Abstract
Over the past several decades, significant advances have been made in our understanding of the basic stages and mechanisms of mammalian brain development. Studies elucidating the neurobiology of brain development span the levels of neural organization from the macroanatomic, to the cellular, to the molecular. Together this large body of work provides a picture of brain development as the product of a complex series of dynamic and adaptive processes operating within a highly constrained, genetically organized but constantly changing context. The view of brain development that has emerged from the developmental neurobiology literature presents both challenges and opportunities to psychologists seeking to understand the fundamental processes that underlie social and cognitive development, and the neural systems that mediate them. This chapter is intended to provide an overview of some very basic principles of brain development, drawn from contemporary developmental neurobiology, that may be of use to investigators from a wide range of disciplines.
Keywords: Brain development; maturation, Magnetic resonance imaging, Diffusion weighted imaging, Genetic patterning of brain, Neurogenesis, Myelination, Effects of experience on connectivity
Human brain development is a protracted process that begins in the third gestational week (GW) with the differentiation of the neural progenitor cells and extends at least through late adolescence, arguably throughout the lifespan. The processes that contribute to brain development range from the molecular events of gene expression to environmental input. Critically, these very different levels and kinds of processes interact to support the ongoing series of events that define brain development. Both gene expression and environmental input are essential for normal brain development, and disruption of either can fundamentally alter neural outcomes. But neither genes nor input is prescriptive or determinative of outcome. Rather brain development is aptly characterized as a complex series of dynamic and adaptive processes that operate throughout the course of development to promote the emergence and differentiation of new neural structures and functions. These processes operate within highly constrained and genetically organized, but constantly changing contexts that, over time, support the emergence of the complex and dynamic structure of the human brain (Waddington 1939; Morange 2001; Stiles 2008).
This paper will review some of the major events that contribute to the development of the human brain from its early embryonic state through adolescence. It begins by examining the foundational changes that occur during the embryonic period, which in humans extends through the eighth week post conception (gestational week eight, or GW8). By the end of the embryonic period the rudimentary structures of the brain and central nervous system are established and the major compartments of the central and peripheral nervous systems are defined (see Fig. 1). The ensuing period of fetal development extends through the end of gestation. During this time there is rapid growth and elaboration of both cortical and subcortical structures, including the rudiments of the major fiber pathways (Kostovic and Jovanov-Milosevic 2006); (Kostovic and Jovanov-Milosevic 2006). Changes in the gross morphology of the prenatal neural system are underpinned by changes occurring at the cellular level. Neuron production in humans begins on embryonic day 42. E42, i.e. 42 days post conception (Bystron et al. 2008; Stiles 2008) and is largely complete by midgestation. As they are produced neurons migrate to different brain areas where they begin to make connections with other neurons establishing rudimentary neural networks. By the end of the prenatal period major fiber pathways, including the thalamocortical pathway, are complete.
Fig. 1.
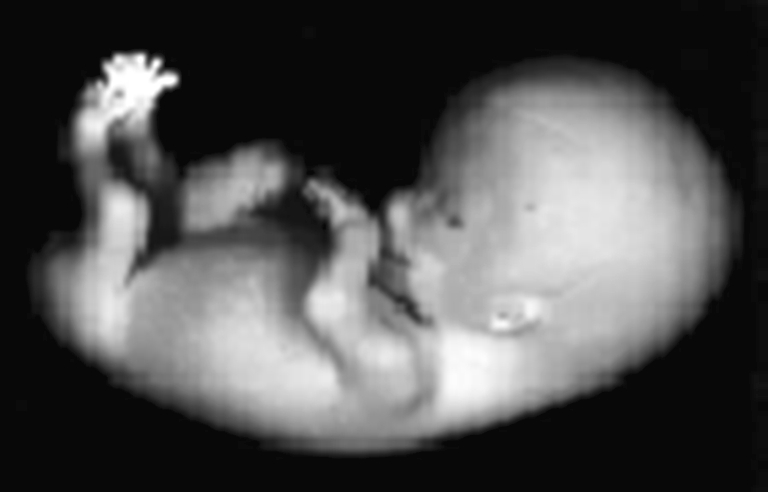
Human embryo at Carnegie Stage 23, the end of the embryonic period (GW8). It is 30 mm long. Image from the Kyoto Collection reproduced with permission of Prof Kohei Shiota, Graduate School of Medicine, Kyoto University, and obtained with permission of Dr. Mark Hill, University of New South Wales, http://embryology.med.unsw.edu.au/embryo.htm
Brain development continues for an extended period postnatally. The brain increases in size by four-fold during the preschool period, reaching approximately 90% of adult volume by age 6 (Reiss et al. 1996; Iwasaki et al. 1997; Courchesne et al. 2000; Kennedy and Dehay 2001; Paus et al. 2001; Kennedy et al. 2002; Lenroot and Giedd 2006). But structural changes in both the major gray and white matter compartments continue through childhood and adolescence, and these changes in structure parallel changes in functional organization that are also reflected in behavior. During the early postnatal period, level of connectivity throughout the developing brain far exceeds that of adults (Innocenti and Price 2005). This exuberant connectivity is gradually pruned back via competitive processes that are influenced by the experience of the organism. These early experience dependent processes underlie the well-documented plasticity and capacity for adaptation that is the hallmark of early brain development.
By way of background, this chapter begins with a consideration of two important concepts that are essential for understanding how brains develop. The first involves gene expression: what genes are and how they play an important role in brain development. The second is the outcome of brain development, the mature brain: what are the major structures and what are the basic principles of brain organization. The chapter then considers some of the major milestones of brain development with the aim of illustrating the dynamic, interactive nature of brain development.
Genes and Gene Products
Genes are the material substance that is passed intergenerationally from parent to offspring. Genes are contained in the nucleotide sequences of DNA that are found in the nucleus of every cell in the body. The expression of a gene has one result: the production of a protein molecule. These molecular products of gene expression are essential for all aspects of development. Genes provide a template for making proteins and it is the proteins that are the active agents in biological development. Thus, while genes contain information that is essential for the development and functioning of the biological organism, genes are basically inert molecules. Genes cannot participate directly in biological processes. They do not directly create blue eyes, disease proclivity, intelligence or behavior. Rather, there is an indirect relationship between the information in a gene and a developmental outcome. The information in the gene sequences must be extracted, recoded and translated into proteins. It is the proteins that enter into the complex, interactive signaling cascades that usually involve many gene products as well as influences from the environment. A particular gene product is thus one of many essential elements that interact to support and guide the complex process of brain development.
The Organization of the Mature Brain
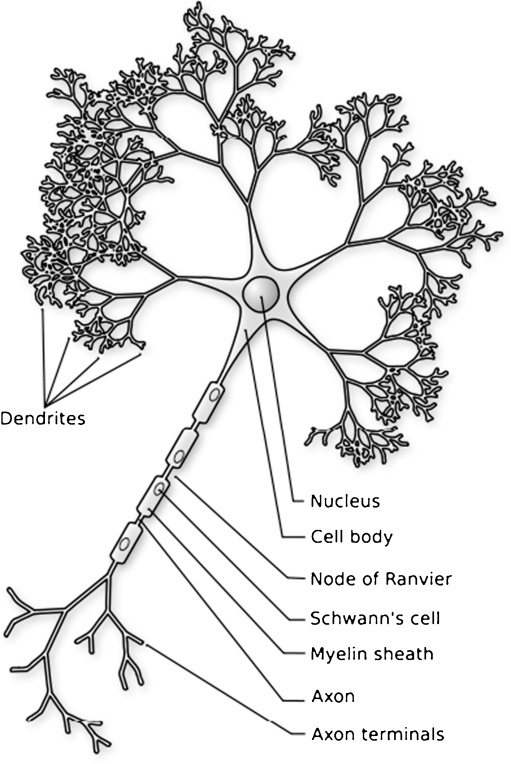
The human brain is arguably the most complex of all biological systems. The mature brain is composed of more than 100 billion neurons (Pakkenberg and Gundersen 1997). Neurons are the information processing cells in the brain (see Fig. 2). There are many different kinds of neurons that vary in their size and shape as well as in their function. Neurons make connections with other neurons to form the information processing networks that are responsible for all of our thoughts, sensations, feelings and actions. Since each neuron can make connections with more than 1,000 other neurons, the adult brain is estimated to have more than 60 trillion neuronal connections. The point of connection between two neurons is called a synapse.
Fig. 2.
Schematic drawing of a neuron. Each neuron a single large axon. At the distal tip of the axon is a growth cone that serves to guide the axon to targeted brain regions. Once the axon reaches the target site, synapses, or points of connection, form between the axon and the target neuron. The synapse allows electrochemical signals to be transmitted to the target neuron. Each neuron also has a complex arbor of dendrites that receive information from other neurons. Image in the public domain uploaded from: http://upload.wikimedia.org/wikipedia/commons/7/72/Neuron-figure-notext.svg. Original image from Nicolas Rougier
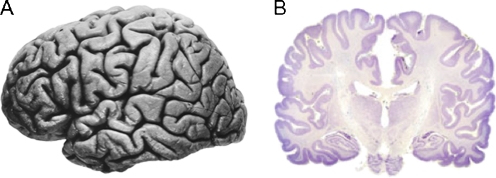
The mature human brain has a characteristic pattern of folds (the sulci) and ridges (the gyri). The enfolding of the mature brain is thought to be an adaptation to the dramatic growth in the size of the brain during the course of evolution. The folding of brain tissue allowed large brains to fit in comparatively small cranial vaults that had to remain small to accommodate the birth process (see Fig. 3a). The largest and most important brain information processing networks involve the neocortex and the subcortical nuclei that relay information to and from the neocortex. The neocortex is a 2–5 mm thick layer of cells that lies on the surface of the brain (the word cortex comes from the Latin term meaning bark, as in the bark of a tree). In the cross-section of the brain shown in Fig. 3b the neocortex is the thin, dark gray strip that follows the brain surface. The subcortical nuclei are clusters of neurons that serve as both signal relay centers communicating between the neocortex and the rest of the body, and as relays among different areas of the cortex. They are located deep in the brain below the cortex and are thus referred to as “subcortical” nuclei. Because both the neocortex and the subcortical nuclei contain the cell bodies of neurons they are gray in appearance, thus giving rise to the term “gray matter”.
Fig. 3.
Two views of the human brain. a. Lateral view (rostral end is left, caudal is right) shows an apparently uniform surface marked by gyri and sulcal folds (Right hemisphere of J. Piłsudski’s brain, lateral view, image in the public domain). b. Coronal cross-section (cut at approximately the level of the dotted line in A) stained for cell bodies that mark neurons. The neocortex is the thin mantel layer (dark purple) on the surface of the brain. The white areas are connecting fiber pathways. Image reproduced with permission from http://www.brains.rad.msu.edu which is supported by the U.S. National Science Foundation. Images obtained with permission from Wiki Commons, http://commons.wikimedia.org/wiki
Populations of neurons are connected to one another by fibers that extend from cell bodies of the individual neurons. There are two kinds of connecting fibers, dendrites and axons (see Fig. 2). Dendrites are arrays of short fibers that look like the branches of a tree; collections of dendrites are often referred to as dendritic arbors. They extend only a short distance away from the neuron cell body. Their main function is to receive the electrochemical input signals from other neurons. Axons are long connecting fibers that extend over long distances and make connections with other neurons, often at the dendrites. Axons act a little like telephone wires in that they are responsible for sending electrochemical signals to neurons located in distant locations. Bundles of individual axons from many different neurons within one region of the brain form fiber tracts that extend to, and make connections with, groups of neurons in other regions of the brain forming the information processing networks. Axons are wrapped in a fatty substance called myelin that, like insulation on a telephone wire, makes the transmission of electrochemical signals between regions efficient. Myelin is white in appearance, thus fiber pathways of brain are often referred to as “white matter”, or “white matter pathways”.
