solution. Brains were extracted and placed in a 30% sucrose formalin solution. Following sufficient sucrose absorption, brains were cut using a cryostat into 40 μm coronal sections and stained with cresyl violet. Lesions were compared with depth records to determine final recording locations. See Figure 2 for an illustration of recording electrode and cannula locations.
Figure 2.
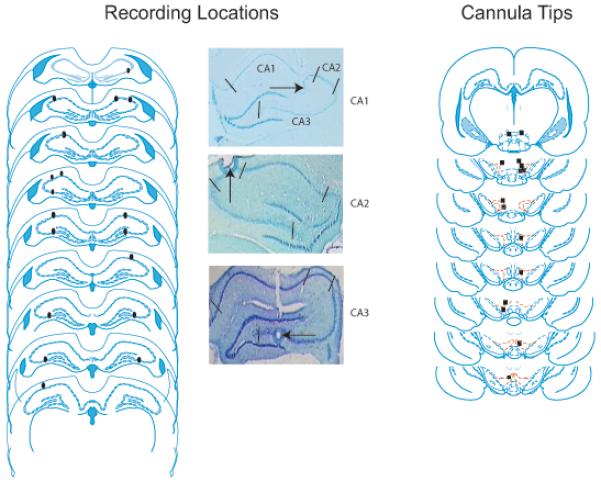
A. Reconstructed HPC recording locations and individual examples of lesions in CA1, CA2, and CA3 subregions of HPC. CA1, CA2, and CA3 subregions of HPC are outlined in the top figure of the CA1 lesion example. B. Locations of VTA cannula tips. The cannula tips in the rat represented by the first plate were not considered to be close enough to VTA, therefore the baclofen infusion data from this rat was excluded from analysis. Reconstructed from Paxinos and Watson, 2009.
Results
Behavior
Behavior was analyzed only from sessions in which well isolated units were recorded. Seventy six sessions from nine rats were analyzed, 24 were Sal/NoSw, 17 were Bac/NoSw, 21 were Sal/RewSw, and 14 were Bac/RewSw. There was a significant difference in the EDS between groups (F(3, 72)= 2.82, p<.05). Tukey’s HSD post-hoc comparisons revealed that in the Bac/NoSw condition (.70 ± .26) rats made significantly more errors in Block 2 than in the Sal/NoSw condition (.08 ± .14), p<.05. There was no significant difference in the EDS between the Sal/NoSw condition and the Sal/RewSw condition (.15 ± .09) or the Bac/RewSw condition (.36 ± .13) (Fig. 3). Thus, the only manipulation that resulted in a change in working memory performance was the baclofen infusion.
Figure 3.
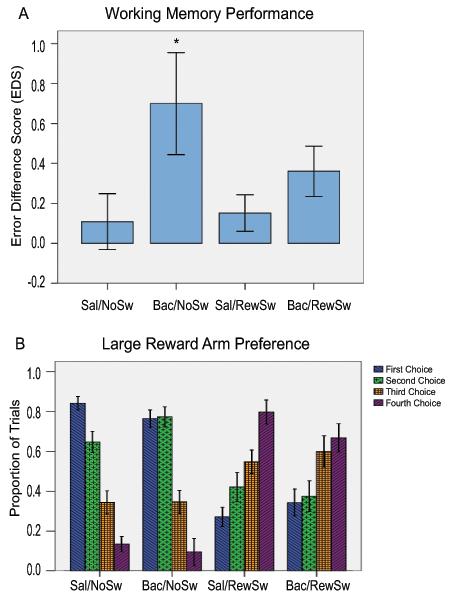
A. Working memory performance in all four conditions presented as difference scores of the average number of errors made in Block 2 minus the average number of errors made in Block 1 (EDS). B. Large reward arm preference. The proportion of trials rats made large reward arm entries during the test phase for their first, second, third, and fourth correct arm entries is presented for all four conditions. These measures of behavioral performance are somewhat independent of each other in that large reward arm entries do not include error entries.
