Abstract
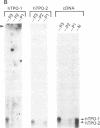
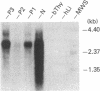
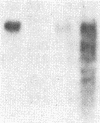
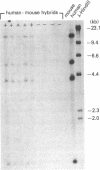
Two forms of human thyroid peroxidase cDNAs were isolated from a lambda gt11 cDNA library, prepared from Graves disease thyroid tissue mRNA, by use of oligonucleotides. The longest complete cDNA, designated phTPO-1, has 3048 nucleotides and an open reading frame consisting of 933 amino acids, which would encode a protein with a molecular weight of 103,026. Five potential asparagine-linked glycosylation sites are found in the deduced amino acid sequence. The second peroxidase cDNA, designated phTPO-2, is almost identical to phTPO-1 beginning 605 base pairs downstream except that it contains 1-base-pair difference and lacks 171 base pairs in the middle of the sequence. This results in a loss of 57 amino acids corresponding to a molecular weight of 6282. Interestingly, this 171-nucleotide sequence has GT and AG at its 5' and 3' boundaries, respectively, that are in good agreement with donor and acceptor splice site consensus sequences. Using specific oligonucleotide probes for the mRNAs derived from the cDNA sequences hTPO-1 and hTPO-2, we show that both are expressed in all thyroid tissues examined and the relative level of two mRNAs is different in each sample. These results suggest that two thyroid peroxidase proteins might be generated through alternate splicing of the same gene. By using somatic cell hybrid lines, the thyroid peroxidase gene was mapped to the short arm of human chromosome 2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnocka B., Ruf J., Ferrand M., Carayon P., Lissitzky S. Purification of the human thyroid peroxidase and its identification as the microsomal antigen involved in autoimmune thyroid diseases. FEBS Lett. 1985 Oct 7;190(1):147–152. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- Degroot L. J., Niepomniszcze H. Biosynthesis of thyroid hormone: basic and clinical aspects. Metabolism. 1977 Jun;26(6):665–718. doi: 10.1016/0026-0495(77)90088-9. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Fang G. H., Kenigsberg P., Axley M. J., Nuell M., Hager L. P. Cloning and sequencing of chloroperoxidase cDNA. Nucleic Acids Res. 1986 Oct 24;14(20):8061–8071. doi: 10.1093/nar/14.20.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya T., Sato I., Hiyama Y., Yoshimura H., Niimi H., Tarutani O. An improved assay method for thyroid peroxidase applicable for a few milligrams of abnormal human thyroid tissues. J Biochem. 1985 Sep;98(3):637–647. doi: 10.1093/oxfordjournals.jbchem.a135320. [DOI] [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Khoury E. L., Hammond L., Bottazzo G. F., Doniach D. Presence of the organ-specific 'microsomal' autoantigen on the surface of human thyroid cells in culture: its involvement in complement-mediated cytotoxicity. Clin Exp Immunol. 1981 Aug;45(2):316–328. [PMC free article] [PubMed] [Google Scholar]
- Kotani T., Umeki K., Matsunaga S., Kato E., Ohtaki S. Detection of autoantibodies to thyroid peroxidase in autoimmune thyroid diseases by micro-ELISA and immunoblotting. J Clin Endocrinol Metab. 1986 May;62(5):928–933. doi: 10.1210/jcem-62-5-928. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T. Y., STEIN W. H., MOORE S., ELLIOTT S. D. THE SEQUENCE OF AMINO ACID RESIDUES AROUND THE SULFHYDRYL GROUP AT THE ACTIVE SITE OF STREPTOCOCCAL PROTEINASE. J Biol Chem. 1965 Mar;240:1143–1149. [PubMed] [Google Scholar]
- Magnusson R. P., Gestautas J., Seto P., Taurog A., Rapoport B. Isolation and characterization of a cDNA clone for porcine thyroid peroxidase. FEBS Lett. 1986 Nov 24;208(2):391–396. doi: 10.1016/0014-5793(86)81055-9. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Battey J., Hollis G. F., Swan D. C., Siebenlist U., Leder P. Localization of human variable and constant region immunoglobulin heavy chain genes on subtelomeric band q32 of chromosome 14. Nucleic Acids Res. 1982 Dec 20;10(24):8155–8170. doi: 10.1093/nar/10.24.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Hieter P. A., Hollis G. F., Swan D., Otey M. C., Leder P. Chromosomal location of human kappa and lambda immunoglobulin light chain constant region genes. J Exp Med. 1982 May 1;155(5):1480–1490. doi: 10.1084/jem.155.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Merry D., Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci U S A. 1986 Jan;83(1):130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Swan D. C., Santos E., Barbacid M., Tronick S. R., Aaronson S. A. Localization of the normal allele of T24 human bladder carcinoma oncogene to chromosome 11. Nature. 1982 Dec 23;300(5894):773–774. doi: 10.1038/300773a0. [DOI] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Yamazaki I., Nakagawa H., Ohtaki S. Steady state kinetics and regulation of thyroid peroxidase-catalyzed iodination. J Biol Chem. 1983 Mar 25;258(6):3837–3842. [PubMed] [Google Scholar]
- Ohtaki S., Kotani T., Nakamura Y. Characterization of human thyroid peroxidase purified by monoclonal antibody-assisted chromatography. J Clin Endocrinol Metab. 1986 Sep;63(3):570–576. doi: 10.1210/jcem-63-3-570. [DOI] [PubMed] [Google Scholar]
- Ohtaki S., Nakagawa H., Nakamura S., Nakamura M., Yamazaki I. Characterization of hog thyroid peroxidase. J Biol Chem. 1985 Jan 10;260(1):441–448. [PubMed] [Google Scholar]
- Omiecinski C. J., Walz F. G., Jr, Vlasuk G. P. Phenobarbital induction of rat liver cytochromes P-450b and P-450e. Quantitation of specific RNAs by hybridization to synthetic oligodeoxyribonucleotide probes. J Biol Chem. 1985 Mar 25;260(6):3247–3250. [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Portmann L., Hamada N., Heinrich G., DeGroot L. J. Anti-thyroid peroxidase antibody in patients with autoimmune thyroid disease: possible identity with anti-microsomal antibody. J Clin Endocrinol Metab. 1985 Nov;61(5):1001–1003. doi: 10.1210/jcem-61-5-1001. [DOI] [PubMed] [Google Scholar]
- Rawitch A. B., Taurog A., Chernoff S. B., Dorris M. L. Hog thyroid peroxidase: physical, chemical, and catalytic properties of the highly purified enzyme. Arch Biochem Biophys. 1979 Apr 15;194(1):244–257. doi: 10.1016/0003-9861(79)90615-5. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M. Coupling of iodotyrosine catalyzed by human thyroid peroxidase in vitro. J Clin Endocrinol Metab. 1985 Jun;60(6):1069–1075. doi: 10.1210/jcem-60-6-1069. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Mudryj M., de Crombrugghe B. A uniquely conserved regulatory signal is found around the translation initiation site in three different collagen genes. J Biol Chem. 1983 Dec 25;258(24):14914–14919. [PubMed] [Google Scholar]