Abstract
A total of 36 consecutive nonambulatory DMD patients underwent scoliosis surgery. Patients were divided into two groups: the autogenous iliac crest bone graft group (the ICBG group; 20 patients) and the allogenous bone graft group (the ALBG group; 16 patients). The mean preoperative curves measured 87° and 31° at the last follow-up in the ICBG group and 83° and 28° in the ALBG group. In the ICBG group, three (15%) patients had intraoperative sacroiliac joint penetration, five (25%) had iliac crest inner cortex penetration and three (15%) had postoperative prolonged wound drainage at the donor site. At three months after surgery, donor site pain caused by bone harvest was found in 50% with severe pain limiting their physical function and causing difficulties in sitting in a wheelchair in 40% of the patients, whereas patients in the ALBG group returned to their preoperative level of function soon after surgery.
Introduction
Posterior spinal fusion in nonambulatory Duchenne muscular dystrophy (DMD) patients has been accepted as the optimal procedure to prevent progression of scoliosis and maintain upright and comfortable sitting [1, 4, 17, 20, 22].
Harvesting autogenous or allogenous bone graft to increase the fusion rate using segmental instrumentation for scoliosis has been a standard procedure. Advantages of autogenous bone graft over allogenous bone graft have been well established. The posterior iliac crest is the most common donor site for autogenous bone graft for spinal fusion. However, donor site morbidity caused by bone harvest remains a concern. Furthermore, this procedure is accompanied by many complications because of longer operative time and greater blood loss [18, 24]. Several studies have reported minor and major complications, with a wide range of incidences varying between 1% and 39% [2, 8, 18, 20, 25].
The aim of this study was to compare the outcome and complication rates of scoliosis surgery in nonambulatory patients with DMD using either autogenous posterior iliac crest bone graft (ICBG) or allogenous bone graft (ALBG).
Materials and methods
From March 2003 to October 2006, a total of 36 consecutive DMD patients underwent posterior spinal fusion and instrumentation (thoracic hooks and lumbar screws) for scoliosis. The patients were prospectively collected and followed. The minimum follow-up period was two years. Patients were divided into two groups: the iliac crest bone graft group (the ICBG group; 20 patients in whom posterior iliac crest bone graft was used) and the allogenous bone graft group (the ALBG group; 16 patients in whom banked allograft bone was used).
Surgical technique
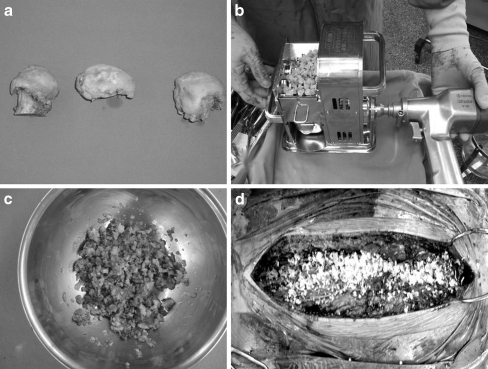
All the patients had standard posterior spinal fusion performed by the same surgeon (M.T.). The posterior elements of the spine were exposed from the upper thoracic spine to the sacrum by stripping the muscles subperiosteally. Spinal cord function was monitored throughout the procedure. Autotransfusion via preoperative storage and intraoperative collection was used. Distal fixation comprised 6-mm pedicle screws in the lumbar spine. Apical and proximal fixation comprised hooks in the thoracic spine. The rods were contoured to recreate the sagittal profile and coronal balance was achieved by sequential reduction of the segments toward the rods. In the ALBG group, allograft bone obtained from Kitasato University Bone Bank (KUBB) was used. The bone was again cleaned of all the attached soft tissue, and the bone mill morselised the grafts and further separated the soft tissue from the bone (Fig. 1). Alternatively, in the ICBG group, a conventional approach to the iliac crest was used to obtain autogenous bone grafts. Grafts were harvested from both iliac crests. The posterior elements were decorticated and bone grafts were placed on the decorticated bed along the length of the instrumentation from T3 or T4 to L5.
Fig. 1.
a Banked allograft bone obtained from Kitasato University Bone Bank. b Banked allograft bone placed into the bone mill. The bone mill morselises the bone graft. c A large quantity of milled bone graft is obtained. d Adequate quantity of bone grafts for the fusion were placed on the decorticated posterior elements of the spine
Clinical and radiological assessments
Assessment was performed clinically and with radiological measurements. All of the patients were reviewed clinically and questioned about whether they felt back pain at one week and at a three-month interval for 24 months after surgery. Intraoperative complications, along with postoperative complications, were recorded.