There were significant differences between conditions in the proportion of trials the rats visited large reward arms on the first (F(3, 72)= 40.55, p<.01), second (F(3, 72)= 7.95, p<.01), third (F(3, 72)= 4.35, p<.01), and fourth choice (F(3, 72)= 39.76, p<.01) of Block 2. Tukey’s HSD post-hoc comparisons revealed that in the Sal/NoSw condition (.84 ± .04) and Bac/NoSw condition (.76 ± .05) rats visited a large reward arm first more than in the Sal/RewSw condition (.27 ± .04) and Bac/RewSw condition (.34 ± .05), p<.01 for all comparisons. The same pattern of results was evident for the second choice; in the Sal/NoSw condition (.65 ± .06) and Bac/NoSw condition (.77 ± .07) rats visited a large reward arm second more than in the Sal/RewSw condition (.42 ± .06) and Bac/RewSw condition (.37 ± .07), p<.01 for all comparisons. For the third choice, in the Sal/NoSw condition (.34 ± .06) rats visited a large reward arm less than in the Bac/RewSw condition (.60 ± .07), p<.05. There were no other significant differences between conditions on the third choice although there were trends for rats in the Sal/RewSw and Bac/RewSw conditions to visit large reward arms on the third choice more often than in the Sal/NoSw and Bac/NoSw conditions, p<.08. For the fourth choice, in the Sal/NoSw condition (.13 ± .05) and Bac/NoSw condition (.09 ± .06) rats visited a large reward arm less than in the Sal/RewSw condition (.80 ± .05) and Bac/RewSw condition (.67 ± .07), p<.01 for all comparisons (Fig. 3). Although, VTA disruption during the reward switch resulted in rats choosing a large reward arm more than saline infused control rats on the third choice, whereas, when VTA was intact during the reward switch, large reward preference on the third choice did not differ from saline infused control rats, there were no statistical differences between any choices between Sal/RewSw and Bac/RewSw conditions.
There were no significant differences between conditions in terms of the change in velocity between Block 1 and Block 2, p>.05.
Place Cells
Comparisons of Baseline Properties of CA1, CA2, and CA3 Place Fields
A grand total of 350 HPC cells were recorded. Of these 261 (74.6%) were classified as place cells, 77 cells were recorded in CA1, 90 cells were recorded in CA2, and 94 cells were recorded in CA3. Place cells recorded across all three regions had broad spike widths (latency difference between the maximum and minimum points of the analog voltage signal), 342.42 ± 2.44 msec, and low average firing rates, .64 ± .02 Hz. There were no statistical differences between place cells recorded in CA1 and CA2 on any measures of place field stability during Block 1 of recording; therefore they were combined to form a single group. CA1/CA2 place fields differed from CA3 place fields in many ways. There were significant differences in in-field firing rates (t(257)=3.52, p<.01): CA3 place cells (12.78 ± .97 Hz) had higher in-field firing rates than CA1/CA2 place cells (9.26 ± .51 Hz). There were significant differences in reliability (t(252)=2.35, p<.05): CA3 place cells (.71± .03) had more reliable place fields than CA1/CA2 place cells (.64 ± .02). There were also significant differences in specificity (t(252)=3.31, p<.01): CA3 place cells (.51± .02) had more specific place fields than CA1/CA2 place cells (.40 ± .02). Finally, there were significant differences in spatial correlation values (t(259)=3.99, p<.01): CA3 place cells (.89± .01) had more stable place fields across trials within Block 1 than CA1/CA2 place cells (.81 ± .01) (Fig 4).
Figure 4.
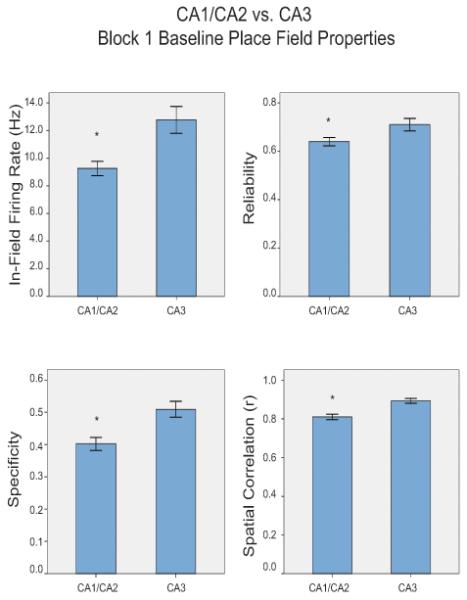
Baseline CA1/CA2 vs. CA3 place field properties. In-field firing rate, reliability, specificity, and spatial correlation values of place cells recorded during Block 1, the baseline period.