At the very center of the brain are a series of interconnected cavities that form the ventricular system of the brain (see Fig. 2b). The ventricular system is filled with a fluid called cerebral spinal fluid that is completely recycled several times per day. The ventricular system has a number of important functions including cushioning and protection of the brain, removal of waste material, and transport of hormones and other substances (Brodal 2010). During brain development the walls of the ventricles are the site of most neuron production.
Although the neocortex of the brain may appear to be relatively uniform in structure (lateral view), it is actually parcellated into structurally and functionally distinct areas. The areas differ in the kinds of neurons they contain, the kinds of input they receive, and in the types of connections they make with other brain areas. These structural differences result in functional differences creating brain areas that are specialized for carrying out different kinds of processes.
Brain Development in the Embryonic and Early Fetal Periods
This section considers some of the major foundational changes that occur during the embryonic period and early fetal period. In humans the embryonic period begins at conception and extends through GW8. By the end of the embryonic period the rudimentary structures of the brain and central nervous system are established and the major compartments of the central and peripheral nervous systems are defined. The early fetal period, which extends to approximately midgestation, is a critical period in the development of the neocortex. Most cortical neurons are generated by that time and many have migrated to their positions in the neocortex and have begun to from essential brain networks for information processing.
The First Step in Brain Development: Differentiation of the Neural Progenitor Cells
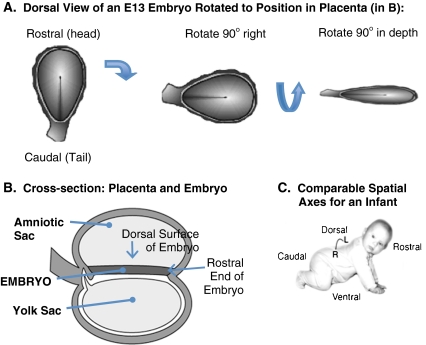
At the end of the second week after conception, the embryo is a simple, oval-shaped, two-layered structure. Figure 4a provides an overview of the major spatial dimensions of the embryo on embryonic day 13 (E13; note during the embryonic period age is often denoted by the number of days after conception, which is referred to as the embryonic day, thus gastrulation begins on embryonic day 13, or E13). Figure 4b orients the embryo within the context of the embryonic placenta, and Fig. 4c shows how the embryonic spatial axes relate to the major spatial dimensions of the infant (see figure caption for details).
Fig. 4.
The major spatial dimensions of the E13 embryo. a. The dorsal surface view of the embryo on E13 is shown in the first panel. The wall of the amniotic sac has been cut away to reveal the dorsal surface (epiblast layer) of the embryo. The rostral (“head”) end of the embryo is on the top of this figure, and the caudal (“tail”) end is at the bottom. b. A lateral cross-section of the embryo and placenta at E13. On E13, the two-layered embryo is located centrally between two major placental sacs. The amniotic sac (which later in development will surround the embryo) is located above the embryo, and the yolk sac is located below. The rostral end of the embryo is to the right in this figure. To place the embryo shown in the first panel of A within the context of the lateral view of the embryo and placenta shown in B, it is necessary to first rotate the embryo so that the rostral end faces right (second panel of A), and then rotate the embryo in depth so that the dorsal surface faces up (last panel of A). C. The comparable rostral-caudal and dorsal-ventral spatial axes of an infant. The spatial axes of a crawling infant are comparable to the position of the embryo in B. Illustrations by Matthew Stiles Davis reprinted by permission of the publisher from THE FUNDAMENTALS OF BRAIN DEVELOPMENT: INTEGRATING NATURE AND NURTURE by Joan Stiles, Cambridge, Mass.: Harvard University Press, Copyright © 2008 by the President and Fellows of Harvard College
Each of the two layers contains a different, very primitive cell type (Fig. 5b). The upper layer contains epiblast cells and the lower layer contains hypoblast cells. By the end of the third week, the embryo is transformed through a set of processes that are referred to collectively as gastrulation into a three-layered structure. While this may seem to be a simple change, the transformations of cell lines that occur during gastrulation set the stage for all subsequent developments in the embryo. The epiblast cells of the upper cell layer will differentiate into the three primary stem cell lines that will eventually give rise to all of the structures in the developing embryo, while the hypoblast cells of the lower layer will form extraembryonic tissues such as the fetal component of the placenta and the connecting stalk. Among the stem cell lines that emerge during gastrulation are the neural stem cells. The neural stem cells are capable of producing all of the different cells that make up the brain and central nervous system, and for this reason the neural stem cells are usually called the neural progenitor cells.
Fig. 5.
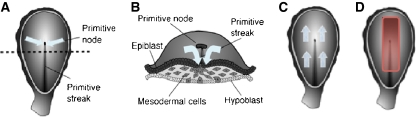
The major events of gastulation occur between E13 and E20. a. The onset of gastrulation is marked by the formation of the primitive streak and the primitive node. The primitive streak provides an opening to deeper embryonic layers. The primitive node is a critical molecular signaling center. On E13, cells from the epiblast layer begin to migrate toward the primitive node and streak (blue arrows). The dotted line indicates the cross-sectional view shown in panel B. b. The migrating cells first move to the primitive streak and then change direction and move down and under the upper layer (blue arrows). As the cells pass the node they receive molecular signals that induce gene expression in the migrating cells. By the end of gastrulation, the hypoblast layer is replaced by the newly formed endodermal layer and the epiblast layer by the ectodermal layer. Between these layers the mesodermal layer forms. c. Once under the upper layer, the cells change direction and begin migrating rostrally under the upper layer (blue arrows). The first cells to migrate form the most rostral regions of the newly forming endodermal and mesodermal layers. Later migrating cells form progressively more caudal regions of the layers. d. Cells that migrate along the axial midline send molecular signals that induce cells in the overlying epiblast layer to differentiate into neuroectodermal cells (red band) which are the neural progenitor cells. Migrating cells also receive a second set of signals from the node that induce anterior or posterior fate in different subpopulations of the neurectodermal cells. Early migrating cells signal anterior fate in the progenitor cells, while late migrating cells signal posterior fate. Illustrations by Matthew Stiles Davis reprinted by permission of the publisher from THE FUNDAMENTALS OF BRAIN DEVELOPMENT: INTEGRATING NATURE AND NURTURE by Joan Stiles, Cambridge, Mass.: Harvard University Press, Copyright © 2008 by the President and Fellows of Harvard College
The first step in the process of gastrulation is signaled by the appearance of a slit-like opening in the upper layer of the embryo called the primitive streak (Fig. 5a). This opening provides access to the lower regions of the embryo. Next, a subset of the epiblast cells detach from the upper layer of the embryo and begin to migrate toward the primitive streak. When they reach the opening they change direction and pass through the primitive streak and under the upper layer (see Fig. 5b). They then change direction again and begin moving toward the rostral end of the embryo (see Fig. 5c). The rostral end of the embryo will develop into the head of the baby. The earliest migrating cells will move to the most rostral positions in the embryo, later migrating cells will move to successively more caudal regions that will develop into the neck and trunk of the body. The migrating cells will form two new embryonic layers. The cells that form the deepest layer will displace the hypoblast cells and form the endodermal stem cell layer which will give rise to structures of the gut and respiratory tract, while the cells that form the new intermediate mesodermal stem cell layer will give rise to structures such as muscle, bone, cartilage and the vascular system. Cells that remain in the epidermal layer are transformed into one of two types of ectodermal layer stem cells. Epidermal ectodermal stem cells will give rise to structures such as skin, nails, and sweat glands, while neurectodermal stem cells will give rise to the brain and central nervous system. The neuroectodermal stem cells are the neural progenitor cells.
Differentiation of all of the embryonic stem cell lines involves complex cascades of molecular signaling. Only the differentiation of the neural stem cells (neural progenitors) will be considered here. At the beginning of gastrulation the epiblast layer cells that will differentiate into neural progenitor cells are located along the rostral-caudal midline of the two-layered embryo (indicated in red in Fig. 5d). The differentiation of these cells into neural progenitor cells is the result of complex molecular signaling that involves multiple gene products (i.e., proteins) that are produced by several different populations of embryonic cells. Recall that at the beginning of gastrulation, epiblast cells begin to migrate toward and then through the primitive streak. As the subset of cells that migrate along the rostral-caudal midline of the embryo approach the opening they pass another structure called the primitive node that is located at the rostral end of the primitive streak (see Fig. 5a, b, c and d). The primitive node is a molecular signaling center. Cells of the primitive node send a molecular signal to the subset of cells that migrate along the rostral-caudal midline of the embryo and that signal, in turn, triggers gene expression in the migrating cells. The gene expression in the migrating cell produces a protein that is secreted into the space between the migrating cells and the cells that remain in the midline region of the upper epiblast layer. The secreted protein binds to receptors on the surface of cells in the upper layer of the embryo and induces the epiblast cells to differentiate into the neural progenitor cells.
Thus, at the end of gastrulation the cells located along the midline of the upper layer of the embryo have transformed into neural progenitor cells (central red rectangle in Fig. 5d). The differentiation of neural progenitor cells requires complex genetic signaling among at least three cell populations: the cells of the node, the migrating cells and the cells that will become the neural progenitors. However, this early signaling is even more complex. In addition to providing the molecular signals that induce the migrating cells to produce proteins that will transform the overlying epidermal cells into neural progenitor cells, the primitive node generates a second set of signals that changes over the course of gastrulation and serves to establish the basic rostral-caudal organization of the embryonic nervous system. Recall that the earliest migrating epidermal cells move to the most rostral end of the embryo and later migrating cells move to successively more caudal locations. The primitive node signals all migrating cells to produce the proteins that signal neural progenitor fate, but each successive wave of migrating cells also receives a second signal that specifies a regional identity for the neural progenitors. Thus, primitive node signals early migrating epidermal cells to produce molecular signals for the cells in the overlying layer to differentiate into neural progenitors capable of producing cells appropriate for forebrain structures, while later migrating cells signal differentiation of neural progenitors capable of producing cells appropriate for hindbrain or spinal cord structures.