The patients in the ICBG group were specifically questioned about whether they had local pain at the donor site at one week and at a three-month interval for 24 months after surgery.
The questions consisted of the following:
Do you have pain in the back hip area where the iliac crest bone graft was harvested? If yes, rate your pain on a scale of 1 to 10.
Do you have any problems with daily activities (difficulties in sitting in a wheelchair) due to the pain?
Sitting anteroposterior and lateral radiographs were taken and evaluated the day before surgery, at one week, three and 24 months after surgery and at the last follow-up. The deformity in the coronal plane was measured by the Cobb angle method. Clinical evaluation was made by the senior author.
Fusion was defined as: first, stable coronal and sagittal alignment over the follow-up period; second, no clinical complaints; third, no evidence of nonunion radiographically; fourth, stable hardware. All four criteria must be present for definition of fusion in this study. CT scans were not available in every patient in this study, and therefore not used for assessment of fusion.
Results
A total of 36 patients (20 in the ICBG group and 16 in the ALBG group) were enrolled in the study. No patients were lost to follow-up. The mean follow-up period was 4.3 years (range, 2.5–6.1 years). Demographic details and surgical parameters are shown in Tables 1 and 2.
Table 1.
Details of the patients and operative parameters in the ICBG group
| Patient number | Age (y) | Scoliotic curvature (°) | Operative time (min) | Intraoperative blood (ml) | Donor site pain (>3 months) and other donor site complications or morbidity | ||||
|---|---|---|---|---|---|---|---|---|---|
| The day before surgery | Immediately after operation | Three months after surgery | Two years after surgery | Last follow-up | |||||
| 1 | 11 | 100 | 38 | 40 | 40 | 42 | 249 | 889 | - |
| 2 | 13 | 60 | 20 | 20 | 22 | 23 | 230 | 860 | Pain for 9 months; sacroiliac joint penetration |
| 3 | 13 | 52 | 18 | 19 | 19 | 20 | 205 | 920 | Pain for 3 months; prolonged wound drainage |
| 4 | 13 | 55 | 13 | 12 | 13 | 15 | 250 | 866 | - |
| 5 | 12 | 93 | 35 | 36 | 37 | 36 | 267 | 867 | - |
| 6 | 12 | 91 | 32 | 33 | 33 | 33 | 271 | 921 | - |
| 7 | 14 | 81 | 27 | 27 | 28 | 30 | 264 | 964 | - |
| 8 | 13 | 93 | 20 | 20 | 22 | 22 | 268 | 868 | - |
| 9 | 14 | 130 | 38 | 40 | 44 | 43 | 327 | 933 | - |
| 10 | 11 | 100 | 38 | 37 | 37 | 37 | 268 | 968 | Pain for 3 months |
| 11 | 17 | 101 | 40 | 40 | 42 | 44 | 256 | 856 | Pain for 9 months; sacroiliac joint penetration |
| 12 | 11 | 81 | 32 | 30 | 32 | 32 | 267 | 767 | - |
| 13 | 13 | 98 | 35 | 35 | 35 | 37 | 266 | 866 | - |
| 14 | 13 | 128 | 28 | 28 | 32 | 32 | 277 | 820 | Pain for 3 months; prolonged wound drainage |
| 15 | 13 | 100 | 35 | 36 | 36 | 38 | 301 | 944 | Pain for 6 months; sacroiliac joint penetration |
| 16 | 12 | 85 | 30 | 32 | 32 | 33 | 266 | 806 | - |
| 17 | 11 | 95 | 35 | 35 | 37 | 37 | 270 | 960 | Pain for 6 months; sacroiliac joint penetration |
| 18 | 12 | 90 | 18 | 20 | 20 | 22 | 276 | 860 | Pain for 6 months; sacroiliac joint penetration |
| 19 | 13 | 50 | 23 | 22 | 22 | 24 | 260 | 860 | Pain for 3 months |
| 20 | 13 | 60 | 20 | 22 | 22 | 23 | 255 | 978 | Pain for 6 months; sacroiliac joint penetration |
| Mean | 13 | 87 | 29 | 29 | 30 | 31 | 253 | 850 | |
ICBG iliac crest bone graft
Table 2.