CA1/CA2 Place Field Responses to VTA Disruption and Reward Manipulations
Of the 167 place cells recorded in CA1 and CA2, 55 cells were recorded in the Sal/NoSw condition, 34 in the Bac/NoSw condition, 54 in the Sal/RewSw condition, and 24 were recorded in the Bac/RewSw condition. There were significant differences in spatial correlation values between conditions (F(3, 163)= 3.65, p<.05). Post-hoc comparisons revealed that cells recorded in the Bac/NoSw condition (.28 ± .05) had significantly lower spatial correlation values than cells recorded in the Sal/NoSw condition (.47 ± .04), p<.05, indicating less spatial consistency after VTA disruption. There were no significant differences among the Sal/RewSw, Bac/RewSw, and Sal/NoSw conditions. There were also no significant differences between groups in terms of specificity, reliability, or in-field firing rate measures (Fig 5). It appears that baclofen treatment resulted primarily in reorganization of the CA1/CA2 place field locations. When the reward locations were switched, baclofen induced reorganization of the CA1/CA2 place field locations was no longer evident.
Figure 5.
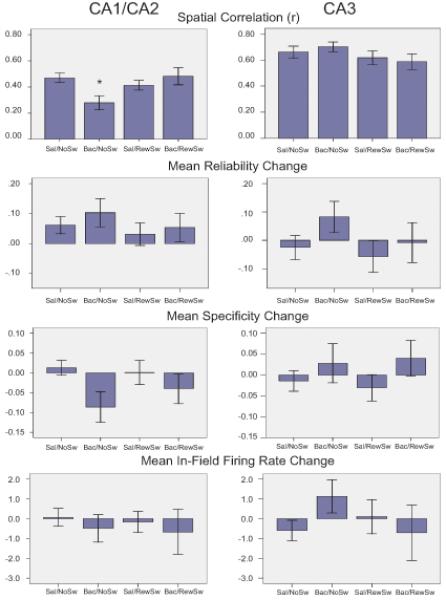
Comparison of Blocks 1 and 2 CA1/CA2 vs. CA3 place field properties. Spatial correlation, reliability, specificity, and in-field firing rate values of CA1/CA2 and CA3 place fields are presented for all four conditions. The only significant effect was a reduction in spatial correlation in CA1 place fields during the Bac/NoSw condition.
CA3 Place Field Responses to VTA Disruption and Reward Manipulations
Of the 94 place cells recorded in CA3, 32 cells were recorded in the Sal/NoSw condition, 19 in the Bac/NoSw condition, 26 in the Sal/RewSw condition, and 17 were recorded in the Bac/RewSw condition. There were no significant differences between groups for any measure of place field stability (Fig 5). See Figure 6 for individual place field examples.
Figure 6.
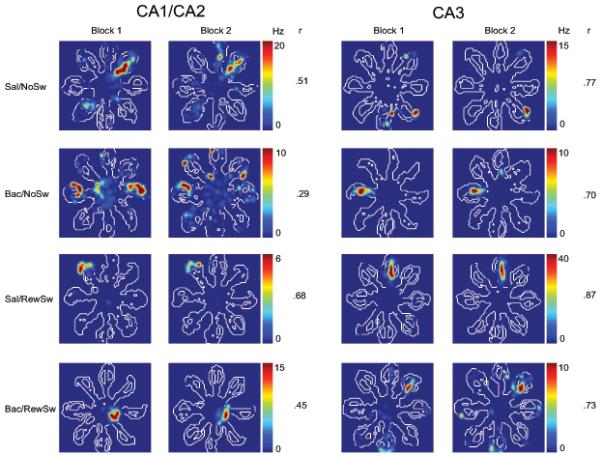
Firing rate map examples from CA1/CA2 and CA3 place cells recorded during all four conditions. Minimum and maximum firing rates (Hz) along with spatial correlation values (r) are presented for each cell.
