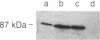Abstract
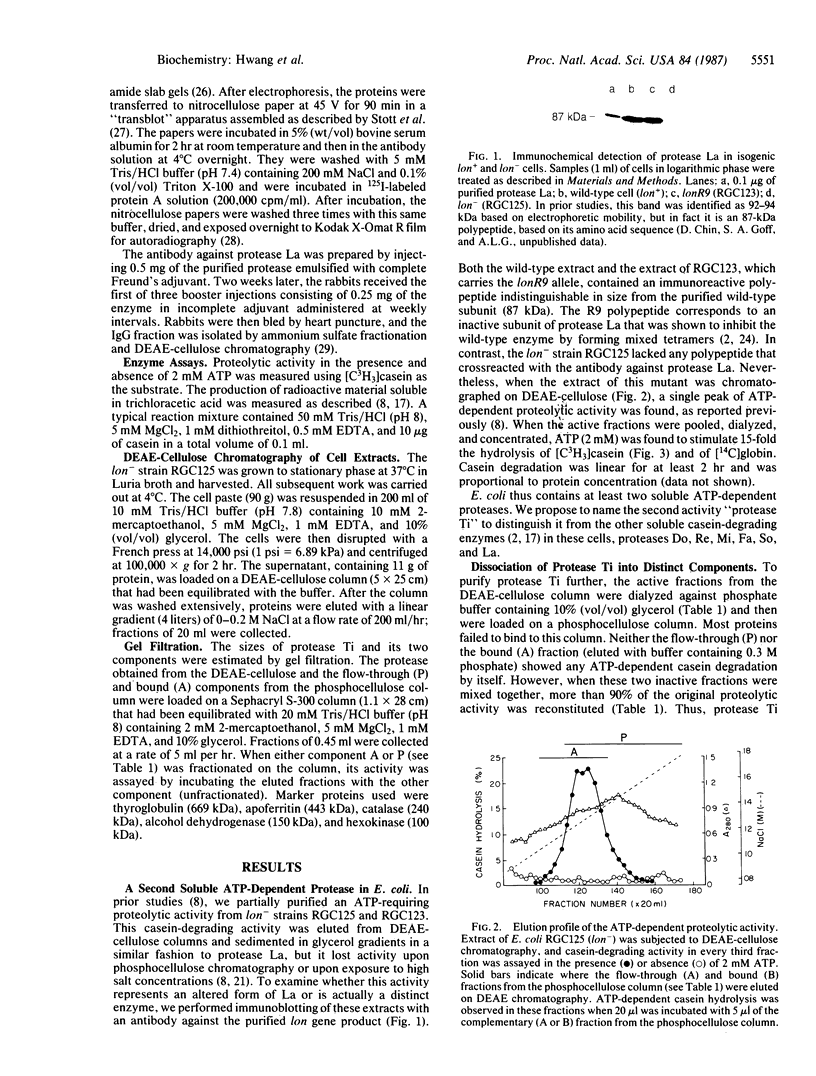
The energy requirement for protein breakdown in Escherichia coli has generally been attributed to the ATP-dependence of protease La, the lon gene product. We have partially purified another ATP-dependent protease from lon-cells that lack protease La (as shown by immunoblotting). This enzyme hydrolyzes [3H]methyl-casein to acid-soluble products in the presence of ATP and Mg2+. ATP hydrolysis appears necessary for proteolytic activity. Since this enzyme is inhibited by diisopropyl fluorophosphate, it appears to be a serine protease, but it also contains essential thiol residues. We propose to name this enzyme protease Ti. It differs from protease La in nucleotide specificity, inhibitor sensitivity, and subunit composition. On gel filtration, protease Ti has an apparent molecular weight of 370,000. It can be fractionated by phosphocellulose chromatography or by DEAE chromatography into two components with apparent molecular weights of 260,000 and 140,000. When separated, they do not show proteolytic activity. One of these components, by itself, has ATPase activity and is labile in the absence of ATP. The other contains the diisopropyl fluorophosphate-sensitive proteolytic site. These results and the similar findings of Katayama-Fujimura et al. [Katayama-Fujimura, Y., Gottesman, S. & Maurizi, M. R. (1987) J. Biol. Chem. 262, 4477-4485] indicate that E. coli contains two ATP-hydrolyzing proteases, which differ in many biochemical features and probably in their physiological roles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. A., Grossman A. D., Gross C. A. A gene regulating the heat shock response in Escherichia coli also affects proteolysis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6779–6783. doi: 10.1073/pnas.81.21.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Kézdy F. J., Markovitz A. Molecular mechanism for dominance of a mutant allele of an ATP-dependent protease. J Mol Biol. 1982 Dec 5;162(2):503–510. doi: 10.1016/0022-2836(82)90541-1. [DOI] [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Markovitz A. ATP hydrolysis-dependent protease activity of the lon (capR) protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4728–4732. doi: 10.1073/pnas.78.8.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. DNA stimulates ATP-dependent proteolysis and protein-dependent ATPase activity of protease La from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(3):795–799. doi: 10.1073/pnas.79.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Waxman L., Goldberg A. L. Studies of the protein encoded by the lon mutation, capR9, in Escherichia coli. A labile form of the ATP-dependent protease La that inhibits the wild type protease. J Biol Chem. 1983 Jan 10;258(1):215–221. [PubMed] [Google Scholar]
- Desautels M., Goldberg A. L. Demonstration of an ATP-dependent, vanadate-sensitive endoprotease in the matrix of rat liver mitochondria. J Biol Chem. 1982 Oct 10;257(19):11673–11679. [PubMed] [Google Scholar]
- Goff S. A., Casson L. P., Goldberg A. L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Swamy K. H., Chung C. H., Larimore F. S. Proteases in Escherichia coli. Methods Enzymol. 1981;80(Pt 100):680–702. doi: 10.1016/s0076-6879(81)80052-3. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Waxman L. The role of ATP hydrolysis in the breakdown of proteins and peptides by protease La from Escherichia coli. J Biol Chem. 1985 Oct 5;260(22):12029–12034. [PubMed] [Google Scholar]
