Abstract
ESI multiple-stage linear ion-trap (LIT) mass spectrometric approaches for a near-complete structural characterization of cardiolipins (CLs), including identification of the fatty acyl substituents, assignment of the fatty acid substituents on the glycerol backbone, and location of the double bond(s) or cyclopropyl group along the fatty acid chain are described. Upon collisionally activated dissociation (CAD) on the [M − 2H + 3Li]+ ions of CL in an ion-trap (MS2), two sets of fragment ions (designated as (a + 136) and (b + 136) ions) analogous to those previously reported for the [M − 2H + 3Na]+ ions were observed, leading to assignment of the phosphatidyl moieties attached to 1'- or 3'-position of the central glycerol. Further dissociation of the (a + 136) (or (b + 136)) ions (MS3) gives rise to the (a + 136 − R1(or 2)CO2Li) (or b + 136 − R1(or 2)CO2Li) ion pairs that identify the fatty acid moieties and their position on the glycerol backbone. This is followed by MS4 on the (a + 136 − R1(or 2)CO2Li) (or b + 136 − R1(or 2)CO2Li) ion to eliminate a tricylic glycerophosphate ester residue (136 Da) to yield the (a − R1(or 2)CO2Li) ion, which is then subjected to MS5. The MS5 spectrum contains the structural information that locates the double bond(s) or cyclopropyl group of the fatty acid substituents. Finally, the subsequent MS6 on the dilithiated fatty acid ions generated from MS5 also yields feature ions that confirm the assignment.
Keywords: Cardiolipin, Diphosphatidylglycerol, Glycerophospholipid, Ion-trap mass spectrometry, ESI, Lithium adduct ion
Introduction
Cardiolipin (CL) (I) is a bis-(1,2-diacyl-sn-glycero-3-phosphoryl)-1',3'-sn-glycerol [1, 2]. The molecular species of cardiolipin are complex, in particular those found in prokaryotic kingdom, due to the different chain lengths and the various degrees of unsaturation of the fatty acid substituents, more importantly, due to the permutations of the four fatty acyl substituents that result in a large number of potential combinations [3, 4]. Therefore, unraveling the structure of cardiolipin has been a very difficult task [4, 5].
Mass spectrometric approaches with or without HPLC separation have been used for qualitative and quantitative analysis of CLs and their structurally related compounds [6–10].. The multiple-stage ion-trap mass spectrometric methods with ESI for characterization of complex CLs as their [M − H]−, [M − 2H + Na]−, [M − 2H + 3Na]+, [M − + 2Na]+ and [M + Na]+ ions afford identification of the fatty acyl substituents and assignment of the fatty acyl groups on the glycerol backbone [8–10]. However, none of the above methods is applicable for location of the double bond(s) of the unsaturated fatty acid substituents. Recently, we developed linear ion-trap (LIT) multiple-stage mass spectrometric approaches for locating the double bond(s) on the unsaturated fatty acids [11], and on the unsaturated fatty acid substituents of glycerophospholipids [12] and of triacylglycerols [13]. In this report, we describe LIT multiple-stage mass spectrometric approach for characterization of CLs that were desorbed as their [M − 2H + 3Li]+ions. The application of MSn advances the structural characterization of CLs to a new level, leading to unveil the location of the double bond(s) and of the cyclopropyl chain of the fatty acyl moieties.