The Formation of the Neural Tube: The First Brain Structure
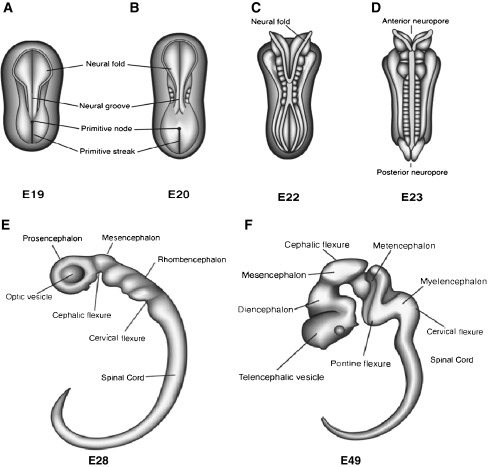
The next major step in brain development involves the formation of the first well-defined neural structure, the neural tube. The neural tube forms during the third week of gestation, between E20-27. As discussed in the last section, by the end of gastrulation the neural progenitor cells have differentiated and are positioned along the rostral-caudal midline of the upper layer of the three-layered embryo. The region of the embryo containing the neural progenitor cells is referred to as the neural plate. The first sign of neural tube development is the appearance of two ridges that form along the two sides of the neural plate on approximately E21 (Fig. 6a). The neural progenitor cells lie between the two ridges. Over the course of several days, the ridges rise, fold inward and fuse to form a hollow tube (Copp et al. 2003). Fusion begins in the center of the developing neural tube and then proceeds in both the rostral and caudal directions (Fig. 6b and c). The anterior neuropore at the most rostral end of the neural tube and the posterior neuropore at the caudal end, are the last segments to close, on E25 and E27, respectively (Fig. 6d). When the neural tube is complete, the neural progenitors form a single layer of cells that lines the center of the neural tube immediately adjacent to its hollow center. In the embryo, the hollow center of the neural tube is cylindrical, like the center of a straw. But as the brain becomes larger and more complex, the shape of the hollow cavity also changes, eventually forming the ventricular system of the brain. Because the neural progenitors are located in the region that will become the ventricles, the region is called the “ventricular zone” (VZ). The neural progenitor cells in the most rostral region of the neural tube will give rise to the brain, while more caudally positioned cells will give rise to the hindbrain and spinal column.
Fig. 6.
Changes in the morphology of the embryo in the embryonic period. The formation of the neural tube occurs between E19 and E29. a. The emergence of the neural ridges is observed on E19. b. The ridges fold over to begin the process of neural tube formation. c. Closure of the neural tube begins on E22 in central regions of the newly forming neural tube. d. Closure continues in rostral and caudal direction. The anterior neuropore closes on E25, and the posterior on E27. e. Following the closure of the neural tube, the embryo begins to expand particularly in anterior regions. The primary vesicles are evident by E28. These include the Prosencephalon, Mesencephalon, and Rhombencephalon. f. By E49 the secondary vesicles emerge. The Prosencephalon differentiates into the Telencephalon and Diencephalon, and the Rhombencephalon into the Metencephalon and Myelencephalon. Illustrations by Matthew Stiles Davis reprinted by permission of the publisher from THE FUNDAMENTALS OF BRAIN DEVELOPMENT: INTEGRATING NATURE AND NURTURE by Joan Stiles, Cambridge, Mass.: Harvard University Press, Copyright © 2008 by the President and Fellows of Harvard College
Although the basic three-dimensional organization of the embryo is evident with the formation of the neural tube, over the next month, the embryo undergoes rapid growth. At the end of neurulation the embryo is 3 to 5 mm long, and by the end of the GW8 it grows to 27 to 31 mm, a tenfold increase. During this period the shape of the primitive nervous system changes dramatically. Just before neural tube closure, the anterior end of the tube begins to expand forming the three primary brain vesicles, or pouches (Fig. 6e). The most anterior of these embryonic brain vesicles is called the “prosencephalon” which is the embryonic precursor of the forebrain. The middle vesicle is the “mesencephalon” which is the precursor of midbrain structures, and the most posterior is the “rhombencephalon” which will become the hindbrain. These three segments further subdivide and by the end of the embryonic period the five secondary brain vesicles are present (Fig. 6f). The prosencephalon divides into the “telencephalon” and the “diencephalon”, and the rhombencephalon divides into the “metencephalon” and “myelencephalon”. The mesencephalon does not further divide. These five subdivisions are aligned along the rostral-caudal axis of the embryo and establish the primary organization of the central nervous system (Stiles 2008).
Neural Patterning in the Embryonic Period
The transformations in the overall shape of the embryo reflect more specific change in neural patterning within all regions of the embryonic nervous system. These changes mark the beginning of a protracted process of neural patterning within the central nervous system that begins in the embryonic period and extends for many years. The changes are gradual and follow an ongoing course of continuous specification and refinement (Sur and Rubenstein 2005). The patterning that emerges in the embryonic period provides only a primitive map of eventual nervous system organization, but it sets the stage for later developments. Embryonic patterning affects all brain regions from the forebrain through the spinal column, such that by the end of the embryonic period in GW8 primitive patterning of sensorimotor regions within the neocortex is established (Bishop et al. 2002), major compartments within diencephalic and midbrain regions have differentiated (Nakamura et al. 2005; Kiecker and Lumsden 2004), and the segmental organization of the hindbrain and spinal column have been specified (Lumsden and Keynes 1989; Gavalas et al. 2003). Space does not permit an extended discussion of embryonic neural patterning. Rather, one example, focused on very early patterning within the developing neocortex, will serve both to define the construct of neural patterning, and to illustrate the idea of continuous specification and refinement of brain areas.
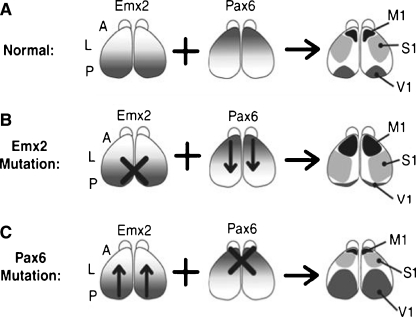
The mature neocortex is partitioned into well-defined structurally and functionally distinct “areas” that are differentiated by their cellular organization and patterns of neuronal connectivity. Initial patterning of neocortex into cortical areas results from different molecular signals present in different regions of the neocortical proliferative zone. Two signaling molecules, Emx2 and Pax6, play an essential role in the early patterning of the presumptive neocortex. Emx2 and Pax6 are transcription factor proteins that are the molecular products of Emx2 and Pax6 gene expression.1 These two signaling molecules are produced in opposite gradients along the anterior-posterior extent of the neocortical proliferative zone (see Fig. 7a). The concentration of Emx2 is highest in posterior and medial regions, and lowest in anterior lateral regions; Pax6 has the opposite expression pattern. The interaction of these two gradients contributes to early patterning of the neocortex (Bishop et al. 2002; Hamasaki et al. 2004). High concentrations of Pax6 combined with low Emx2 induces progenitors to produce neurons appropriate for motor cortex (M1), while the reverse concentrations induce production of neurons for visual cortex (V1). At intermediate levels of both factors somatosensory cortices (S1) emerge.
Fig. 7.
The effects of different concentrations of Emx2 and Pax6 on the development of sensorimotor cortical areas. It is the combination of the specific concentration of each molecule that determines the identity of the cortical region. Mutations that affect the quantities of either molecule alter cortical patterning. Adapted with permission from Bishop et al. (2002). "Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex." J Neurosci 22(17): 7627–7638, Fig. 1
Studies of mutant mice, for which expression of either Emx2 or Pax6 is blocked (thus altering the balance of signals across the cortical proliferative zone), show systematic shifts in the organization of cortical areas (Bishop et al. 2000; Bishop et al. 2002). These studies confirm that it is the interaction of the two signaling molecules that induces change in the surrounding cell populations. When Emx2 expression is blocked, visual areas shrink and somatosensory and motor areas enlarge (Fig. 7b); when Pax6 expression is blocked, visual areas enlarge while somatosensory and motor areas shrink (Fig. 7c). Thus it is the effect of the particular level of one molecular signal in combination with the particular level of another signal that produces the classical pattern of sensorimotor organization in the developing cortex. Since these original reports of the role of Pax6 and Emx2 signaling in neocortical patterning, it has become clear that the interactions are more complex. At least two additional molecules have been identified, Coup-TF1 and SP8. Both are produced in gradients. Coup-TF1 is expressed in greatest concentration in caudal-lateral regions, while SP8 is expressed in rostral-medial regions. As was the case with Pax6 and Emx2, blocking the expression of these genes results in dramatic alteration in the sensorimotor organization of the neocortex (O’Leary et al. 2007; Zembrzycki et al. 2007; O’Leary and Sahara 2008; Sansom and Livesey 2009).
These graded patterns of molecular signaling occur in regions of the neocortical proliferative zone that during gastrulation had been specified as “anterior”. Thus this later patterning constitutes a regional elaboration or refinement of an earlier phase of neural patterning. As will be discussed later, patterning within these regions is far from complete at the end of the embryonic period. Fundamental organizational features of the sensory and motor cortices will not arise until the late fetal period. In addition, across the period of fetal and early postnatal development the structural and functional identity of these basic brain areas remains malleable and subject to the effects of input and experience.
Brain Development in the Fetal Period
The fetal period of human development extends from the ninth gestational week through the end of gestation. The gross morphology of the developing brain undergoes striking change during this time. The human brain begins as a smooth, “lissencephalic” structure and gradually develops the characteristic mature pattern of gyral and sulcal folding. The formation of gyri and sulci follows an orderly sequence. Primary sulci are first seen as grooves positioned in specifically targeted brain regions, secondary branches then begin to form off the primary sulci, followed later by the tertiary branches. The first fissure to form is the longitudinal fissure that separates two cerebral hemispheres. Its development begins in rostral regions as early as GW8 (Chi et al. 1977) and proceeds caudally until it is complete at GW22. Other primary sulci form between GW14-26. These include: Sylvian, Cingulate, Parieto-Occipital and Calcarine (GW14-16); Central and Superior Temporal (GW20-24); and Superior Frontal, Precentral, Inferior Frontal, Postcentral, and Intraparietal (GW25-26). Secondary sulci emerge between GW30-35; formation of tertiary sulci begins during GW36 and extends well into the postnatal period.
The changes that occur in the gross anatomy of the fetal brain reflect dramatic changes occurring at the cellular level. Neuron production begins in the embryonic period on E42, and extends through midgestation in most brain areas. Regions of the brain that contain the cell bodies of neurons are gray in appearance, hence the name. Different populations of neurons form gray matter structures in many regions of the brain including hindbrain and spinal column, cerebellum, midbrain structures, deep subcortical nuclei and the neocortex. Soon after they are produced, neurons migrate away from the proliferative regions of the VZ. The neurons that will form the neocortex migrate in an orderly fashion forming the six-layered neocortical mantel. Once positioned in cortex neurons begin to differentiate producing neurotransmitter and neurotrophic factors, and extending the dendritic and axonal processes that form fiber pathways of the brain neural networks. The major fiber pathways make up the brain white matter. The efficiency of information transmission in the pathways is greatly enhanced by myelin which ensheaths the axons. Myelin is a fatty substance that is white in appearance, hence the name white matter. Much of brain development in the fetal period centers around the processes of neuron production, migration and differentiation. The remainder of this section will consider these processes in greater detail.