Details of the patients and operative parameters in the ALBG group
| Patient number | Age (y) | Scoliotic curvature (°) | Operative time (min) | Intraoperative blood loss (ml) | ||||
|---|---|---|---|---|---|---|---|---|
| The day before surgery | Immediately after operation | Three months after surgery | Two months after surgery | Last follow-up | ||||
| 1 | 12 | 60 | 26 | 26 | 27 | 28 | 192 | 580 |
| 2 | 14 | 62 | 23 | 22 | 22 | 24 | 205 | 460 |
| 3 | 16 | 62 | 15 | 15 | 16 | 16 | 240 | 800 |
| 4 | 19 | 64 | 27 | 25 | 25 | 27 | 280 | 970 |
| 5 | 13 | 66 | 25 | 27 | 27 | 27 | 210 | 450 |
| 6 | 12 | 78 | 20 | 20 | 20 | 22 | 295 | 660 |
| 7 | 15 | 85 | 30 | 30 | 34 | 34 | 235 | 1150 |
| 8 | 13 | 96 | 20 | 20 | 22 | 24 | 280 | 670 |
| 9 | 13 | 130 | 38 | 40 | 41 | 42 | 225 | 733 |
| 10 | 13 | 82 | 19 | 19 | 20 | 20 | 228 | 777 |
| 11 | 13 | 65 | 9 | 12 | 12 | 12 | 165 | 1010 |
| 12 | 13 | 101 | 40 | 40 | 42 | 44 | 235 | 656 |
| 13 | 20 | 60 | 15 | 15 | 19 | 19 | 215 | 900 |
| 14 | 13 | 82 | 25 | 25 | 27 | 27 | 255 | 850 |
| 15 | 12 | 110 | 38 | 38 | 40 | 41 | 230 | 865 |
| 16 | 14 | 120 | 30 | 30 | 34 | 34 | 236 | 875 |
| Mean | 13 | 83 | 25 | 25 | 27 | 28 | 233 | 775 |
ALBG allogenous bone graft
There were no significant differences in age, the preoperative and postoperative Cobb angles, or the number of levels fused between the two groups. The operative time and intraoperative blood loss were higher in the ICBG group than in the ALBG group (253 min. versus 233 min.; 850 ml vs. 775 ml).
The ICBG group
There were three (15%) instances of sacroiliac joint penetration and five (25%) instances of iliac crest inner cortex penetration possibly due to a poor pelvis. Prolonged wound drainage at the donor site was observed in three (15%) patients, and treated only with observation resolving in two weeks. There were no neurological complications or infections. There was no instrumentation failure during the follow-up period. Paralytic ileus occurred in four (20%) patients, and cleared after 48 hours following observation without oral intake. There were no secondary operations for any reason, nor any second hospital admissions related to surgery.
At one week after surgery, all of the patients reported back pain in the region of the instrumentation surgery. At three months after surgery, 30% (6/20) reported back pain. At six months, 5% (1/20) reported back pain and at nine months 5% (1/20) reported back pain. At 12 months after surgery, none of the patients reported back pain. Therefore, no clinical complaints in the region of the instrumentation surgery were noted at one year after surgery.
Ninety percent (18/20) of the patients reported donor site pain at one week after surgery. The self-reported pain values ranged from 4 to 7 with a mean of 6. All of the patients with documented pain reported that they had problems with daily activities (difficulties in sitting in a wheelchair) due to pain. At three months after surgery, 50% (10/20) of the patients reported continued pain at the donor site and 40% (8/20) reported problems with daily activities. The self-reported pain values ranged from 1 to 7 with a mean of 5. At six months after surgery, 40% (8/20) of the patients reported continued pain at the donor site and 30% (6/20) reported problems with daily activities. The self-reported pain values ranged from 1 to 7 with a mean of 5. At nine months after surgery, 15% (3/20) of the patients reported continued pain at the donor site and 5% (1/20) reported problems with daily activities. The self-reported pain values were from 1 to 3 with a mean of 2. At 12 months after surgery, none of the patients reported continued pain and problems with daily activities.