Materials and Methods
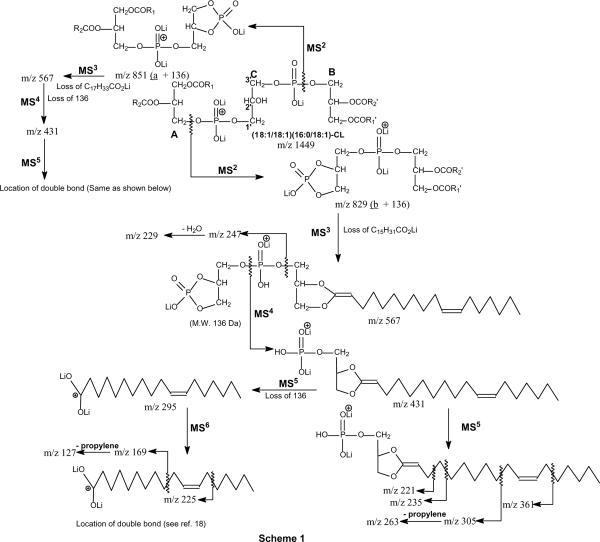
Cardiolipins isolated from Mycobacterium Bovis BCG (M. Bovis BCG) were prepared as previously described [14]; CLs from E. coli and bovine heart were purchased from Avanti Polar Lipid (Alabaster, AL); 9,10-methyleneoctadecanoic acid (dihydrosterculic acid) was purchased from Metreya LLC (Pleasant Gap, PA). All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA). The CL solution (10 pm/ul) was prepared by adding 3 ul saturated methanolic LiOH to 100 ul CL. The three glycerol moieties on CLs are designated as A, B, and central glycerol (Scheme 1) as previously described [8].. Abbreviation of cardiolipin, such as (16:0/16:1)(18:0/18:1)-CL signifies that the 16:0-, 16:1-, 18:0-, and 18:1-fatty acyl substituents are located at sn-1, sn-2, sn-1' and sn-2', respectively. Cyclopropane fatty acid such as 9,10-methyleneoctadecanoic acid was abbreviated as Prc18:0(9)-fatty acid. Low-energy CAD tandem mass spectrometry experiments conducted on a Finnigan (San Jose, CA) LTQ linear ion-trap mass spectrometer (ITMS) were described previously [10].
Scheme 1.
The proposed fragmentation pathways leading to characterization of CLs. The m/z values as shown represent the ions observed in the various MSn spectra arising from the [M − 2H + 3Li]+ ions of (Δ1118:1/ Δ1118:1)(16:0/ Δ1118:1)-CL at m/z 1449.
Results and Discussion
Characterization of cardiolipin from Escherichia coli (E. coli)
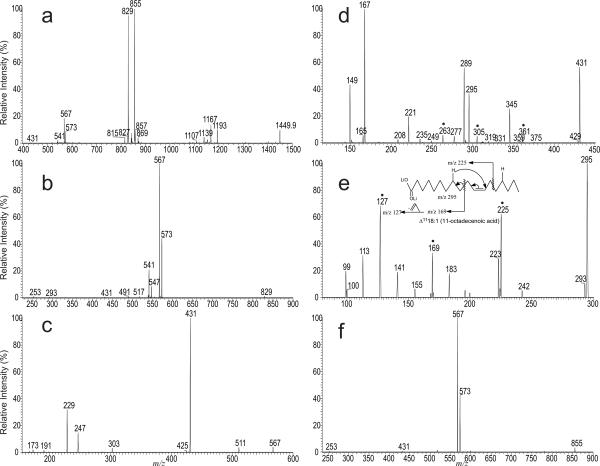
Earlier studies with LIT MSn on the [M − 2H + 3Na]+ ions showed that CL yields two prominent ions containing A or B glycerol from cleavages of the C(3B)O-P and the C(3A)O-P bonds, following resonance excitation in an ion-trap. Similar results were also observed for the [M − 2H + 3Li]+ ions (Scheme 1) [10]. For example, the LIT MS2-spectrum of the [M − 2H + 3Li]+ ions of the major E. coli CL species containing unsaturated fatty acid substituents [9] seen at m/z 1449.9 (Figure 1a) is dominated by the ions at m/z 855 (a + 136) and 829 (b + 136), analogous to the ions of m/z 903 (a + 136) and 877 (b + 136) seen for the [M − 2H + 3Na]+ ions [8, 10]. The ion at m/z 855 is more abundant than the ion at m/z 829, consistent with the earlier notion that the (18:1/18:1)- and 16:0/18:1-diacyl-sn-glycero-3-phosphoryl residues attached to the 1' and 3' of the central glycerol, respectively [8]. This assignment is supported by further dissociation of the ion of m/z 829 (1449 → 829; Panel b), which gave rise to the ions at m/z 573 and 567 arising from losses of 16:0-fatty acid substituent as free acid and as lithium salt, respectively. The spectrum also contains the ions at m/z 547 and 541, arising from the analogous losses of 18:1-fatty acid moiety. The former ion pairs are respectively more abundant than those of the latter, indicating that the ions at m/z 829 (b + 136) consists of a 16:0/18:1-diacyl residue [10, 15]. The MS4 spectrum of the ion at m/z 567 (1449 → 829 → 567; Panel c) is dominated by the ion at m/z 431, probably arising from loss of a tricylic glycerophosphate ester residue (136 Da) [9, 10, 16]. The results are consistent with the observation of the ions at m/z 247, representing a lithiated diphosphorylglycerol ion, and at m/z 229 (247 – H2O) (Scheme 1).