Neuron Production
The human brain contains billions of neurons most of which are produced by mid-gestation (Bayer et al. 1993; Rakic 1995). The pool of neural progenitor cells that is specified at the end of gastrulation is far too small to accommodate neuron production on this scale. Thus, the first step in neuron production involves increasing the size of the neural progenitor cell population. Neural progenitors are a mitotic population of cells, that is, they can divide to form new cells. Neurons are post-mitotic cells; once formed they are no longer capable of dividing and producing new cells. From the end of gastrulation through approximately E42 in humans, the population of neural progenitor cells divides by what is described as a “symmetrical” mode of cell division. Symmetrical cell division produces two identical neural progenitor cells. Over multiple rounds of cell division between E25 and E42, symmetrical cell division provides the means for augmenting the size of the neural progenitor pool.
Beginning on E42, the mode of cell division begins to shift from symmetrical to asymmetrical. During asymmetrical cell division, two different types of cells are produced. In neural progenitors, asymmetrical cell division produces one neural progenitor and one neuron (Wodarz and Huttner 2003). The new progenitor cell remains in the proliferative zone and continues to divide, while the postmitotic neuron leaves the proliferative zone to take its place in the developing neocortex. The shift to asymmetrical cell division among the progenitor population is gradual and initially includes only a small proportion of progenitors, but those numbers increase dramatically by the end of cortical neurogenesis. In humans cortical neurogenesis is complete by approximately E108 (Clancy et al. 2001).
Neuron Migration
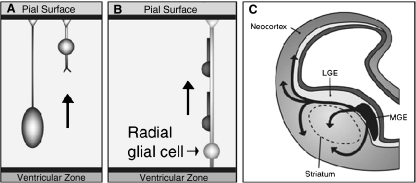
Most neurons are produced in the VZ and migrate radially from the VZ in the center of the brain out to the developing neocortex (see Fig. 8a and b). Very early in neocortical development the distances the neuron must traverse are small. Thus the earliest produced neurons can use a mode of migration referred to as somal translocation (Nadarajah and Parnavelas 2002). In somal translocation the neuron extends a long basal process, which is an extension of the cell’s body, just beyond the edge of the VZ into the outer region of the brain compartment (see Fig. 8a). The basal process attaches to the pial surface, which is the outer surface of the developing brain (Miyata et al. 2001). The nucleus of the cell then moves through cytoplasm of the basal process. As the nucleus moves up the process becomes shorter and thicker but remains attached to the pial surface. At the end of somal translocation the nucleus of the cell has moved out of the VZ and into the embryonic cortex.
Fig. 8.
Different modes of neuronal migration to the neocortex. a. Neuron migration by somal translocation where cell extends a cytoplasmic process and attaches to the outside of the brain compartment (pial surface), and then the nucleus moves up into the brain area. b. Neuron migraton radial glial guide. Radial glial provides scaffold for neuron to migrate along. c. Neuron migration from second proliferative zone in ganglionic eminences by tangential migration (arrows indicate direction of migration for different neuron populations). Figures A and B adapted with permission from Nadarajah et al. (2003). Neuronal Migration in the Developing Cerebral Cortex: Observations Based on Real- time Imaging. Cerebral Cortex, 13, 607–611. Figure 5. Figure C adapted from illustrations by Matthew Stiles Davis reprinted by permission of the publisher from THE FUNDAMENTALS OF BRAIN DEVELOPMENT: INTEGRATING NATURE AND NURTURE by Joan Stiles, Cambridge, Mass.: Harvard University Press, Copyright © 2008 by the President and Fellows of Harvard College
As development proceeds, the brain becomes larger and the primary mode of neuronal migration from the VZ changes. Because of the greater distances, neurons require what was originally identified as a special population of cells within the VZ called “radial glial guides” to support their migration (Rakic 1972). Much like neurons migrating via somal translocation, radial glial guides extend a basal process that attaches to the pial surface of the brain. However, the nucleus of the radial glial cells remains in the VZ, and the basal process forms a kind of scaffolding along which neurons can migrate (see Fig. 8b). The migrating neurons attach themselves to the radial glial guide and move along the cellular scaffold out into the developing cortical plate (Nadarajah and Parnavelas 2002). Each glial scaffold can support the migration of many neurons. Although the radial glial guides were originally thought to be a special, transient population of cells, it has recently been discovered that the cells that provide the scaffolding are actually the neural progenitor cells (Noctor et al. 2001; Noctor et al. 2002; Parnavelas et al. 2002; Weissman et al. 2003).
Very recent studies have identified a second proliferative zone located in the region of the ventral telencephalon that will later develop into the basal ganglia (see Fig. 8c). During embryonic and fetal development three compartments in this region, the medial, lateral and caudal ganglionic eminences, are the source of an important class of inhibitory cortical interneurons (Anderson et al. 2001; Corbin et al. 2001; Nery et al. 2002). Unlike neurons migrating from the VZ, these neurons traverse long distances using a mode of migration that has been termed “tangential migration”, because the route of migration traverses the contour of developing cortical mantle tangentially. Tangential migration involves a variety of signaling pathways not seen in radial migration. Neurons use a number of guidance molecules produced in local regions along their migratory route to direct their movement into the cortex (Marin and Rubenstein 2001; Huang 2009; Valiente and Marin 2010).
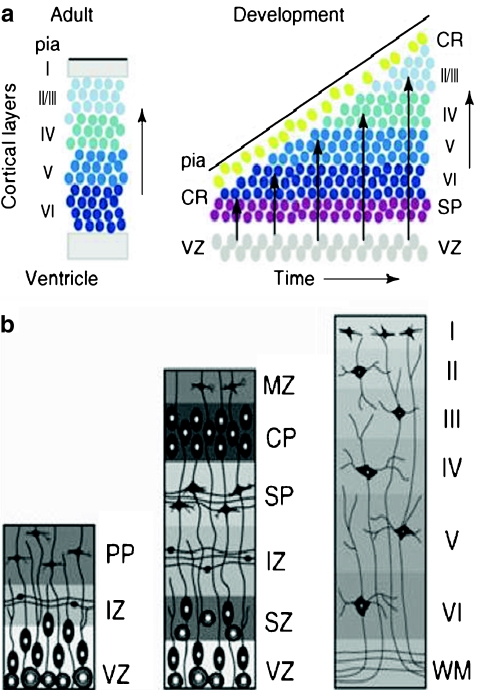
The migration of neurons into the developing neocortex results in the formation of an orderly 6-layered structure (Cooper 2008). With one exception, earlier migrating neurons form deepest layers of cortex and later migrating neurons form successively more superficial layers (see Fig. 9a) such that the order of migration has been described as inside-out. The exception to the inside-out rule is the very earliest set of migrating neurons. These first neurons to leave the proliferative zone initially form a primitive structure called the preplate (PP; see Fig. 9b, first panel). Once the preplate is complete, the next wave of migrating neurons splits the preplate into two separate regions, the marginal zone (MZ) and the subplate (SP). These neurons begin to form a new region between the MZ and SP that is the emerging cortical plate (CP; see Fig. 9b, second panel). The first neurons to arrive in the CP are the cells that will form cortical layer 6, the deepest layer of cortex, subsequently migrating cells will form progressively more superficial layers of cortex.
Fig. 9.
a. The earliest produced neurons migrate to the deepest cortical layers (dark blue). Subsequently migrating neurons migrate to successively more superficial layers (lighter blues) creating an inside out order of migration. Adapted with permission from Cooper (2008). Trends in Neuroscience, 31(3), 113-19. b. As shown in the first panel, the first neurons migrate from the ventricular zone (VZ) to form the preplate (PP). As shown in the second panel, the next neurons split the PP into the marginal zone (MZ) an the subplate (SP), both transient brain structures. The mature brain, shown in the third panel, has six well developed cortical layers (I-VI), but none of the embryonic structures (MZ, SP, VZ). The intermediate zone (IZ) has become a mature white matter layer (WM). Illustrations by Matthew Stiles Davis reprinted by permission of the publisher from THE FUNDAMENTALS OF BRAIN DEVELOPMENT: INTEGRATING NATURE AND NURTURE by Joan Stiles, Cambridge, Mass.: Harvard University Press, Copyright © 2008 by the President and Fellows of Harvard College
Both the MZ and the SP are transient brain layers that play a critical role in the development of the cortex, but both largely disappear by the end of the fetal period (see Fig. 9b, third panel). The MZ contains an important class of cells, the Cajal-Retzius cells (CR), that control the positioning of neurons into the correct layers of cortex. The CR cells produce a molecular signal, called Reelin, that is part of the pathway that signals neurons when to stop migrating and take up their positions in cortex (Bielle et al. 2005; Huang 2009; Valiente and Marin 2010). Each new wave of migrating neurons bypasses the previous wave of neurons such that each new wave of migrating cells assumes the most superficial position within the developing cortex. As each new wave of neurons reaches the top of the cortical plate, it moves into the zone of Reelin signaling and receives the cue to stop. The cortex of animals with defects in Reelin signaling lack laminar structure; the preplate fails to split, and the neurons simply conglomerate under the abnormal preplate (Rice and Curran 2001). Finally, neurons in the subplate layer do not participate in the formation of cortical layers, but as will be discussed later, they are essential for establishing the primary sensory inputs to the developing neocortex.
Neuron Differentiation
The different layers of cortex contain different types of neurons. One question is how these different classes of neurons are derived. This question was addressed by McConnell and colleagues (McConnell and Kaznowski 1991; Frantz and McConnell 1996; Desai and McConnell 2000) in a series of studies that asked whether different subsets of progenitor cells produce specific kinds of neurons, or if the same progenitor is capable of producing multiple neuron types? They found that early in corticogenesis neural progenitor cells are capable of producing any neuron type, but that with development they became more and more restricted in the types of neurons they can produce. McConnell and colleagues used progenitor cell transplant studies to examine this question. They took dividing progenitor cells from a host fetus of a particular age, and transplanted the cells into a donor animal of a different age. The key question was, what kinds of neurons do the transplanted progenitors produce in the host environment? In the first experiment, progenitor cells were taken from young donor animals (if left in the donor these progenitors would produce neurons for layer 6 of cortex), and transplanted them into an older host (whose progenitors were producing layer 2–3 neurons). The transplanted progenitors produced layer 2–3 neurons suggesting that some kind of signaling from the host induced a change in the type of neurons being produced by the transplanted progenitors. However, when the timing was reversed and progenitors from an older host were transplanted to a younger animal, the progenitors continued to produce cells appropriate for the donor animal. It appears that early in corticogenesis progenitor cells can receive signals to produce any neural cell line, but as development proceeds and these early cell types are no longer needed, the progenitor loses the capacity to generate those cells, exhibiting what is termed fate restriction. While there is evidence that fate restriction may be at least in part controlled by cell intrinsic signaling (Shen et al. 2006; Leone et al. 2008) the particular signaling pathways that induce these shifts in the progenitor population remain poorly defined (Molyneaux et al. 2007).