The mean preoperative deformity in the coronal plane was 87°. The mean postoperative deformity was 29° at three months after surgery, 30° at 24 months and 31° at the last follow-up. There was a significant difference in the preoperative and postoperative Cobb angles. We noticed less than 4° in the loss of correction at the last follow-up.
The ALBG group
There were no neurological complications or infections. There was no instrumentation failure during the follow-up period. Paralytic ileus occurred in 19% (3/16) of the patients, which was treated by observation without oral intake and cleared in 48 hours. Transient tachycardia, possibly due to the underlying cardiomyopathy, occurred in 19% (3/16) of the patients, and resolved with adequate medication. There have been no further operations for any reason, nor any second hospital admissions related to surgery.
At one week after surgery, all the patients reported back pain in the region of the surgery. At three months after surgery, 31% (5/16) reported back pain. At six months, 13% (2/16) reported back pain, and at nine months none of the patients reported back pain. Therefore, no clinical complaints in the region of the instrumentation surgery were noted at nine months after surgery. None reported pain in the pelvis over the follow-up period. All the patients sat in their wheelchairs by the third postoperative day and returned to their preoperative level of function by the seventh postoperative day.
The mean preoperative deformity in the coronal plane was 83°. The mean postoperative deformity was 25° at three months after surgery, 27° at 24 months and 28° at the last follow-up. There was a significant difference in the preoperative and postoperative Cobb angles. No significant loss of correction was noted between postoperative time and the last follow-up.
Figure 2 shows a radiographic example in the ALBG group.
Fig. 2.
a and b Radiograph of a 13-year-old boy. Sitting anteroposterior preoperative radiograph demonstrates a significant thoracolumbar curve of 80° from T9 to L5. c and d At the time of surgery, banked allograft bone was used. Postoperative radiograph at 1 week after surgery is demonstrated. The postoperative deformity measures 19°. e and f Radiograph at a long-term follow-up of 3.2 years after surgery demonstrates the absence of loss of correction and instrumentation failure, which indicates the probability of the absence of pseudarthrosis
Discussion
Scoliosis is almost universal in DMD patients. Natural history studies on DMD demonstrated an almost invariable progression in the scoliosis with progression of the underlying disease [1, 4, 14, 20, 22]. Posterior spinal fusion for scoliosis in DMD has been widely accepted as the optimal procedure to prevent progression of scoliosis and maintain sitting balance and comfort [1, 4, 14, 20, 22].
The posterior iliac crest is the most common donor site for autogenous bone graft for posterior spinal fusion. However, donor site morbidity caused by bone harvest, which can interfere with daily activity of wheelchair-dependent patients, has remained a concern.
The advantages of autogenous bone graft over allograft have been well recognised. Large quantities and good quality of corticocancellous bone graft may be obtained from the iliac crest. However, harvesting ICBG involves an additional surgical procedure, blood loss, postoperative morbidity and complications. The complications of harvesting ICBG have been also well recognised [12]. The complication rate reported in the literature varies widely, ranging from 1% to 25 % for major complications and 9.4% to 39% for minor complications [2, 8, 18, 21]. Major complications included large haematoma, wound infection, reoperation, unsightly scar, severe pain interfering with daily activity, and chronic pain limiting physical function [2, 18, 25]. Minor complications included dysaesthesia, prolonged wound drainage, and superficial infection [2, 18, 25]. Robertson et al. [16] reported on donor site morbidity, significant pain, tenderness, and local sensory loss, which were worst at six months after surgery and improved by one year. Although the major complication rate in most of the reports is relatively small, the minor complication rate is significant.
Donor site pain after harvesting from iliac crest is common. Lehmann et al. [13] reported this complication in 55% of the adult patients. Skaggs et al. [19] found this complication to be 24% in children. They reported pain severe enough to interfere with daily activity was noted in 15% in children at a more than four-year follow-up. In our series, at three months after surgery, 50% (10/20) of the patients reported continued pain at the donor site, and 40% (8/20) reported problems with daily activities and difficulties in sitting in a wheelchair due to the pain in the back hip area even though the pain improved gradually by one year.
Indeed, in adolescent idiopathic scoliosis surgery, a large quantity and good quality of corticocancellous bone may be obtained from the iliac crest. However, in our experience, the pelvis in this group of patients with DMD who cannot walk is often small. We felt the amount of bone obtained from the iliac crest, even if bone grafts were harvested from both iliac crests, were inadequate for long posterior spinal fusion in DMD scoliosis. Also, a high rate of intraoperative sacroiliac joint and iliac crest inner cortex penetration was observed. These complications may have led to continued postoperative donor site pain and prolonged wound drainage.