Figure 1.
The LIT MS2-spectra of the [M − 2H + 3Li]+ ions of (18:1/18:1)(16:0/18:1)-CL at m/z 1449.9 (a) isolated from E. coli., the sequential MS3 spectrum of the ion at m/z 829 (1449 → 829) (b), MS4 spectrum of the ion at m/z 567 (1449 → 829 → 567) (c), its MS5 spectrum of the ion at m/z 431 (1449 → 829 → 567 → 431) (d), MS6 spectrum of the ion at m/z 295 (1449 → 829 → 567 → 431 → 295) (e), and the MS3 spectrum of the ion at m/z 855 (1449 → 855) (f). The MSn spectra together determine the (Δ1118:1/Δ1118:1)(16:0/Δ1118:1)-CL structure. The ions marked with “•” (Panel d and e) are feature ions that locate the double bond at C(11).
The assignment of the position of double bond of the 18:1-fatty acid moiety was achieved by MS5 on the ion of m/z 431 (1449 → 829 → 567 → 431; Panel d), which yields ions at m/z 361, 305 and 263, arising from charge-remote fragmentations (CRF) involving β-cleavage with γ-H shift as previously reported [12], indicating that the double bond is located at C11. The spectrum also contains the ions at m/z 295 and 287 representing a dilithiated and a monolithiated ions of 18:1-fatty acid, respectively, along with ions at m/z 167 and 149, arising from further losses of 18:1-fatty acid as a ketene and as an acid, respectively. These ions clearly show the presence of 18:1-fatty acid substituent. The location of the double bond is confirmed by the MS6 spectrum of the ion at m/z 295 (1449 → 855 → 567 → 431 →295; Panel e), which contains the ions at m/z 225, 169 and 127, arising from similar CRF involving β-cleavage with γ-H shift. These ions are analogous to the ions at m/z 361, 305 and 263 seen in Figure 1d and the spectrum (Figure 1e) is identical to that arising from Δ1118:1-fatty acid standard [11]. The above results indicate that the CL species consists of a 16:0/Δ1118:1-diacyl-sn-glycero-3-phosphoryl residue attached to 3' of the central glycerol.
The MS3 spectrum of the ion at m/z 855 (a + 136) (1449 → 855; Figure 1f) is dominated by the ion pairs at m/z 573 (loss of 18:1-acid) and 567 (loss of 18:1-Li salt), indicating the presence of 18:1/18:1-diacylglycerol moiety. The position of double bond of the 18:1-fatty acid attached to the 18:1/18:1-diacylglycero-phosphoryl residue is determined by the subsequent MS4 on the ion at m/z 567 (1449 → 855 → 567) and MS5 on the ion at m/z 431 (1449 → 855 → 567 → 431), together with the MS6 on the ion at m/z 295 (1449 → 855 → 567 → 431→ 295) (data not shown). The spectra are respectively identical to those shown in Panels c–e, indicating that the double bond is also located at C11. Taken together, the results indicate that the m/z 1449 ion represent mainly a (Δ1118:1/Δ1118:1)(16:0/Δ1118:1)-CL.