Once they have reached their target region of cortex, the young neurons need to become part of information processing networks. In order to become integrated into neural networks, the neurons need to develop neuronal processes (axons and dendrites) that allow them to communicate with other neurons. Axons are the principal means of sending signals from the neuron, while dendrites are major sites for receiving input from other neurons. Each cell has many dendrites that form dense “arbors” in the immediate vicinity of the cell, and a single axon that can extend for some distance away from the cell. At the tip of each axon is a structure called a growth cone. The growth cone is the site of axon elongation and extension (Brown et al. 2001). As the axon is extended, the growth cone samples the local environment for guidance molecules that direct the axon toward its target. Some guidance cues are attractive and signal movement toward a source, others are repulsive and guide movement away. Once the axon has reached its target, connections called synapses are formed with the target cell. Synapses allow for the transmission of electrochemical information which is the essential means of communication in the brain.
Two of the most important pathways in the brain are the ones that transmit sensorimotor information, the thalamocortical (TC) and corticothalamic (CT) pathways. The TC relays sensory and motor information from the receptors in the retina, cochlea, muscle or skin to the sensorimotor regions of the neocortex via the major subcortical sensorimotor relay, the thalamus. The CT pathway completes the feedback loop by transmitting information from cortex back to the thalamus. These essential pathways begin to form in the later part of the second trimester in humans, and are complete by GW 26 (Kostovic and Jovanov-Milosevic 2006). The cells of the transient subplate layer of the developing brain (see Fig. 9b) play an essential role in establishing these pathways. When TC axons arrive at the developing cortex during GW22 they do not immediately make connections with neurons in the primary input layer of cortex (layer 4). Rather, they initially make connections with the neurons of the subplate layer. The TC-subplate connections last for approximately 4 weeks, during which time the subplate neurons make connections with neurons in cortical layer 4. The subplate neurons appear to provide instructive input to the TC neurons during this period. In the absence of subplate neuron signaling, normal patterns of connectivity between TC axons and layer 4 cortical neurons do not develop. A similar pattern of instructive connectivity is seen in the development of the CT pathway. Prior to the establishment of connections between neurons from the deep layers of cortex (layers 5 and 6) and the thalamus, subplate neurons extend and establish connections with thalamic neurons. It is thought that the subplate connections may serve to guide the CT axons to their positions in the thalamus. Once the TC and CT pathways are complete, the subplate neurons retract their connections and the cells themselves gradually die off.
Regressive Events in Prenatal Brain Development
While most neurodevelopmental events involve the proliferation of neural elements, two important processes involve substantial loss of neural elements. These two processes include naturally occurring cell death, which involves the normal loss of 50% or more of the neurons within a brain region; and synaptic exuberance and pruning in which there is massive excess production of connections followed by the systematic elimination of up to 50% of those connections. Both of these processes reflect nonpathological events that play an essential role in establishing the complex networks of the developing brain. The timescales of these two sets of events are different. Most naturally occurring cell death in neuronal populations occurs prenatally, while both cell death in glia populations and the events involving exuberant production and pruning of connections are largely postnatal events. This section will consider cell death in neural populations during the prenatal period. The major postnatal regressive events will be discussed in the next section.
There are two broad categories of cell death. Necrotic cell death is a pathological process that follows insult or injury to a population of cells and is a mechanism for eliminating damaged tissue from the biological system. Apoptosis is a distinct form of cell death that reflects a highly regulated sequence of physiological events. Apoptosis is a well-understood cell-intrinsic process. It involves a cascade of gene expression that ultimately results in the breakdown of nuclear chromatin (DNA and support proteins) and the fragmenting of the cell. All neurons and neural progenitor cells (as well as many other types of cells) have this intrinsic “suicide” program. The set of genes involved in the apoptotic cascade is large, but very specific, with each molecular signal triggering the next step in the cascade. A wide variety of cell intrinsic and environmental factors can influence the apoptotic process. Some trigger cell death, while others protect the cell by preventing the cascade. Apoptosis has been documented within all of the neuronal and neural progenitor cell compartments in the human brain (Rakic and Zecevic 2000). Across the cortex, rates of apoptosis within all layers is high, reaching 70% in some regions (Rabinowicz et al. 1996).
One factor that protects against the apoptosis cascade is uptake of neurotrophic substances (Levi-Montalcini 1964; Oppenheim 1989). Neurotrophic factors are produced by target neurons at synaptic sites, and are taken up by the afferent neurons that make effective connections with the targets (Huang and Reichardt 2001). During development it is thought that neurons compete for neurotrophic resources. According to the neurotrophic hypothesis (Oppenheim 1989), neurons that establish effective connections are able to obtain more neurotrophic factor and are more likely to survive. Thus one important function of cell death in brain development is its role in regulating the establishment of effective and functional neural circuits (Buss et al. 2006). In addition, a number of other functions for cell death have been proposed. Although the evidence is somewhat limited, cell death may serve as a mechanism for correcting errors in neuronal production or migration (Buss and Oppenheim 2004). There is substantial evidence that cell death plays an essential role in eliminating cell populations that serve only a transient function in brain development, such as cells of the MZ or SP. Importantly there is strong evidence of high levels of cell death in the neural progenitor population (de la Rosa and de Pablo 2000; Yeo and Gautier 2004). Rates of apoptosis in the VZ increase across the period of corticogenesis suggesting gradual elimination of this important but transient cell population. Note that the mechanism for triggering cell death in the MZ, SP or progenitor cell populations must differ from those discussed for neurons. None of these populations contain cells that enter neural networks, thus the specific effects of neurotrophin availability are not likely associated with the apoptotic pathways in these cells groups.
Brain Development in the Postnatal Period
Though the production and migration of neurons are largely prenatal events, proliferation and migration of glial progenitors continues for an extended period after birth, and the differentiation and maturation of these cells continue throughout childhood. The full scope of neuron-glia interactions is still not fully defined, but it is clear that these interactions play an important role in functional organization of neural circuits during postnatal life. Importantly, estimates of the developmental time course in humans of the postnatal processes outlined below are derived by extrapolation from data acquired in other species, often rodents, and from very limited human postmortem material. Unfortunately, the result is much remaining uncertainty about the temporal extent of proliferation, migration, differentiation, and regression during the postnatal period in humans, and about the timing of these processes relative to each other. In vivo brain imaging of children is providing important clues about the time course of age-related biological alterations in the brain, and provides an opportunity to link these changes to evolving behavior.
Postnatal Proliferation and Migration
In the postnatal period, neurogenesis continues to only a very limited degree; however, in the subventricular zone, new neurons continue to emerge and migrate to the olfactory bulb, and neurons are also produced in the dentate gyrus of the hippocampus, where they migrate from the subgranular layer only as far as the nearby granular layer. These exceptional forms of neurogenesis appear to continue throughout adult life but produce only a small percentage of the neuronal population. In contrast, proliferation and migration of glial progenitors, while beginning prenatally, continue for a protracted period as oligodendrocytes and astrocytes differentiate; in fact, glial progenitors (particularly oligodendrocyte progenitor cells, or OPCs) appear to persist indefinitely in the adult brain in a wide anatomical distribution, and can differentiate in response to injury. Glial progenitors proliferate in the forebrain subventricular zone and migrate outward into the overlying white matter and cortex, striatum, and hippocampus, where they differentiate into oligodendrocytes and astrocytes. Unlike neural progenitors, glial progenitors continue to proliferate as they migrate (Cayre et al. 2009).
Myelination
Upon reaching its destination, an OPC begins to differentiate by extending processes and increasing myelin protein expression. The processes then begin to form membrane wraps around nearby axons. Eventually the oligodendrocyte forms tightly wrapped multi-layered sheaths from which most of the cytoplasm has been extruded. The dramatic increase in axonal conduction velocity associated with myelination is well known. However, recent research suggests that functional interactions between oligodendrocytes and neurons extend far beyond the effects of the electrically insulating sheath. Oligodendrocytes synthesize a number of trophic factors that appear to contribute to the maintenance of axonal integrity and neuronal survival, and neuron-oligodendrocyte interactions have been shown to influence neuronal size and axon diameter (McTigue and Tripathi 2008). An intriguing new line of evidence also suggests that a subset of the OPCs dispersed throughout the brain form excitatory and inhibitory connections with neurons, and thus may contribute actively and directly to neural signaling (Lin and Bergles 2004).
In summary, proliferation and migration of glial precursors and differentiation of astrocytes and oligodendrocytes are largely postnatal processes. While there is little doubt that these processes play a critical role in the functional maturation of developing neural circuits, the full scope of their impact on neural dynamics may be much greater than was previously appreciated. Ongoing research continues to uncover additional molecular interactions between neurons, oligodendrocytes, and astrocytes. The existence of these interactions implies that the late maturation of glial populations probably has widespread functional implications.
Regressive Events in the Postnatal Period
Cell Death in Glial Populations
As described above, brain development involves overproduction of neurons and glial cells, neural processes, and synapses. Although neural apoptosis has its peak during prenatal life, apoptosis in glial cell populations has a time course corresponding to the protracted postnatal time course of differentiation from glial precursors. During the period of initial myelination, many excess oligodendrocytes undergo apoptosis a few days after differentiating, and there is evidence that this process depends on signals from nearby axons, such that the number of surviving oligodendrocytes matches the local axonal surface area (see McTigue and Tripathi 2008, for review).
Synaptic Exuberance and Pruning
Although the development of neural networks requires the formation of precise connections between developing neurons and their targets, it is well documented that initial patterns of connectivity in the developing brain are exuberant in terms of both the numbers of connections formed and their topography. This exuberance can be observed on two very different time scales that appear to support different aspects of the process of emerging connectivity in the developing brain. At a macroscopic level, exuberance and pruning can be observed within major brain areas and pathways on timescales that extend over months or even years. But at a microscopic level very rapid formation and retraction of connections can be observed at the level of individual neurons over periods of minutes or hours.
At the macroscropic level, studies of both monkeys and humans have documented widespread exuberant production of connections throughout all brain regions in the early postnatal period (Zecevic et al. 1989; Bourgeois and Rakic 1993; Bourgeois et al. 1994; Huttenlocher and de Courten 1987; Huttenlocher and Dabholkar 1997). Across brain areas, the number of synapses plateaus at levels nearly twice as high as those observed in the adult brain, and then slowly declines to normal adult levels across the period of childhood and adolescence (see Fig. 10a and b). But, the exuberance of connectivity extends beyond the sheer numbers of connections within a brain region. Early in development transient connections form throughout the brain, which are not observed in adults. Exuberant connectivity has been documented in pathways as diverse as the corpus callosum, thalamocortical pathways, corticospinal tract and pathways linking the temporal lobe and the limbic system (Stanfield et al. 1982; Stanfield and O’Leary 1985; Innocenti and Price 2005). Many factors affect the retention or elimination of pathways. Competition for resources such as neurotrophic factors plays a significant role in selection of pathways. Importantly, afferent input plays a critical role in modulating the stabilization or elimination of pathways.