To resolve the problems, we began to use banked allograft bone for posterior spinal fusion in DMD scoliosis. We have routinely used banked allograft bone obtained from the Kitasato University Bone Bank (KUBB). Banked allograft bone has both osteoinductive and osteoconductive activities, although less active than those of autografts [10, 11, 24]. In a study of 87 consecutive patients who underwent idiopathic scoliosis surgery, Grogan et al. [9] showed the ability of allograft bone to produce reliable results compared with autograft bone. Fabry [7] concluded that there was no significant difference in the clinical and radiographic results (loss of correction, fusion rate, complication) between the two groups of scoliosis patients operated using either autograft (iliac crest) or allograft (femoral heads) and recommended the use of allograft bone to prevent problems of discomfort at the donor site scar. Dadd et al. [6] concluded that even in the presence of adequate iliac crest, the use of bank bone is superior for grafting in adolescent idiopathic scoliosis surgery. Thus, allograft bone seems to give good results [3]; however, banked allograft bone is not always available in many countries for spine surgery. Potential risks of bacterial contamination and viral transmission have also been suggested, although such a risk is very small [5, 15, 23].
In Japan, there are two kinds of bone banks—regional bone banks and institutional bone banks. A regional bone bank retrieves, processes and preserves bone and supplies bones to other institutions. Our bone bank (KUBB) is one of the regional bone banks, of which there are two such institutions in Japan. Therefore, banked allograft bone is always available for spine surgery at any time in Japan.
To the best of our knowledge, there have been no previous articles in the literature comparing autogenous ICBG and banked allograft bone in instrumented posterior spinal fusion in DMD scoliosis. This study shows a comparable overall radiological fusion rate and clinical outcome using either autogenous ICBG or banked allograft bone but with reduced morbidity, complications, operative time and blood loss in the ALBG group. Curve correction and maintenance of correction were excellent in the both groups, with minimal loss of correction. There was no pseudarthrosis, infection or instrumentation failure in either group. However, intra/postoperative morbidity or complications that could be attributed to the donor site were observed in the ICBG group. Scoliosis surgery in nonambulatory DMD patients should be aimed at maximising function and improving QOL. However, donor site pain in the ICBG group was severe enough to interfere with daily activity and caused difficulties in sitting for nonambulatory DMD patients, although the incidence of pain decreased with time. With the results of this study, we do not recommend the use of autogenous ICBG for posterior spinal fusion in nonambulatory DMD patients.
Study limitation
There are some limitations to this study. The number of cases in both groups was relatively small. Radiological assessment of fusion was never perfect. Determination of fusion has been a difficult issue, with no methods having been shown as reliable. In this study, a definition that combined radiological minimal loss of correction and no clinical complaints was selected. However, the lack of progression of spinal deformity and the absence of instrumentation failure during a minimum two-year follow-up period indicate the probability of the absence of pseudarthrosis.