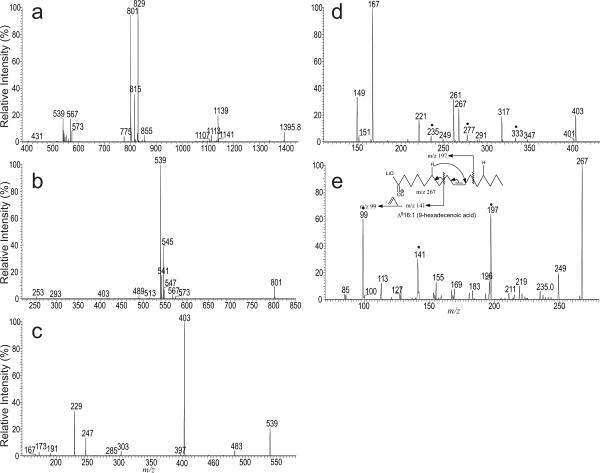
Similarly, the LIT MS2-spectrum of the [M − 2H + 3Li]+ ions at m/z 1395.8 (Figure 2a) from the same CL extract is dominated by the ions at m/z 829 (a + 136) and 801 (b + 136). The LIT MS3-spectrum of the ion of m/z 829 and the subsequent MS4 spectrum of m/z 567, the MS5 spectrum of m/z 431, and the MS6 spectrum of m/z 295 (data not shown) are identical to those shown in Figure 1, indicating that the molecule consists of the identical 16:0/Δ1118:1 diacylglycerol phosphoryl moiety as seen earlier. By contrast, the LIT MS3-spectrum of the ion of m/z 801 (1395 → 801; Figure 2b) contains ions at m/z 545 and 539, arising from losses of the 16:0-fatty acid substituent as an acid and as a lithium salt, respectively, together with the ions at m/z 547 and 541, arising from the analogous losses of the 16:1-fatty acid moiety. The preferential losses of the 16:0-fatty acid moieties than the similar losses of the 16:1-fatty acid moieties indicate that the 16:0- and 16:1-fatty acids are located at sn-1' and sn-2' of the glycerol backbone, consistent with the notion that the ion at m/z 1395 represents mainly a (16:0/18:1)(16:0/16:1)-CL isomer [9].
Figure 2.
The LIT MS2-spectra of the [M − 2H + 3Li]+ ions of (16:0/18:1)(16:0/16:1)-CL at m/z 1395.8 (a) isolated from E. coli., the subsequent MS3 spectrum of the ion at m/z 801 (1395 → 801) (b), MS4 spectrum of the ion at m/z 539 (1395 → 801 → 539) (c), its MS5 spectrum of the ion at m/z 403 (1395 → 801 → 539 → 403) (d), and MS6 spectrum of the ion at m/z 267 (1395 → 801 → 539 → 403 → 267) (e). The combined information from the MSn spectra leads to assign the (16:0/Δ1118:1)(16:0/Δ916:1)-CL structure. The ions marked with “•” (Panel d and e) are feature ions that locate the double bond on the 16:1-fatty acyl substituent..
Further dissociation (MS4) of the ion at m/z 539 (1395 → 801 → 539; Figure 2c) gave rise to the prominent ion at m/z 403 (539 − 136) arising from loss of a tricylic glycerophosphate ester residue, and the ion at m/z 303 arising from loss of 16:1-fatty acyl ketene, together with ions at m/z 247 (lithiated diphosphorylglycerol) and 229 (247 – H2O) as described earlier. The presence of 16:1-fatty acyl residue and the position of its double bond is also determined by the sequential MS5 on the ion of m/z 431 (1395 → 801 → 539 → 403; Figure 2d) and MS6 on the ion of m/z 267 (1395 → 801 → 539 → 403 → 267; Figure 2e). Again, the MS5 spectrum of the ion of m/z 403 (Figure 2d) is dominated by the ions at m/z 167 (loss of C15H29CO2H) and 149 (loss of C15H29CO2Li), and the ions at m/z 267 and 261, representing the dilithiated and monolithiated 16:1-fatty acid ions. The spectrum also contains the ions at m/z 333, 291, and 235, arising from the CRF processes involving β-cleavage with γ-hydrogen shift. The results indicate that the double bond of the 16:1-fatty acid moiety is located at C9. This assignment is further confirmed by the observation of the ions at m/z 197, 141 and 99 in the MS6 spectrum of the ion of m/z 267 (Figure 2e and inset). These ions are analogous to the ions at m/z 333, 277 and 235 (Figure 2d) and are 136 Da lighter. The above results give assignment of a (16:0/Δ1118:1)(16:0/Δ916:1)-CL structure.