Discussion
We hypothesized that VTA disruption would be detrimental to spatial working memory performance and that under these conditions place fields would become less stable. Our results are consistent with this hypothesis: VTA disruption caused CA1/CA2 place fields to reorganize and resulted in impairments in spatial working memory performance. Based on the known differential input pattern from VTA to HPC, we also predicted that CA1/CA2 place field stability would be more susceptible than CA3 to VTA disruption. Our results are consistent with this prediction: VTA disruption caused place fields in CA1/CA2 to reorganize and did not affect CA3 place field stability. We predicted that changes in the expected reward locations would affect place field stability and working memory performance. This was not the case, place fields did not respond to changes in the expected reward locations, nor was working memory performance affected by this manipulation. Interestingly, changes in the expected reward locations ‘rescued’ place field stability and spatial working memory performance when VTA was disrupted. What follows is a discussion of possible mechanisms by which VTA disruption affected place field stability and behavior. Pilot data from the current study investigating the behavioral effects of different doses of baclofen in VTA revealed 5 ng of baclofen to be too high of a dose for rats to reliably navigate the maze. However, 6-10 ng in VTA has been found to disrupt performance and unit activity in striatum more so than 2.5 ng (Yun et al., 2004). Therefore, the current dose of 2.5 ng more likely rendered VTA neurons hypotonic rather than completely inactivating their activity. In addition, a small infusion volume of .5 μL was used to minimize spread; therefore the drug may not have affected the entirety of VTA. Nevertheless, VTA disruption was induced via local infusion of Baclofen, a drug that preferentially silences DA neurons (Xi et al., 1998). Therefore it is reasonable to discuss VTA disruption effects in terms of a temporary loss of DA input to HPC.
Behavior
VTA disruption impaired performance by causing rats to make more working memory errors. This finding is consistent with previous studies that linked DA with spatial working memory functions (Xu et al., 2009; Martig et al., 2009; Wisman et al., 2008). This impairment was not related to motivational or movement problems associated with disrupting VTA as there were no differences in movement velocity before and after VTA disruption. Consistent with previous studies using the differential reward spatial working memory task (Pratt and Mizumori, 2001), rats preferentially selected the large reward arms first during the test phase. Changing the reward context did not affect working memory performance; however, this manipulation resulted in animals making fewer errors when VTA was disrupted. This “behavioral rescue” may have been mediated by other structures that were recruited by the reward switch. A likely possibility is the substantia nigra pars compacta, which contributes a small portion of the DA input to the temporal or lateral regions of HPC (Gasbarri et al., 1994). Presumably, DA release was facilitated by the unexpected changes in reward magnitude and this extra DA release was enough to support accurate performance on the task. In addition, given that VTA was not fully inactivated, it is possible that DA neurons within VTA were recruited by the reward switch and provided a sufficient amount of DA to HPC to support working memory performance. Prefrontal cortex (PFC) is another structure known for its involvement in working memory. Furthermore, DA levels in PFC are critically involved in working memory (Sawaguchi and Goldman-Rakic, 1991; Seamans et al., 1998), and PFC-HPC circuitry supports performance on spatial working memory tasks via a D1 receptor dependent mechanism (Seamans et al. 1998). It is possible that DA release, induced by the reward switch, recruited prefrontal cortex to support working memory performance. However, the current study utilized a non-delay HPC dependent spatial working memory task whereas PFC-HPC connections have been shown to be selectively involved in delay-dependent spatial working memory tasks (Seamans et al., 1998). Therefore, it is less likely that the “behavioral rescue” was mediated by this circuitry.
VTA disruption did not affect large reward arm preferences. This finding is consistent with the literature supporting a role for DA in new learning. Since our rats had already learned the locations of the large and small reward arms, VTA was no longer needed to support this preference. The large reward arm preference was well learned, as demonstrated when the reward locations were switched in the Sal/RewSw and Bac/RewSw conditions, and rats continued to go to the reward arms that previously held large rewards first. The pattern of arm entries was consistent with rats having a preference for the previous large reward arms until the third choice was made. On the third arm entry, performance in the Sal/RewSw condition did not differ from performance in the Sal/NoSw and Bac/NoSw conditions indicating that when rats were performing in the Sal/RewSw condition, they were learning to choose large reward arms earlier as they experienced more training sessions with the reward locations reversed. This was not the case for the Bac/Rew condition however, third choice preference did not differ between the Sal/RewSw and Bac/RewSw conditions which suggests a minimal effect of VTA disruption on large reward preference during this manipulation. More studies are needed to fully examine the role of VTA in this component of the task. For example, VTA has been found to be critically involved in learning driven by unexpected outcomes (Takahashi et al., 2009). This study did not test the rat’s ability to learn the new locations of large and small rewards; rather this condition was presented randomly and used as a change in context. A future VTA inactivation study investigating the development of large reward choice preference would provide more insight into the role of VTA in the differential reward working memory task.