- Gottesman S., Gottesman M., Shaw J. E., Pearson M. L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981 Apr;24(1):225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama-Fujimura Y., Gottesman S., Maurizi M. R. A multiple-component, ATP-dependent protease from Escherichia coli. J Biol Chem. 1987 Apr 5;262(10):4477–4485. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):110–119. doi: 10.1042/bj0690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M. R., Trisler P., Gottesman S. Insertional mutagenesis of the lon gene in Escherichia coli: lon is dispensable. J Bacteriol. 1985 Dec;164(3):1124–1135. doi: 10.1128/jb.164.3.1124-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. S., Waxman L., Goldberg A. L. The energy utilized in protein breakdown by the ATP-dependent protease (La) from Escherichia coli. J Biol Chem. 1987 Jan 15;262(2):722–726. [PubMed] [Google Scholar]
- Mizusawa S., Gottesman S. Protein degradation in Escherichia coli: the lon gene controls the stability of sulA protein. Proc Natl Acad Sci U S A. 1983 Jan;80(2):358–362. doi: 10.1073/pnas.80.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Voellmy R., Goldberg A. L. Protein degradation is stimulated by ATP in extracts of Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8194–8200. [PubMed] [Google Scholar]
- Phillips T. A., VanBogelen R. A., Neidhardt F. C. lon gene product of Escherichia coli is a heat-shock protein. J Bacteriol. 1984 Jul;159(1):283–287. doi: 10.1128/jb.159.1.283-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Effects of protease inhibitors on protein breakdown in Escherichia coli. J Biol Chem. 1972 May 25;247(10):3341–3352. [PubMed] [Google Scholar]
- Schoemaker J. M., Markovitz A. Identification of the gene lon (capR) product as a 94-kilodalton polypeptide by cloning and deletion analysis. J Bacteriol. 1981 Jul;147(1):46–56. doi: 10.1128/jb.147.1.46-56.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John A. C., Goldberg A. L. Effects of reduced energy production on protein degradation, guanosine tetraphosphate, and RNA synthesis in Escherichia coli. J Biol Chem. 1978 Apr 25;253(8):2705–2711. [PubMed] [Google Scholar]
- St John A. C., Goldberg A. L. Effects of starvation for potassium and other inorganic ions on protein degradation and ribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1980 Sep;143(3):1223–1233. doi: 10.1128/jb.143.3.1223-1233.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott D. I., McLearie J., Marsden H. S. A gel transfer tank for immunoblotting and its application for analysis of nuclear protein antigens. Anal Biochem. 1985 Sep;149(2):454–460. doi: 10.1016/0003-2697(85)90597-4. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Waxman L., Goldberg A. L. ATP serves two distinct roles in protein degradation in reticulocytes, one requiring and one independent of ubiquitin. J Cell Biol. 1983 Jun;96(6):1580–1585. doi: 10.1083/jcb.96.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H. Structures and activities of protease inhibitors of microbial origin. Methods Enzymol. 1976;45:678–695. doi: 10.1016/s0076-6879(76)45058-9. [DOI] [PubMed] [Google Scholar]
- Voellmy R. W., Goldberg A. L. ATP-stimulated endoprotease is associated with the cell membrane of E. coli. Nature. 1981 Apr 2;290(5805):419–421. doi: 10.1038/290419a0. [DOI] [PubMed] [Google Scholar]
- Waxman L., Goldberg A. L. Protease La from Escherichia coli hydrolyzes ATP and proteins in a linked fashion. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4883–4887. doi: 10.1073/pnas.79.16.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman L., Goldberg A. L. Selectivity of intracellular proteolysis: protein substrates activate the ATP-dependent protease (La). Science. 1986 Apr 25;232(4749):500–503. doi: 10.1126/science.2938257. [DOI] [PubMed] [Google Scholar]
- Zehnbauer B. A., Markovitz A. Cloning of gene lon (capR) of Escherichia coli K-12 and identification of polypeptides specified by the cloned deoxyribonucleic acid fragment. J Bacteriol. 1980 Aug;143(2):852–863. doi: 10.1128/jb.143.2.852-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]