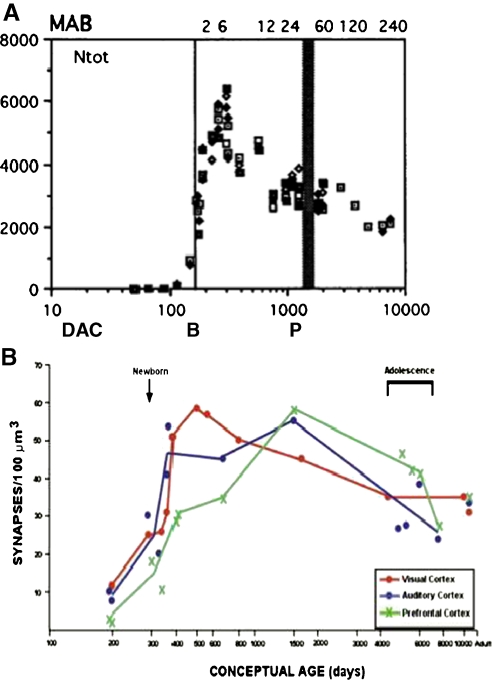
Fig. 10.
Synaptic connectivity in the primate brain exhibits initial exuberant production followed by gradual pruning. a. In primate brain, the number of synaptic contacts per probe was plotted along a logarithmic scale as a function of days after conception (DAC). Months after birth (MAB) are indicated along the top of the graph, birth (B) at 166 days post conception is indicated by the thin vertical line and puberty (P) at 3–4 years by the thick vertical line. Reprinted with permission from Bourgeois and Rakic (1993). “Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage.” Journal of Neuroscience 13(7): 2801–2820, Fig. 3. b. In human brains, counts of the number of synapses per constant volume of tissue were measured as a function of pre- and postnatal age. Adapted with permission from Huttenlocher and Dabholkar (1997). Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology, 387, 167–178, Fig. 2
Recent studies using real time imaging have begun to document the processes of exuberance and pruning at a more microscopic level. These studies suggest that as axons seek their targets they very rapidly sample the surrounding space forming and retracting synaptic connections in a dynamic, ongoing and balanced fashion (Hua and Smith 2004). Thus at the level of individual neurons the processes associated with exuberant production and retraction of connections provide rapid sampling of the local environment and serve to support axon guidance and target detection.
Imaging Studies of Brain Morphology
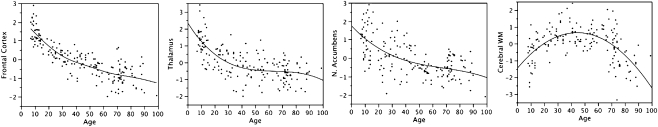
Since MRI is a safe technique for use in children it has now been applied widely in pediatric imaging, and it reveals dramatic changes in the tissues of the developing brain during the postnatal brain growth spurt. These MRI signal changes reflect alterations in tissue chemistry that are presumed to mark the proliferation of oligodendrocytes and deposition of myelin, and they reveal much about the timing and anatomical distribution of these processes (Barkovich 2000; Barkovich 2005). The visual appearance of the brain on MR images changes appreciably over the first 2 to 3 years of life, mirroring an orderly pattern of myelination in white matter regions. However the changes in gross brain structure that continue past this age are subtler, and were not well described until after quantitative morphometry techniques were applied. Early MR morphometry studies comparing brain morphology in children and adults showed that gray matter volumes, both in the cerebral cortex and in subcortical nuclei, were considerably larger in school-aged children than in young adults (Jernigan and Tallal 1990; Jernigan et al. 1991; Pfefferbaum et al. 1994). This suggested that tissue alterations related to brain maturation might be much more protracted during childhood than was generally supposed, and that some of these alterations might be regressive; that is, they might involve tissue loss. These findings were confirmed and extended by later studies (see Toga et al. 2006 for a review), but the underlying tissue alterations remain a matter of speculation. The size of the cranial vault increases dramatically after birth but very little after the first decade. However, the MRI results suggest that throughout childhood and adolescence effects of waning progressive changes, associated with continuing maturation of glial populations and neurotrophic effects, are opposed by concurrent regressive changes, perhaps associated with “pruning” of neuronal processes. These observations are consistent with ample histological evidence for ongoing myelination across this period (Yakovlev and Lecours 1967), and more limited, but persuasive, evidence for reduction of synaptic density in cortex during childhood (Huttenlocher and Dabholkar 1997), but it remains unclear to what extent these factors, and perhaps others, contribute to the changing morphology observed with MRI. Data shown in Fig. 11, plotting estimated volumes of brain structures across the lifespan, illustrate that during childhood and adolescence changes in brain structure are at least as dramatic as those at the end of life. The plots illustrate results from an extended age-range for volumes of particular brain structures (modified from Jernigan and Gamst 2005). Shown are continuous age-related decreases in volume of frontal cortex, thalamus, and nucleus accumbens across the lifespan, and increases in cerebral white matter volume during childhood and early adulthood that give way to decreases later in life. All volumes are normalized for cranial volume—which does not change appreciably over this age range.
Fig. 11.
Estimated volumes of brain structures in normal volunteers are plotted against age. The volumes in the figures are presented as standardized residuals (removing variability associated with volume of the supratentorial cranial vault). They are, from left, volumes of frontal cortex, thalamus, nucleus accumbens, and cerebral white matter. Note the rapid age-related change (and striking individual differences) in the childhood and adolescent age-range. (Figures modified from Jernigan & Gamst, Neurobiology of Aging, 26 (9), 1271–1274, 2005)
More recent MR morphometry studies have provided more anatomical detail by employing mapping methods for visualizing the pattern of age-related change (Giedd, Snell and et al. 1996; Giedd, Vaituzis and et al. 1996; Sowell et al. 1999a; Sowell et al. 1999b; Sowell et al. 2002). Studies of developing children describe the protracted course of postnatal white matter growth and establish that before adolescence the volume of tissue with the MR signal characteristics of “gray matter” begins to decline concurrently in locations throughout the brain, e.g., in cerebral cortex and deep nuclei. The most detailed studies, employing both high-resolution mapping techniques and longitudinal assessments (Gogtay et al. 2004; Sowell et al. 2004) have revealed a modal pattern of childhood and adolescent change in the cerebral cortex that includes not only widespread, regionally specific, apparent cortical thinning, but more limited areas of cortical thickening as well. On average, cortical thinning appears to occur first in primary sensory-motor cortex and then to progress into secondary, then multimodal, and then supramodal cortical areas throughout childhood and adolescence. A recent study [Ostby et al. 2009] confirmed these observations in a large cross-sectional sample and provided concurrent estimates of cortical surface area and cortical thickness. This is an important contribution since studies of cortical volume conflate these factors, and no previous studies had addressed whether the changes in cortical thickness are accompanied by alterations of surface area as well. Ostby et al. (2009) report that between the ages of 8 and 30 years more modest decreases in cortical surface area accompany robust decreases in cortical thickness.
An important issue germane to the interpretation of these effects is their relationship to myelination. At the most basic level, cortical “thinning” could simply reflect increased myelination in the white matter tracts coursing within and near the deepest layer of cortex. In other words the “gray” signal of the unmyelinated fibers could simply be becoming more “white” as myelin is deposited. This is clearly a part of what is measured as cortical thinning with morphometry, especially in younger children. However, there is evidence that true regressive changes also occur in some structures—probably due to loss or simplification of neuronal processes (dendrites and/or axons). This can be inferred from the fact that the progressive changes that would be expected to result from continuing myelination do not seem to increase cranial volume in late childhood (as though they were opposed by some regressive factor); and from the fact that there are modest but significant CSF volume increases adjacent to the cortical surface and in the ventricular system over this age-range, as might be expected, ex vacuo, in the wake of the loss of neural elements in the adjacent tissues (Jernigan et al. 1991; Sowell et al. 2002).
Using mapping methods, Sowell et al. (2004) reported similarities in the patterns of brain growth and cortical density reductions and interpreted this as evidence that local cortical thinning might bear a direct relationship to myelination of nearby fiber tracts; but the nature of this relationship remains unclear. It is possible that functional changes resulting from maturation of fiber tracts stimulate cortical thinning (or thickening), or, conversely, that increasing activity due to intrinsic cortical maturation stimulates myelination of the axons in the maturing network. Neuron-glia signaling mechanisms mediating effects of action potentials on oligodendrocyte differentiation and myelination have been reported (see Fields and Burnstock 2006 for review); therefore it is plausible that increasing activity in neural circuits plays a role both in myelination and in stimulating intracortical structural alterations.. However, the interactions among these factors in developing brain tissues are still poorly understood. In summary, MR morphometry studies reveal a complex pattern of development in brain structure during childhood and hint that ongoing maturation of fiber tracts probably plays a key role. Only recently, however, has it been possible to examine the maturation of fiber tracts directly, using diffusion tensor imaging (DTI) (Basser et al. 1994; Mori and van Zijl 1995).
Diffusion Imaging of Fiber Tract Development
Diffusion imaging measures the diffusion of water molecules through the tissue. A common use of diffusion imaging involves fitting, for each voxel, a mathematical function called a tensor, that estimates proton diffusion (motion) along each of 3 orthogonal spatial axes. Tensors from voxels in the brain with high water content, such as in ventricles, exhibit high levels of proton diffusion that has no preferred direction; i.e., the diffusion is random, or isotropic. Diffusion in gray matter voxels is lower but also relatively isotropic. However, in voxels that contain fiber bundles, the diffusion is higher along the long axis of the fibers. This directionality of the diffusion is usually measured as an index of anisotropy, usually as fractional anisotropy (FA). It has been shown that proton diffusion in the cerebral white matter of human newborns is high, and exhibits low anisotropy (Hermoye et al. 2006). As the fiber tracts mature, and myelination proceeds, diffusion declines, and anisotropy (or FA) increases. By examining the change in detail, i.e., by measuring the three tensor eigenvalues (each a measure of the amount of diffusion along one of the spatial axes), it has been shown that developmental increase in FA often reflects a decrease in all three diffusivities, which is, however, smaller in the principal eigenvalue (i.e., in diffusion along the long axis of the tracts). The interpretation (Suzuki et al. 2003) is that unrestricted water in extra-axonal space declines, decreasing tissue diffusivity overall, while diffusion within and/or along the membranes of the axons remains relatively constant or increases. The denser packing of axons that results from myelination and increases in axonal diameter are likely to reduce diffusion by decreasing extra-axonal water. How alterations of fiber morphology or intra-axonal diffusion contribute to changing tensor values is less well understood. Nevertheless, there is growing evidence that alterations reflected in and measurable with diffusion imaging continue throughout childhood and adolescence (Schneider et al. 2004; Barnea-Goraly et al. 2005; Snook et al. 2005). The pattern of FA increases, for example, suggests that FA reaches asymptote earliest in long projection, then commissural, and finally association fibers, the latter continuing to exhibit age-related FA increases well into adulthood (see Huppi and Dubois 2006; Mukherjee and McKinstry 2006 for reviews; Cascio et al. 2007).