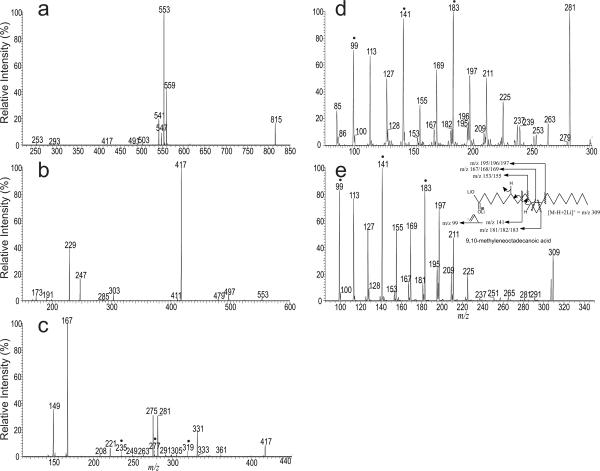
In Figure 2a, the ion at m/z 815 was also observed. Further dissociation of the ion at m/z 815 (Figure 3a) and the subsequent MS4 on the ions of m/z 553 (1395 → 815 → 553) (Figure 3b) and MS5 on the ions of 417 (1395 → 815 → 553 → 417) (Figure 3c) confirm the presence of (16:0/17:1)(16:0/17:1)-CL as a minor isomer. The spectrum (Figure 3c) also contains the ion at m/z 281, which is equivalent to an [M − H + 2Li]+ ion of 17:1-fatty acid together with the ion at m/z 275, corresponding to a monolithiated ion of 17:1-fatty acid. The MS6 spectrum of the ion at m/z 281 (1395 → 815 → 553 → 417→ 281; Figure 3d) contains abundant ions at m/z 141, 183, along with ions at 197 and 195, which is similar to those seen in the LIT MS2 spectrum of the [M − H + 2Li]+ ion of 9,10-methyleneoctadecanoic acid standard (Figure 3e and inset), indicating that the 17:1-fatty acid may represent a 9,10-methylene hexadecanoic acid (Prc16:0(9)), a cyclopropane fatty acid rather than a unsaturated fatty acid. This structure assignment is further supported by the observation of the analogous ions at m/z 235, 277, and 319 in Figure 3c. These ions are 136 Da heavier, consistent with the notion that the precursor ion at m/z 417 is 136 Da heavier than the ion at m/z 281. The structure assignment is also consistent with the notion that 9,10-methylene hexadecanoic acid is present in the phospholipids isolated from E. coli [17].
Figure 3.
The LIT MS3 spectrum of the ion at m/z 815 (1395 → 815) (a) and the subsequent MS4 spectrum of the ions of m/z 553 (1395 → 815 → 553) (b), MS5 spectrum of the ions of m/z 417 (1395 → 815 → 553 → 417) (c), MS6 spectrum of the ion at m/z 281 (1395 → 815 → 553 → 417→ 281) (d), and the LIT MS2 spectrum of the [M − H + 2Li]+ ion of 9,10-methyleneoctadecanoic acid standard at m/z 309 (e). Spectrum (d) contains ions similar to those seen in Panel (e) from standard, indicating that the ion at m/z 281 may represent a dilithiated 9,10-methylene hexadecanoic acid ion, rather than a dilithiated 17:1-fatty acid ion. The structural information from the combined MSn spectra indicate that the ion of m/z 1395 also represents a (16:0/ Prc16:0(9))(16:0/ Prc16:0(9))-CL. The suggested bond cleavages leading to formation of the feature ions (marked with “•” in Panel c, d and e) that locate the propane group are shown in inset of Panel e.
Characterization of the major cardiolipin species isolated from Mycobacterium bovis BCG and Bovine heart (the related figures were prepared as supplemental materials)
As shown in Figure S1a, the MS2 spectrum of the [M − 2H + 3Li]+ ion of (18:1/16:0)(18:1/16:0)-CL) at m/z 1423 from M. bovis BCG is dominated by the ion at m/z 829 ((a + 136) & (b + 136) ion) arising from the cleavages of the CH2-OP bond. Further dissociation of the ion at m/z 829 (1423.9 → 829; Figure S1b) gave rise to prominent ions at m/z 547 and 541, arising from losses of 18:1-fatty acyl as an acid and as a lithium salt, respectively; whereas ions at m/z 573 and 567 arising from the analogous losses of 16:0-fatty acid are less abundant. The abundances of the ion pairs (i.e., 547/541 vs. 573/567) are reversed from those seen in Figure 1b, consistent with the presence of the 18:1/16:0-diacylphosphoryl residue.
