Abstract
The purpose of this study was to determine if the cartilage from loose osteochondral fragments remains viable. Five patients with OCD of the knee who had undergone surgical treatment (arthroscopic reduction and internal fixation of the loose body) were included. The average age of patients was 13 years (range 10–14 years). Cartilage samples were obtained from the loose body fragments before reattachment was performed (study group) and from the healthy native cartilage (intercondyle area, control group) from each of the five patients. Tissue viability was assessed using live-dead assay in both groups. All five loose osteochondral fragments showed similar viability to the healthy native cartilage group, with 88% cell viability (95% CI 50–100) in loose body fragments versus 92% viability (95% CI 50–100) from healthy cartilage. This study showed that cartilage from detached OCD fragments remains viable before reattachment is performed.
Introduction
Treatment of symptomatic unstable osteochondral fragments in osteochondritis dissecans (OCD) with arthroscopic reduction and internal fixation (ARIF) has been shown to have good clinical results in 90% of the cases [1–5]. Fixation with autogenous osteochondral grafts (OATS) has also been suggested as an alternative fixation technique for the treatment of unstable osteochondral fragments [6]. Most of these loose osteochondral fragments had been detached for a couple of months before reduction and fixation was performed, probably affecting cartilage viability while deprived from the subchondral bone nutrition. Histological and cell biological studies of these osteochondral fragments are controversial. Some authors argue that chondrocytes from these osteochondral fragments retain viability and have a normal homeostasis while others suggest that degeneration is observed after the fragment is detached [7–9].
Recent studies have shown that cell death may contribute to the subsequent development of osteoarthritis [10, 11]. Innovative therapies are focussing on the survival of the chondrocytes in order to prevent cartilage degeneration [10–12]. Viable cells would be desired when reduction and fixation of the osteochondral fragment is performed, potentially reducing the chances of progressive cartilage degeneration.
The purpose of this study was to determine if chondrocytes isolated from osteochondral loose body fragments are viable before reattachment is performed.
Methods
Five patients (five knees) with clinical diagnosis of OCD of the knee were included. Demographic patient data can be found in Table 1. All patients underwent surgical treatment with arthroscopic reduction and internal fixation of the loose body. All patients were male with a mean age of 13.6 years (range 10–15 years) at the time of the arthroscopic treatment. Preoperative MRI was performed in all patients, providing information about the osteochondral fragment, the underlying subchondral bone, the integrity and condition of the articular surface and the structure of the interface between the osteochondral fragment and the subjacent bone. The lesions were classified according to De Smet et al. [13] based on T2-weighted images: a line of high signal intensity of at least 5 mm in length between the fragment and the underlying bone (class 1), an area of increased homogeneous signal at least 5 mm in diameter beneath the lesion (class 2), a focal defect of 5 mm or more on the surface of the articular cartilage (class 3), and a high signal traversing the subcondral bone into the lesion (class 4). Under arthroscopy the lesions were classified according to the International Cartilage Repair Society (ICRS) whereby ICRS OCD I was a stable lesion with continuous but softened area covered by intact articular cartilage, ICRS OCD II was a lesion with partial articular cartilage discontinuity but stable when probed, ICRS III was a lesion with a complete articular cartilage but not dislocated “dead in situ”, and ICRS IV was a dislocated fragment, loose within the bed or empty defect. Also the osteochondral fragments were examined for integrity of the superficial area and stability. This was performed gently and manually in order to minimise the mechanical induction of cell death.
Table 1.
Demographic data
| Case | Age (years) | Gender | Localisation | MRI classification | ICRS classification |
|---|---|---|---|---|---|
| 1 | 10 | Male | MFC | 3 | IV |
| 2 | 15 | Male | MFC | 3 | IV |
| 3 | 14 | Male | MFC | 4 | IV |
| 4 | 15 | Male | MFC | 3 | IV |
| 5 | 14 | Male | MFC | 3 | IV |
ICRS International Cartilage Repair Society, MFC medial femoral condyle
In all knees the osteochondral fragment was dislocated from the subchondral bed (ICRS IV). Curettage of the lesion was performed to remove fibrous tissue followed by arthroscopic reduction and internal fixation of the osteochondral fragment. With a curette, three to four biopsies were harvested from the cartilage of the detached osteochondral fragment (study). A control tissue sample was obtained from the intercondylar notch (control) (Figs. 1, 2, 3, 4). Samples were kept moist and taken to the laboratory for cell extraction and live-dead assay.