Place Cells
Comparison of CA1/CA2 and CA3 Place Fields during the Baseline Period
CA3 place fields were more specific, reliable, stable, and had higher in-field firing rates than fields in CA1 and CA2. These findings are consistent with previous studies that have found CA3 place fields to be smaller and more specific than CA1 place fields (Barnes et al., 1990; Mizumori et al., 1996). Computational theories further support the view that CA1 and CA3 make different contributions to HPC-dependent behaviors (Rolls and Kesner, 2006). These theories are based on known anatomical differences within CA1 and CA3 (Koene and Hasselmo, 2008). CA1 receives strong input from layer III of entorhinal cortex and projections from CA3, which may predispose CA1 to function as a novelty detector (Lisman and Otmakhova, 2001). CA1 can compare the current environment via the entorhinal projection with memory representations from CA3. CA3 on the other hand receives projections from dentate gyrus and layer II of entorhinal cortex. Also, within CA3 is a recurrent collateral system, which likely sustains activated memory representations. Thus, CA3 is thought to compare cortical and dentate inputs with memory representations in the recurrent collateral system to determine whether the expected environment has changed (Lisman and Otmakhova, 2001). Consistent with this theory, CA3 place fields tend to reorganize more readily than CA1 place fields during large changes in context than CA1 place fields, whereas CA1 place fields reorganize more during smaller changes in context (Guzowski et al. 2004). Together with anatomical differences, these findings indicate that CA1 may be more involved in novelty detection whereas CA3 may be more suited to determine if the current context matches an expected context.
CA1/CA3 place field differences have primarily been studied in context-processing and novelty detection frameworks. Although significant CA1 and CA3 differences were found, the current study did not directly test the hypothesis that CA1 is more involved in novelty detection and CA3 is more involved in context discrimination. It is worth noting that distinguishing between processes involved in novelty detection and context discrimination may not be straightforward since a single process (i.e. context comparison) could underlie both phenomena (Mizumori et al., 2007). Nevertheless, our finding that CA3 place fields were more stable than CA1 place fields while animals were performing a spatial working memory task is intriguing; it is consistent with previous work that demonstrated that CA1 place fields responded more than CA3 place fields to small changes in context (Guzowski et al. 2004). Here, a different set of maze arms is presented to the rat every trial during the spatial working memory task and this may elicit trial-by-trial reorganization in CA1 place fields whereas in CA3, place fields may be more stable because the environment is not sufficiently changed to elicit reorganization.
Few studies have investigated place field properties in CA2. Anatomically, CA2 is situated in between CA3 and CA1 and has been thought of as an interface between the two regions (Amaral and Witter, 1989). Like CA3 and dentate gyrus, CA2 receives strong entorhinal cortex projections from layer II. CA2 also receives input from CA3 and projects to CA1 (Amaral and Witter, 1989). This pattern of connections suggests that CA2 also acts as a mismatch detector of sorts, possibly by comparing input from entorhinal cortex and CA3. The results from this study showed that CA2 place fields responded more similarly to CA1 place fields while rats were performing a spatial working memory task. In other words, like CA1 place fields, CA2 place fields were more sensitive to small changes in context than cells in CA3. More work investigating CA2 place field responses to large and small changes in context relative to CA1 and CA3 place field responses is needed to provide a more comprehensive account of CA2 function. Overall, however these results are congruent with a large body of electrophysiological data that suggest HPC performs match/mismatch comparisons to determine whether the expected context is sufficiently different from the current context (Mizumori et al., 2007).