The subsequent MS4 on the ion of m/z 567 (1423 → 829 → 567; Figure S1c) gave rise to the prominent ion at m/z 431, arising from loss of a tricylic glycerophosphate ester residue, and the ion at m/z 303, deriving from loss of 18:1-ketene, along with ions at m/z 247 and 229 as seen earlier. The location of double bond along the 18:1-fatty acyl chain was further deduced by the subsequent MS5 on the ion of m/z 431 (1423 → 829 → 567 → 431; Figure S1d), which contains the ion series at m/z 333, 277, 235, and 221, indicating that the double bond is located at C(9); whereas the ions at m/z 295 and 289 represent the dilithiated and monlithiated 18:1-fatty acid cations, respectively. The assignment of the double bond is further confirmed by the MS6 spectrum of the ion of m/z 295 (1423 → 829 → 567 → 431 → 295; Figure 4e and inset), which is identical to that arising from the Δ918:1-fatty acid standard [11]. The combined structural information from the above MSn spectra give assignment of (Δ918:1/16:0)(Δ918:1/16:0)-CL.
The major bovine heart CL was observed at m/z 1467.9, corresponding to the [M − 2H + 3Li]+ ions of (18:2/18:2)(18:2/18:2)-CL. This structural assignment is supported by the LIT MS2 spectrum of the ion at m/z 1467.9 (Figure S2a), which is dominated by the ion at m/z 851 ((a + 136) and (b + 136) ions), and by its subsequent MS3 spectrum of the ion at m/z 851 (1467 → 851; Figure S2b), which contained the ions at m/z 571 and 565, arising from loss of 18:2-fatty acid substituents as an acid and as a lithium salt, respectively, consistent with the presence of two identical 18:2/18:2-diacylglycero phosphoryl groups. The assignment of 18:2-fatty acid substituents is deduced by the subsequent MS4 on the ion at m/z 565 (1467 → 851 → 565; Figure S2c), which contained the dilithiated and monolithiated 18:2-fatty acid ions at ions at m/z 293 and 287, respectively, together with the ions at m/z 167 and 149 arising from losses of the 18:2-fatty acid as a ketene and as an acid, respectively; whereas the location of double bonds is dedued by the sequential MS5 on the ion at m/z 429 (1467 → 851 → 565→ 429; Figure S2d and inset), which contained ions at m/z 373 and 277, arising from the CRF processes involving β-cleavage with γ-hydrogen shift, and ions at m/z 331 (allylic cleavage) and 319 (vinylic cleavage), together with ions at 221, 235. These ions clearly locate the double bonds at C(9) and C(12) [11, 12]. This assignment is further supported by the MS6 spectrum of the ion at m/z 293 (1467 → 851 → 565→ 429 → 293) (data not shown), which is identical to the MS2 spectrum of the dilithiated ion of Δ9,1218:2 fatty acid [11]. The combined structural information indicate that the CL species is a (Δ9,1218:2/Δ9,1218:2)(Δ9,1218:2/Δ9,1218:2)-CL.
Conclusions
We demonstrated LIT MSn method for characterization of CL isolated from several biological specimens without laborious chromatographic separation and chemical reaction. However, higher stage tandem mass spectrometry with MS5 and MS6 are required for advancing the analysis for locating the double bond(s) or the cyclopropyl group, resulting in significant decline in sensitivity and thus extended time for signal average is often necessary for confident assignment. The analogous fragment ions were seen among the MSn (n=2–4) spectra from [M − 2H + 3Li]+, [M − 2H + 3Na]+, or [M − H]− ions of CL, indicating that the fragmentation processes under low-energy CAD for all the above adduct ions are similar [10,11]. Nonetheless, structural information for locating the double bonds or the cyclic group on the fatty acid chain is absent employing LIT MSn on the sodiated (i.e., [M − 2H + 3Na]+ and [M − 2H + Na]−) or [M − H]− ions of CL. This lack of information locating the double bond(s) for the sodiated ions has been previously reported [13], attributable to the fact that metal ions such as Na+ and K+ that form adduct precursor ions were lost upon CAD, before CRF processes take place [13]. The differences in the profiles between the spectra arising from the cyclopropane fatty acids (Figure 3d and 3e) and those arising from unsaturated fatty acid (Figure 1e and 2e) suggest that it may be applicable for distinction a double bond from a cyclopropyl group on the fatty acid substituents using the present approaches..