References
- 1.Askin GN, Hallettv R, Hare N, Webb JK. The outcome of scoliosis surgery in the severely handicapped child. An objective and subjective assessment. Spine. 1997;22:44–50. doi: 10.1097/00007632-199701010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Banwart JC, Asher MA, Hasanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Blanco JS, Sears CJ. Allograft bone use during instrumentation and fusion in the treatment of adolescent idiopathic scoliosis. Spine. 1997;22:1338–1342. doi: 10.1097/00007632-199706150-00011. [DOI] [PubMed] [Google Scholar]
- 4.Bridwell KH, Baldus C, Iffrig TM, Lenke LG, Blanke K. Process measures and patient/parent evaluation of surgical management of spinal deformities in patients with progressive flaccid neuromuscular scoliosis (Duchenne’s muscular dystrophy and spinal muscular atrophy) Spine. 1999;24:1300–1309. doi: 10.1097/00007632-199907010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DG, Li P, Oakeshott RD. HIV infection of human cartilage. J Bone Joint Surg Br. 1996;78:22–25. [PubMed] [Google Scholar]
- 6.Dodd CA, Fergusson CM, Freedmann L, Houghton GR, Thoman D. Allograft versus auto graft bone in scoliosis surgery. J Bone Joint Surg Br. 1988;70:431–434. doi: 10.1302/0301-620X.70B3.3286656. [DOI] [PubMed] [Google Scholar]
- 7.Fabry G. Allograft versus auto graft bone in idiopathic scoliosis surgery: a multivariate statistical analysis. J Pediatr Orthop. 1991;4:465–468. doi: 10.1097/01241398-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–1480. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Grogan DP, Kalen V, Ross TI, Guidera KJ, Pugh LI. Use of allograft bone for posterior spinal fusion in idiopathic scoliosis. Clin Orthop. 1999;369:273–278. doi: 10.1097/00003086-199912000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Sakano S, Sato K, Sugiura H, Iwata H, Murata Y, Seo H. Sensitivity of osteoinductive activity of demineralized and defatted rat femur to temperature and duration of heating. Clin Orthop Relat Res. 1995;316:267–275. [PubMed] [Google Scholar]
- 11.Izawa H, Hachiya Y, Kawai T, Muramatsu K, Narita Y, Ban N, Yoshizawa H. The effect of heat-treated human bone morphogenetic protein on clinical implantation. Clin Orthop Relat Res. 2001;390:252–258. doi: 10.1097/00003086-200109000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts: a review of complications and techniques. Spine. 1989;14:1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann TR, Spratt KF, Tozzi JE, Weinstein JN, EL-Khoury RSJ, GY CH. Long-term follow-up of lower lumbar fusion in patients. Spine. 1987;12:97–104. doi: 10.1097/00007632-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Miller RG, Chalmers AC, Dao H, Filler-Katz A. The effects of spine fusion on respiratory function in Duchenne muscular dystrophy. Neurology. 1991;41:38–45. doi: 10.1212/wnl.41.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Nemzek JA, Arnoczky SP, Swenson CL. Retroviral transmission in bone allotransplantation. The effects of tissue processing. Clin Orthop. 1996;324:275–282. doi: 10.1097/00003086-199603000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Robertson PA, Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine. 2001;26:1473–1476. doi: 10.1097/00007632-200107010-00018. [DOI] [PubMed] [Google Scholar]
- 17.Sakai DN, Hsu JD, Bonnett DA, Brown JC. Stabilization of the collapsing spine in Duchenne muscular dystrophy. Clin Orthop. 1977;128:256–260. [PubMed] [Google Scholar]
- 18.Sengupta DK, Truumees E, Patel CK, Kazmierczak C, Hughes B, Elders G, Herkowitz HN. Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine. 2006;31:985–991. doi: 10.1097/01.brs.0000215048.51237.3c. [DOI] [PubMed] [Google Scholar]
- 19.Skaggs DL, Samuelson MA, Hale JM, Kay RM, Tolo VT. Complications of posterior iliac crest bone grafting in spine surgery in children. Spine. 2000;25:2400–2402. doi: 10.1097/00007632-200009150-00021. [DOI] [PubMed] [Google Scholar]
- 20.Smith A, Koreska J, Mosley CF. Progression of scoliosis in Duchenne muscular dystrophy. J Bone Joint Surg Am. 1989;71:1066–1074. [PubMed] [Google Scholar]
- 21.Summers BN, Eisentein SM. Donor site pain from the ilium. J Bone Joint Surg Am. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 22.Takaso M, Nakazawa T, Imura T, Takahira N, Itoman M, Takahashi K, Yamazaki M, Otori S, Akazawa T, Minami S, Kotani T (2009) Surgical management of severe scoliosis with high-risk pulmonary dysfunction in Duchenne muscular dystrophy. Int Orthop. doi:10.1007/s00264-009-0764-7 [DOI] [PMC free article] [PubMed]
- 23.Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63A:244–248. [PubMed] [Google Scholar]
- 24.Uchiyama K, Ujihira M, Mabuchi K, Takahira N, Komiya K, Itoman M. Development of heating method by micro-wave for sterilization of bone allografts. J Orthop Science. 2005;10:77–83. doi: 10.1007/s00776-004-0857-5. [DOI] [PubMed] [Google Scholar]
- 25.Violas P, Chapuis M, Bracq H. Local autograft bone in the surgical management of adolescent idiopathic scoliosis. Spine. 2004;29:189–192. doi: 10.1097/01.BRS.0000105536.65164.B1. [DOI] [PubMed] [Google Scholar]