Place Field Responses to VTA disruption and Reward Context Manipulations
Place field reorganization or remapping is traditionally thought to be a manifestation of the mechanism through which HPC executes context discrimination (Mizumori et al., 2007). Remapping can be divided into two types, global and rate remapping. Global remapping occurs when place fields change locations whereas rate remapping occurs when place field firing rates change without a change in location (Leutgeb et al., 2005). Global and rate remapping are thought to signal different types of context changes: large changes in the context induce global remapping and small changes in the context cause rate remapping (Colgin et al., 2008). CA1 and CA2 place fields responded to VTA disruption by reorganizing locations. In-field firing rates, reliability, and specificity measures remained stable. This shows that HPC cells continue to code spatial qualities of a context even when VTA input is compromised. The location of the place field however, appears to be regulated by VTA (presumably DA). Perhaps HPC DA helps to identify the saliency of locations. To the extent that place field remapping reflects a perceived context change, inappropriate reorganization by CA1 and CA2 place fields during the Bac/NoSw condition (in which the context was constant) may have contributed to the rats’ poor performance.
When VTA was intact, CA1 and CA2 place fields did not respond to changes in the reward context. This finding seems to contrast with the robust changes in CA1 place fields following reward switches on a T-Maze (Smith and Mizumori, 2006a). The apparent discrepancy could be related to the fact that during the current experiment reward was always available at expected reward locations; only the magnitude of reward was switched. In contrast, during the reward switch on the T-Maze (Smith and Mizumori, 2006a), the reward was either present or not present at expected reward locations. Changing the reward magnitude alone may not have been sufficient to induce remapping in HPC place fields. Instead, it appears that HPC responds to changes in reward only when the location of the reward per se is manipulated. This suggests that HPC place cells code for spatial properties of reward processing. The combination of a reward switch and VTA disruption, however, produced an unexpected result. The VTA disruption-induced destabilization of CA1/CA2 place fields was blocked when the reward context was changed at the same time. Similar to the “behavioral rescue” seen in this condition (described above), “rescue” of place field stability during VTA disruption could have been enhanced by activation of VTA itself or other structures such as substantia nigra.
CA3 place fields did not respond to VTA disruption nor did they respond to changes in the reward context. These results are consistent with anatomical projections from VTA to HPC. VTA projects more heavily to CA1 and CA2 regions of HPC and we saw greater reorganization in response to VTA inactivation in CA1 and CA2 place fields than in CA3 place fields.
Interactions Between VTA DA and HPC
VTA and HPC appear to operate as a circuit (Lisman and Grace, 2005; Mizumori et al., 2004) whereby DA from VTA likely enables HPC dependent functions such as spatial learning and memory, novelty detection, and context discrimination presumably by enhancing plasticity-promoting cellular signaling mechanisms in HPC. For example, DA facilitates both early and late LTP (for review see Jay 2003), which are thought to be important for short and long-term memory formation (Vertes, 2005), respectively. This study supports the hypothesis that DA regulates HPC dependent memory formation and plasticity. Disrupting DA transmission was detrimental to spatial working memory performance, a test of HPC dependent short term-memory formation. In addition, place fields exhibited inappropriate remapping when DA was removed, which suggests that plasticity processes that normally stabilize place field locations during spatial working memory performance were affected. While the data from the current study provides insight into VTA-to-HPC functional connections during a HPC dependent task, more studies investigating VTA DA neuron physiology while animals are engaged in HPC dependent tasks will contribute greatly to our understanding of DA influences on HPC dependent learning and memory (e.g. Puryear et al., submitted).
Considering the reciprocal direction of the VTA-HPC functional loop, HPC is thought to facilitate DA release from VTA (Lisman and Grace, 2005). Indeed, HPC has been found to influence VTA DA neurophysiology in anesthetized rats (Floresco et al., 2001). There are several routes by which HPC can affect VTA processing. HPC projects to ventral pallidum via nucleus accumbens (Lisman and Grace, 2005). Ventral pallidum in turn projects to VTA and pedunculo-pontine nucleus (Lisman and Grace, 2005). HPC dependent excitation of VTA neurons is blocked by TTX injections into nucleus accumbens (Floresco et al., 2001) and both ventral pallium and pedunculo-pontine nucleus have been shown to regulate DA neuron burst firing in VTA (Floresco et al., 2003; Pan and Hyland, 2005; Lodge and Grace, 2006). A challenge lies in determining whether these structures also contribute to HPC dependent processing.