Supplementary Material
Figure S1 The LIT MS2-spectra of the [M − 2H + 3Li]+ ions of (Δ918:1/16:0)(Δ918:1/16:0)-CL at m/z 1423.9 (a) isolated from M. Bovis BCG., and the subsequent MS3 spectrum of the ion at m/z 829 (1423 → 829) (b), MS4 spectrum of the ion at m/z 567 (1423 → 829 → 567) (c), MS5 spectrum of the ion at m/z 431 (1423 → 829 → 567 → 431) (d), and the MS6 spectrum of the ion at m/z 295 (1423 → 829 → 567 → 431 → 295) (e) that locate the double bond along the 18:1-fatty acid chain. The inset in Panel e illustrates the proposed fragmentation pathways leading to the ions (marked with “•” in Panel d and e) that locate the double bond at C(9).
Figure S2 The LIT MS2-spectra of the [M − 2H + 3Li]+ ions of (18:2/18:2)(18:2/18:2)-CL at m/z 1467.9 (a) isolated from Bovine heart, and the subsequent MS3 spectrum of the ion at m/z 851 (1467 → 851) (b), MS4 spectrum of the ion at m/z 565 (1467 → 851 → 565) (c), and its MS5 spectrum of the ion at m/z 429 (1467 → 851 → 565→ 429) (d). The MSn spectra combined give assignment of the (Δ9,1218:2/Δ9,1218:2)(Δ9,1218:2/Δ9,1218:2)-CL structure. The ions labeled with “◆” (vinyl cleavage) “*” (allylic cleavage) and “•” (β-cleavage) lead to locate the double bonds along the 18:2-fatty acid chain (fragmentation pathway see Ref 19).
Acknowledgements
This research was supported by US Public Health Service Grants P41-RR-00954, R37-DK-34388, P60-DK-20579, and P30-DK-56341. We acknowledge Dr. Elizabeth R. Rhoades for preparation of lipid samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeCocq J, Ballou CE. On the Structure of Cardiolipin. Biochemistry. 1964;155:976–980. doi: 10.1021/bi00895a023. [DOI] [PubMed] [Google Scholar]
- 2.Pangborn MC. The Composition of Cardiolipin. J. Biol. Chem. 1947;168:351–361. [PubMed] [Google Scholar]
- 3.Malhotra A, Xu Y, Ren M, Schlame M. Formation of Molecular Species of Mitochondrial Cardiolipin. 1. A Novel Transacylation Mechanism to Shuttle Fatty Acids between Sn-1 and Sn-2 Positions of Multiple Phospholipid Species. Biochim. Biophys. Acta. 2009;1791:314–320. doi: 10.1016/j.bbalip.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlame M. Formation of Molecular Species of Mitochondrial Cardiolipin: 2. A Mathematical Model of Pattern Formation by Phospholipid Transacylation. Biochim. Biophys. Acta. 2009;1791:321–325. doi: 10.1016/j.bbalip.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlame M, Otten D. Analysis of Cardiolipin Molecular Species by High-Performance Liquid Chromatography of Its Derivative 1,3-Bisphosphatidyl-2-Benzoyl-Sn-Glycerol Dimethyl Ester. Anal. Biochem. 1991;195:290–295. doi: 10.1016/0003-2697(91)90332-n. [DOI] [PubMed] [Google Scholar]
- 6.Rezanka T, Siristova L, Melzoch K, Sigler K. Direct Esi-Ms Analysis of O-Acyl Glycosylated Cardiolipins from the Thermophilic Bacterium Alicyclobacillus Acidoterrestris. Chem. Phys. Lipids. 2009;161:115–121. doi: 10.1016/j.chemphyslip.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Tyurin VA, Tyurina YY, Jung M-Y, Tungekar MA, Wasserloos KJ, BayIr H, Greenberger JS, Kochanek PM, Shvedova AA, Pitt B, Kagan VE. Mass-Spectrometric Analysis of Hydroperoxy- and Hydroxy-Derivatives of Cardiolipin and Phosphatidylserine in Cells and Tissues Induced by Pro-Apoptotic and Pro-Inflammatory Stimuli. Journal of Chromatography B. 2009;877:2863–2872. doi: 10.1016/j.jchromb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu FF, Turk J, Rhoades ER, Russell DG, Shi Y, Groisman EA. Structural Characterization of Cardiolipin by Tandem Quadrupole and Multiple-Stage Quadrupole Ion-Trap Mass Spectrometry with Electrospray Ionization. J. Am. Soc. Mass Spectrom. 2005;16:491–504. doi: 10.1016/j.jasms.