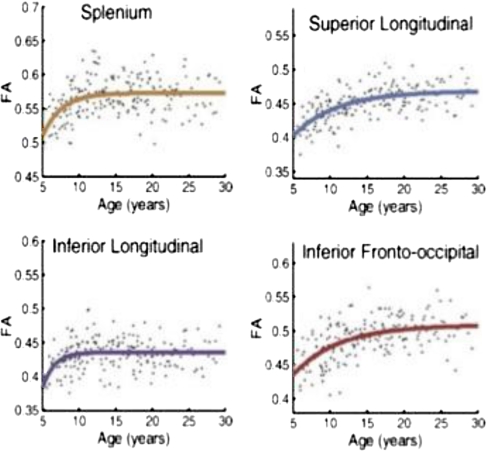
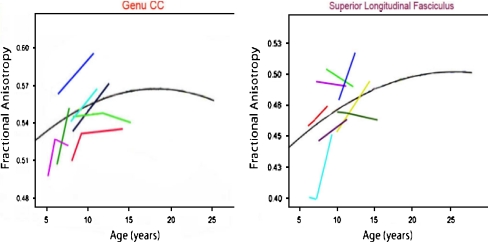
Lebel et al. (2008, Lebel and Beaulieu 2009) reported diffusion imaging results in a large group of typically developing children and young adults. Robust increases in FA across the age-range from 5–12 years were observed within fiber tracts defined with manual and semiautomated tractography. In Fig. 12 the cross-sectional results are shown for 4 major tracts: the corpus callosum (splenium), the inferior and superior longitudinal fasciculi, and the inferior fronto-occipital fascicului. These plots reveal the rapid change in FA in young school-aged children and also demonstrate that different tracts vary in the pace with which adult values of FA are approached. This group recently reported individual trajectories of tract FA obtained with repeated imaging of school-aged children. Some of the results are shown in Fig. 13: These data were obtained from sessions spaced 2 to 4 years apart, but they clearly demonstrate that over this age range substantial increases in FA occur within individual children, and they suggest wide individual differences in the pace of these changes.
Fig. 12.
Cross sectional data from Lebel et al. (2008, Lebel and Beaulieu 2009) showing robust FA increases in 4 major fiber tracts; note rapid change in FA in young school-aged children and variability in the pace at which FA in the different tracts approaches asymptote. Reprinted with permission from Lebel et al. (2008). “Microstructural maturation of the human brain from childhood to adulthood.” Neuroimage 40(3): 1044–1055
Fig. 13.
Individual trajectories for sequential measurements of FA in the genu of the corpus callosum (left) and the superior longitudinal fasciculus (SLF) (right), redrawn from Lebel et al. (2008, Lebel and Beaulieu 2009), illustrating individual differences
In summary, in vivo brain imaging is opening a window on continuing brain development during infancy and childhood. As the imaging techniques mature, and the biological significance of the signals they record are more firmly established, these techniques promise to reveal much more about the dynamic interactions within human brain tissues that attend the molecular and microstructural events described in this review.
The Role of Experience in Brain Development
The events of the prenatal period serve to establish the core compartments of the developing nervous system from the spinal cord and hindbrain to the cortical structures of the telencephalon. These early events also provide initial patterning within each of the major subdivisions of the brain, but this early patterning, particularly in the neocortex, is both underspecified and malleable. The mature organization of the neocortex emerges over a protracted time during the postnatal period, and it requires diverse forms of input. Some of this input arises from within the organism in the form of molecular signaling and cross-regional activity. But the specific experience of the individual organism also plays an essential role in establishing the mature organization of the neocortex. The development of normal brain organization requires input via all of the major sensory systems. When specific aspects of input are lacking, alternative patterns of brain organization can and do emerge. These alternative patterns of organization reflect the effects of altered profiles of neural competition and capture a fundamental property of mammalian brain development, the capacity for plastic adaptation.
The Role of Input on Brain Development
Greenough introduced the term “experience expectant” development to capture the idea that the early experience of the organism plays an essential role in normal brain development, particularly in the early postnatal period (Greenough et al. 1987). Although cortical patterning begins in the embryonic period it remains malleable for an extended period of time. Typical, expected, postnatal experience is necessary for the emergence of normal patterns of neocortical organization. When that input is lacking brain areas develop differently, and the specific pattern of development reflects the kinds of input that the organism actually received. At later ages, the developing—and even the mature—nervous system continues to require input to acquire new knowledge and to develop functional neural systems. Greenough has termed this later phase of development “experience dependent” learning. These two important constructs suggest that throughout development experience plays an essential role in establishing and refining neural organization in ways that allow the organism to adapt to the contingences of the world in which it lives. Studies that systematically manipulate the specific experience of the young organism provide insight into the dynamic and adaptive nature of brain development.
Two simple ways to alter input are enrichment and deprivation. Both have dramatic effects on the structural and functional organization of the developing brain. Greenough has shown that simply rearing animals in either impoverished (standard laboratory cage) or enriched environments (large enclosures with interesting and changing landmarks and multiple littermates) affects the development of a wide range of brain structures and functions (Black et al. 1987; Greenough and Chang 1988; Jones and Greenough 1996; Markham and Greenough 2004). Animals reared in complex environments show enhancement in density of cortical synapses, increases in the number of brain support cells, and even augmentation of the complexity of the brain vascular system. Further, many of the effects of rearing in the complex environment persist even when the animal is returned to more impoverished conditions.
Sensory deprivation has more selective effects that target particular cortical sensory systems. The seminal studies of Hubel and Weisel (Hubel and Wiesel 1977; Hubel et al. 1977) showed that monocular visual deprivation in the early postnatal period can substantially alter basic patterns of organization within primary visual cortex (PVC). Within the typical primary visual pathway, inputs from the two eyes remain segregated from the retina to the thalamus to PVC. In PVC the inputs from the two eyes form a distinctive banded pattern, called ocular dominance columns (ODC), that give the input layer of PVC a striped appearance (see Fig. 14a). When patterned input to one eye is blocked by suturing the eyelid closed the effect of this altered experience on ODC organization is striking (see Fig. 14b). The bands representing the active eye widen and expand into the territory of the deprived eye; while the bands representing the deprived eye shrink to thin stripes. The monocular reduction in activity introduced by the suturing procedure alters the competitive balance of the input from the two eyes. The inputs from the active eye invade and subsume territory that would normally have received input from the deprived eye.
Fig. 14.
Autoradiographs of the ocular dominance columns (ODC) in two young monkeys. A radioactive transneuronal dye was injected into one eye and taken up by neurons in the input layer of primary visual cortex (PVC). a. The normal patterning of the ODC in a 6-week old monkey. ODCs from each eye are equal and adultlike. b. ODC patterning from an animal that was monocularly deprived at 2 weeks of age. The nondeprived eye was injected with the tracer at 18 months of age. ODC for the nondeprived eye (light bands) expand and while those of the deprived eye (dark bands) shrink showing clear dominance of the nondeprived eye in PVC. Adapted from LeVay et al. (1980). Journal of Comparative Neurology, 191, 1–51, Figs. 5 and 6
Neural Pathology and Input
The enrichment and deprivation studies provide powerful evidence of the role of experience on brain development. The enrichment studies suggest widespread effects of experience on the complexity and function of the developing system, while the deprivation studies document the capacity for neural reorganization within particular sensory systems But experimental studies can be more invasive, introducing procedures that directly affect or eliminate specific brain areas. These studies provide evidence that plasticity in developing neural systems can extend to the capacity to develop fundamentally different patterns of organization and function in the face of injury. For example, Sur and colleagues (Sur et al. 1988; Pallas et al. 1990), surgically eliminated the major input pathway to primary auditory cortex (PAC) in one day old ferrets to determine what would happen to this important sensory area in the absence of input. In the normal course of early development the visual pathway from the retina extends what are typically transient connections to PAC, in addition to the normal connections to PVC. The retina-PAC connections are typically pruned as part of the normal competitive processes. However, in the absence of competition, the inputs from the retina stabilize and form a functional visual pathway to PAC. PAC takes on patterns of internal organization that, while cruder, are characteristic of PVC (Sur and Leamey 2001) and the “rewired” PAC functions as a visual area in behavioral testing (von Melchner et al. 2000). Thus the altered early experience of the organism results in fundamental functional and structural reorganization of a primary sensory area, providing robust evidence for the role of neural plasticity in early brain development.
Summary and Conclusions
Over the past three decades there has been tremendous progress in our understanding of the basic principles of neural development. This progress has changed our fundamental models of how brains develop. Strongly deterministic models have given way to more dynamic and interactive models anchored in the process of development, itself. As suggested by the examples presented in this paper, the processes that underlie and guide brain development involve the ongoing interplay of genetic and environmental factors. Brains do not develop normally in the absence of critical genetic signaling and they do not develop normally in the absence of essential environmental input. Rather, at each point in development, organism intrinsic and environmental factors interact to support the increasingly complex and elaborate structures and functions of the brain. During the embryologic period the interactive processes are most prominent at the level of cell-cell interactions where gene expression in one population of cells generates molecular signals that alter the developmental course of another population of cells. However, even during this earliest period, interactions involving factors in the external environment also play essential roles in the development of the embryonic brain. During the fetal and postnatal periods, organism intrinsic factors continue to play a critical role in development, but across this extended period a wide array of factors in the external world influence the course of brain development in increasingly prominent ways.
Although nothing in neural development appears to be “predetermined”, the process of development is nonetheless orderly and follows very regular patterns over time. The regularity of developmental process arises from constraints imposed by both genetic and environmental factors. Genes provide the templates for creating particular proteins that are essential to the developmental process; the environment provides essential input that shapes and influences the direction of the emerging neural networks. A third essential constraint arises from the fact that the developmental process unfolds over time. The integrity of the developmental process depends absolutely upon the availability of the right neural elements appearing at the appropriate moment in developmental time. Often the emergence of a new element depends upon developmental events that immediately precede its appearance. For example, the differentiation of the neural progenitor cells along the axial midline of the neural plate during gastrulation sets the stage for the formation of the ventricular zone during neurulation. Furthermore, at each point in developmental time the organism has both a state and a history that limit which factors can influence its development. Visual and auditory signals have little effect on the gastrulating embryo, but both are essential for the typical development of vision and audition in the newborn. The constructs of “progressive differentiation” and “progressive commitment” capture important aspects of the temporal nature of brain development and can account for the regularities that are observed (Stiles 2008). At all levels of the neural system, progressive differentiation of specific elements and structures coupled with progressive commitment of those elements to functional systems appear to be the governing principles of brain development.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Acronym List
- CP
cortical plate
- CR
Cajal-Retzius cells
- CT
corticothalamic pathway
- DTI
diffusion tensor imaging
- E#
embryonic day (number of days post conception, e.g. E25)
- FA
fractional anisotrophy
- GW#
gestational week (number of weeks post conception, e.g. GW8)
- M1
primary motor cortex
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MZ
marginal zone
- ODC
ocular dominance columns
- OPC
oligiodendrocyte progenitor cells
- PAC
primary auditory cortex
- PVC
primary visual cortex
- S1
somatosensory cortex
- SP
subplate
- TC
thalamocortical pathway
- V1
primary visual cortex
- VZ
ventricular zone
Footnotes
Note that by convention gene names are italicized, and the name of proteins that are the products of gene expression are not.
This work was supported by grants to Joan Stiles from the National Institute of Child Health and Human Development: R01-HD25077, R01 HD060595, and to Terry Jernigan from the National Institute of Drug Abuse: RC2DA029475 and the Lundbeck Foundation: R32-A3161. The authors would also like to acknowledge the support of the UCSD Kavli Institute for Brain and Mind.