Conclusions
The current study provides evidence that VTA (presumably DA) activity is important for accurate spatial working memory and place field stability in CA1 and CA2. In particular, DA may help to determine the specific location of place fields. Consistent with the known anatomical pattern of VTA innervation of HPC; this study also provides evidence for a dissociation between place field responses to VTA inactivation across CA1/CA2 and CA3 subregions since CA3 place fields did not respond to VTA inactivation. These findings provide powerful support for selective DA modulation of HPC dependent behavior and place field stability in freely behaving animals.
Acknowledgements
This work was supported by NIMH Grant MH 58755. We would like to thank Emily Clark and Min Jung Kim for their helpful comments and reviews of the manuscript. We would also like to thank Graham Jones and Kelsey Smith for behavioral testing of animals, data entry, and histological processing and Chris Higginson for custom analysis routines.
Grant Sponsor: NIMH
Grant Number: MH 58755
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–91. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL, Mizumori SJ, Leonard BW, Lin LH. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. doi: 10.1016/s0079-6123(08)61257-1. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–77. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O’Dowd BF, George SR. Insights into the role of dopamine receptor systems in learning and memory. Rev Neurosci. 2007;18:37–66. doi: 10.1515/revneuro.2007.18.1.37. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–22. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Gill KM, Mizumori SJ. Context-dependent modulation by D(1) receptors: differential effects in hippocampus and striatum. Behav Neurosci. 2006;120:377–92. doi: 10.1037/0735-7044.120.2.377. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668:71–9. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Innocenzi R, Pacitti C, Brioni JD. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–44. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–4. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–90. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–95. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Koene RA, Hasselmo ME. Reversed and forward buffering of behavioral spike sequences enables retrospective and prospective retrieval in hippocampal regions CA3 and CA1. Neural Netw. 2008;21:276–88. doi: 10.1016/j.neunet.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–23. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nature Neuroscience. 2003;6:526–31. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–68. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–61. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Martig AK, Jones GL, Smith KE, Mizumori SJ. Context dependent effects of ventral tegmental area inactivation on spatial working memory. Behav Brain Res. 2009;203:316–20. doi: 10.1016/j.bbr.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Lavoie AM, Kalyani A. Redistribution of spatial representation in hippocampus of aged rats performing a spatial memory task. Behav Neurosci. 1996;110:1006–1016. doi: 10.1037//0735-7044.110.5.1006. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Smith DM, Puryear CB. Hippocampal and neocortical interactions during context discrimination: electrophysiological evidence from the rat. Hippocampus. 2007;17:851–62. doi: 10.1002/hipo.20317. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Yeshenko O, Gill KM, Davis DM. Parallel processing across neural systems: implications for a multiple memory system hypothesis. Neurobiol Learn Mem. 2004;82:278–98. doi: 10.1016/j.nlm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;24:547–53. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Pan WX, Hyland B. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–32. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 2009. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res. 2001;123:165–83. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Learning-related development of context-specific neuronal responses to places and events: the hippocampal role in context processing. J Neurosci. 2006a;26:3154–63. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006b;16:716–29. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–92. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Stalnaker TA, Haney RZ, Calu DJ, Taylor AR, Burke KA, Shoenbaum G. The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron. 2009;62:269–280. doi: 10.1016/j.neuron.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AH, Uwano T, Kimura T, Hori E, Katsuki M, Nishijo H, Ono T. Dopamine D1 receptor modulates hippocampal representation plasticity to spatial novelty. J Neurosci. 2008;28:13390–400. doi: 10.1523/JNEUROSCI.2680-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–35. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Wisman LA, Sahin G, Maingay M, Leanza G, Kirik D. Functional convergence of dopaminergic and cholinergic input is critical for hippocampus-dependent working memory. J Neurosci. 2008;28:7797–807. doi: 10.1523/JNEUROSCI.1885-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Stein EA. Nucleus accumbens dopamine release modulation of mesolimbic GABAA receptors- an in vivo electrochemical study. Brain Research. 1998;798:156–65. doi: 10.1016/s0006-8993(98)00406-5. [DOI] [PubMed] [Google Scholar]
- Xu H, Yang HJ, Zhang Y, Clough R, Browning R, Li XM. Behavioral and neurobiological changes in C57BL/6 mice exposed to cuprizone. Behav Neurosci. 2009;123:418–29. doi: 10.1037/a0014477. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–33. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