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Hsu FF, Turk J. Characterization of Cardiolipin from Escherichia Coli by Electrospray Ionization with Multiple Stage Quadrupole Ion-Trap Mass Spectrometric Analysis of [M − 2h + Na]- Ions. J. Am. Soc. Mass Spectrom. 2006;17:420–429. doi: 10.1016/j.jasms.2005.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu FF, Turk J. Characterization of Cardiolipin as the Sodiated Ions by Positive-Ion Electrospray Ionization with Multiple Stage Quadrupole Ion-Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2006;17:1146–1157. doi: 10.1016/j.jasms.2006.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu FF, Turk J. Elucidation of the Double-Bond Position of Long-Chain Unsaturated Fatty Acids by Multiple-Stage Linear Ion-Trap Mass Spectrometry with Electrospray Ionization. J. Am. Soc. Mass Spectrom. 2008;19:1673–1680. doi: 10.1016/j.jasms.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu FF, Turk J. Structural Characterization of Unsaturated Glycerophospholipids by Multiple-Stage Linear Ion-Trap Mass Spectrometry with Electrospray Ionization. J. Am. Soc. Mass Spectrom. 2008;19:1681–1691. doi: 10.1016/j.jasms.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu FF, Turk J. Electrospray Ionization Multiple-Stage Linear Ion-Trap Mass Spectrometry for Structural Elucidation of Triacylglycerols: Assignment of Fatty Acyl Groups on the Glycerol Backbone and Location of Double Bonds. J. Am. Soc. Mass Spectrom. 2010;20 doi: 10.1016/j.jasms.2010.01.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhoades E, Hsu F, Torrelles JB, Turk J, Chatterjee D, Russell DG. Identification and Macrophage-Activating Activity of Glycolipids Released from Intracellular Mycobacterium Bovis Bcg. Mol. Microbiol. 2003;48:875–888. doi: 10.1046/j.1365-2958.2003.03473.x. [DOI] [PubMed] [Google Scholar]
- 15.Hsu F-F, Turk J. Electrospray Ionization with Low-Energy Collisionally Activated Dissociation Tandem Mass Spectrometry of Glycerophospholipids: Mechanisms of Fragmentation and Structural Characterization. Journal of Chromatography B. 2009;877:2673–2695. doi: 10.1016/j.jchromb.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu FF, Turk J. Charge-Driven Fragmentation Processes in Diacyl Glycerophosphatidic Acids Upon Low-Energy Collisional Activation. A Mechanistic Proposal. J. Am. Soc. Mass Spectrom. 2000;11:797–803. doi: 10.1016/S1044-0305(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaneshiro T, Marr AG. Cis-9,10-Methylene Hexadecanoic Acid from the Phospholipids of Escherichia Coli. J. Biol. Chem. 1961;236:2615–2619. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The LIT MS2-spectra of the [M − 2H + 3Li]+ ions of (Δ918:1/16:0)(Δ918:1/16:0)-CL at m/z 1423.9 (a) isolated from M. Bovis BCG., and the subsequent MS3 spectrum of the ion at m/z 829 (1423 → 829) (b), MS4 spectrum of the ion at m/z 567 (1423 → 829 → 567) (c), MS5 spectrum of the ion at m/z 431 (1423 → 829 → 567 → 431) (d), and the MS6 spectrum of the ion at m/z 295 (1423 → 829 → 567 → 431 → 295) (e) that locate the double bond along the 18:1-fatty acid chain. The inset in Panel e illustrates the proposed fragmentation pathways leading to the ions (marked with “•” in Panel d and e) that locate the double bond at C(9).
Figure S2 The LIT MS2-spectra of the [M − 2H + 3Li]+ ions of (18:2/18:2)(18:2/18:2)-CL at m/z 1467.9 (a) isolated from Bovine heart, and the subsequent MS3 spectrum of the ion at m/z 851 (1467 → 851) (b), MS4 spectrum of the ion at m/z 565 (1467 → 851 → 565) (c), and its MS5 spectrum of the ion at m/z 429 (1467 → 851 → 565→ 429) (d). The MSn spectra combined give assignment of the (Δ9,1218:2/Δ9,1218:2)(Δ9,1218:2/Δ9,1218:2)-CL structure. The ions labeled with “◆” (vinyl cleavage) “*” (allylic cleavage) and “•” (β-cleavage) lead to locate the double bonds along the 18:2-fatty acid chain (fragmentation pathway see Ref 19).