References
- Anderson SA, Marin O, et al. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128(3):353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR. American Journal of Neuroradiology. 2000;21(6):1099–1109. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ. Magnetic resonance techniques in the assessment of myelin and myelination. Journal of Inherited Metabolic Disease. 2005;28(3):311–343. doi: 10.1007/s10545-005-5952-z. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, et al. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, et al. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14(1):83–144. [PubMed] [Google Scholar]
- Bielle F, Griveau A, et al. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nature Neuroscience. 2005;8(8):1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, et al. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288(5464):344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Rubenstein JL, et al. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. The Journal of Neuroscience. 2002;22(17):7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, et al. Complex experience promotes capillary formation in young rat visual cortex. Neuroscience Letters. 1987;83(3):351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, et al. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. The Journal of Neuroscience. 1993;13(7):2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal P. The central nervous system: Structure and function. New York: NY, Oxford University Press; 2010. [Google Scholar]
- Brown M, Keynes R, et al. The developing brain. Oxford: Oxford University Press; 2001. [Google Scholar]
- Buss RR, Oppenheim RW. Role of programmed cell death in normal neuronal development and function. Anatomical Science International. 2004;79(4):191–197. doi: 10.1111/j.1447-073x.2004.00088.x. [DOI] [PubMed] [Google Scholar]
- Buss RR, Sun W, et al. Adaptive roles of programmed cell death during nervous system development. Annual Review of Neuroscience. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, et al. Development of the human cerebral cortex: boulder committee revisited. Nature Reviews. Neuroscience. 2008;9(2):110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Gerig G, et al. Diffusion tensor imaging: Application to the study of the developing brain. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(2):213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- Cayre M, Canoll P, et al. Cell migration in the normal and pathological postnatal mammalian brain. Progress in Neurobiology. 2009;88(1):41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, et al. Gyral development of the human brain. Annals of Neurology. 1977;1(1):86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, et al. Translating developmental time across mammalian species. Neuroscience. 2001;105(1):7–17. doi: 10.1016/S0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends in Neurosciences. 2008;31(3):113–119. doi: 10.1016/j.tins.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, et al. The genetic basis of mammalian neurulation. Nature Reviews. Genetics. 2003;4(10):784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Nery S, et al. Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nature Neuroscience. 2001;4(Suppl):1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127(13):2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends in Neurosciences. 2000;23(10):454–458. doi: 10.1016/S0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nature Reviews. Neuroscience. 2006;7(6):423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17(1):55–61. doi: 10.1016/S0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Ruhrberg C, Livet J, Henderson CE, Krumlauf R. Neuronal defects in the hindbrain of Hoxa1, Hoxb2 and Hoxb2 mutants reflect regulatory interactions among these Hox genes. Development. 2003;130(23):5663–5679. doi: 10.1242/dev.00802. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. The Journal of Comparative Neurology. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Chang FF. Plasticity of synapse structure and pattern in the cerebral cortex. In: Peters A, Jones EG, editors. Cerebral cortex. New York: Plenum; 1988. pp. 391–440. [Google Scholar]
- Greenough WT, Black JE, et al. Experience and brain development. Child Development. 1987;58(3):539–559. doi: 10.2307/1130197. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, et al. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43(3):359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hermoye L, Saint-Martin C, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29(2):493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nature Neuroscience. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huang Z. Molecular regulation of neuronal migration during neocortical development. Molecular and Cellular Neurosciences. 2009;42(1):11–22. doi: 10.1016/j.mcn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual Review of Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier Lecture: Functional architecture of macaque monkey visual cortex. Proceedings of the Royal Society of London. Series B. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, et al. Plasticity of ocular dominance columns in monkey striate cortex. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1977;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Seminars in Fetal & Neonatal Medicine. 2006;11(6):489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Human Neurobiology. 1987;6(1):1–9. [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nature Reviews. Neuroscience. 2005;6(12):955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Iwasaki N, Hamano K, et al. Volumetric quantification of brain development using MRI. Neuroradiology. 1997;39:841–846. doi: 10.1007/s002340050517. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Tallal P. Late childhood changes in brain morphology observable with MRI. Developmental Medicine and Child Neurology. 1990;32(5):379–385. doi: 10.1111/j.1469-8749.1990.tb16956.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age--consistency and interpretation of observed effects. Neurobiol Aging. 2005;26(9):1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, et al. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(Pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Jones TA, Greenough WT. Ultrastructural evidence for increased contact between astrocytes and synapses in rats reared in a complex environment. Neurobiology of Learning and Memory. 1996;65(1):48–56. doi: 10.1006/nlme.1996.0005. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Dehay C. Gradients and boundaries: limits of modularity and its influence on the isocortex. [Commentary] Developmental Science. 2001;4(2):147–148. [Google Scholar]
- Kennedy DN, Makris N, et al. Basic principles of MRI and morphometry studies of human brain development. Developmental Science. 2002;5(3):268–278. doi: 10.1111/1467-7687.00366. [DOI] [Google Scholar]
- Kiecker C, Lumsden A. Hedgehog signaling from ZLI regulates diencephalic regional identity. Nature Neuroscience. 2004;7(11):1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks’ gestation. Seminars in Fetal & Neonatal Medicine. 2006;11(6):415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal diffusion tensor imaging of healthy brain development in children. Honolulu: ISMRM Annual Meeting; 2009. [Google Scholar]
- Lebel C, Walker L, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leone DP, Srinivasan K, et al. The determination of projection neuron identity in the developing cerebral cortex. Current Opinion in Neurobiology. 2008;18(1):28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. Journal of Comparative Neurology. 1980;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor. Annals of the New York Academy of Sciences. 1964;118:149–170. doi: 10.1111/j.1749-6632.1964.tb33978.x. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nature Neuroscience. 2004;7(1):24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337(6206):424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nature Reviews. Neuroscience. 2001;2(11):780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1(4):351–363. doi: 10.1017/S1740925X05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254(5029):282–285. doi: 10.1126/science.1925583. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. Journal of Neurochemistry. 2008;107(1):1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, et al. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31(5):727–741. doi: 10.1016/S0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, et al. Neuronal subtype specification in the cerebral cortex. Nature Reviews. Neuroscience. 2007;8(6):427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Morange M. The misunderstood gene. Cambridge: MA, Harvard University Press; 2001. [Google Scholar]
- Mori S, van Zijl PC. Diffusion weighting by the trace of the diffusion tensor within a single scan. Magnetic Resonance in Medicine. 1995;33(1):41–52. doi: 10.1002/mrm.1910330107. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clinics of North America. 2006;16(1):19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Pernavelas JG. Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cerebral Cortex. 2003;13(6):607–611. doi: 10.1093/cercor/13.6.607. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nature Reviews. Neuroscience. 2002;3(6):423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Katahira T, Matsunaga E, Sato T. Isthmus organizer for midbrain and hindbrain development. Brain Research Reviews. 2002;49(2):120–126. doi: 10.1016/j.brainresrev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, et al. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nature Neuroscience. 2002;5(12):1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409(6821):714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, et al. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. The Journal of Neuroscience. 2002;22(8):3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Current Opinion in Neurobiology. 2008;18(1):90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Chou SJ, et al. Area patterning of the mammalian cortex. Neuron. 2007;56(2):252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Oppenheim, R. W. (1989). The neurotrophic theory and naturally occurring motoneuron death [see comments]. Trends in Neurosciences, 12(7). [DOI] [PubMed]
- Ostby Y, Tamnes CK, et al. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of Neuroscience. 2009;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. The Journal of Comparative Neurology. 1997;384(2):312–320. doi: 10.1002/(SICI)1096-9861(19970728)384:2<312::AID-CNE10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Pallas SL, Roe AW, et al. Visual projections induced into the auditory pathway of ferrets. I. Novel inputs to primary auditory cortex (AI) from the LP/pulvinar complex and the topography of the MGN-AI projection. The Journal of Comparative Neurology. 1990;298(1):50–68. doi: 10.1002/cne.902980105. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Alifragis P, et al. The origin and migration of cortical neurons. Progress in Brain Research. 2002;136:73–80. doi: 10.1016/S0079-6123(02)36008-4. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, et al. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–266. doi: 10.1016/S0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, de Courten-Myers GM, et al. Human cortex development: estimates of neuronal numbers indicate major loss late during gestation. Journal of Neuropathology and Experimental Neurology. 1996;55(3):320–328. doi: 10.1097/00005072-199603000-00007. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. The Journal of Comparative Neurology. 1972;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Corticogenesis in human and nonhuman primates. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge: The MIT Press; 1995. pp. 127–145. [Google Scholar]
- Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. The European Journal of Neuroscience. 2000;12(8):2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, et al. Brain development, gender and IQ in children: a volumetric imaging study. Brain. 1996;119(5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annual Review of Neuroscience. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Sansom SN, Livesey FJ. Gradients in the brain: the control of the development of form and function in the cerebral cortex. Cold Spring Harb Perspect Biol. 2009;1(2):a002519. doi: 10.1101/cshperspect.a002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, et al. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46(4):258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nature Neuroscience. 2006;9(6):743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, et al. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, et al. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9(6 Pt 1):587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, et al. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, et al. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44(1):4–16. doi: 10.1017/S0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield, B. B., & D. D. O'Leary (1985). The transient corticospinal projection from the occipital cortex during the postnatal development of the rat. Journal of Comparative Neurology, 238(2). [DOI] [PubMed]
- Stanfield, B. B., D. D. O'Leary, et al. (1982). Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature, 298(5872). [DOI] [PubMed]
- Stiles J. The fundamentals of brain development: Integrating nature and nurture. Cambridge: MA, Harvard University Press; 2008. [Google Scholar]
- Sur M, Garraghty PE, et al. Experimentally induced visual projections into auditory thalamus and cortex. Science. 1988;242(4884):1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nature Reviews. Neuroscience. 2001;2(4):251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310(5749):805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Matsuzawa H, et al. Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR in Biomedicine. 2003;16(5):257–260. doi: 10.1002/nbm.848. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, et al. Mapping brain maturation. Trends in Neurosciences. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Marin O. Neuronal migration mechanisms in development and disease. Current Opinion in Neurobiology. 2010;20(1):68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- von Melchner L, Pallas SL, et al. Visual behaviour mediated by retinal projections directed to the auditory pathway. Nature. 2000;404(6780):871–876. doi: 10.1038/35009102. [DOI] [PubMed] [Google Scholar]
- Waddington CH. An introduction to modern genetics. New York: Macmillan; 1939. [Google Scholar]
- Weissman T, Noctor SC, et al. Neurogenic Radial Glial Cells in Reptile, Rodent and Human: from Mitosis to Migration. Cerebral Cortex. 2003;13(6):550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Huttner WB. Neurogenic Radial Glial Cells in Reptile, Rodent and Human: from Mitosis to Migration. Cerebral Cortex. 2003;13(6):550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- Yeo W, Gautier J. Early neural cell death: dying to become neurons. Developmental Biology. 2004;274(2):233–244. doi: 10.1016/j.ydbio.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, et al. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Research. Developmental Brain Research. 1989;50(1):11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Zembrzycki A, Griesel G, et al. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Development. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]