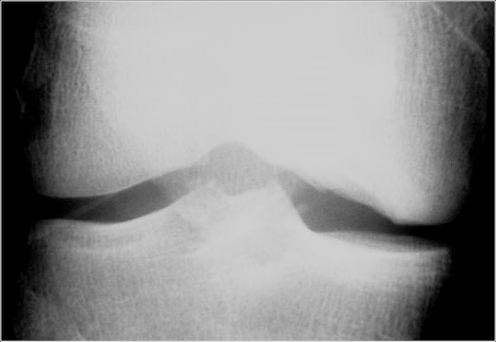
Fig. 1.
Preoperative anteroposterior radiograph showing the ostechondral lesion in the medial femoral condyle
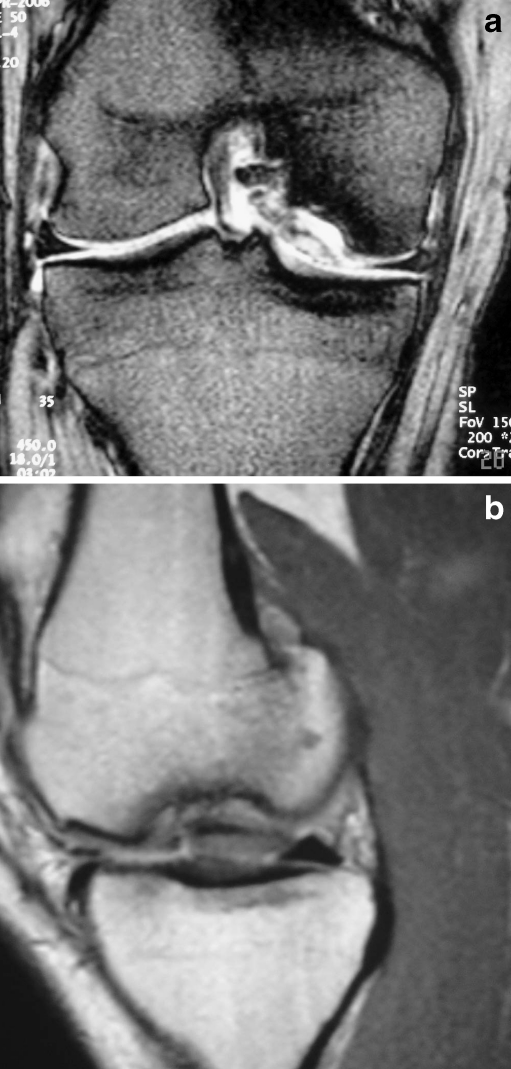
Fig. 2.
a Coronal view showing completely detached osteochondral fragment. b Sagittal view. Fluid between the osteochondral fragment and the subjacent subchondral bone is evident, suggesting an unstable fragment
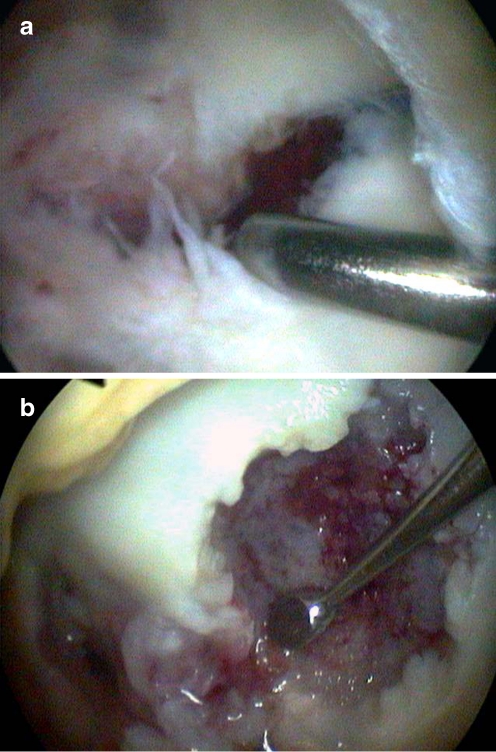
Fig. 3.
a Intraoperative arthroscopic view of the detached osteochondral fragment. b Intraoperative arthroscopic debridement of the subjacent subchondral bone
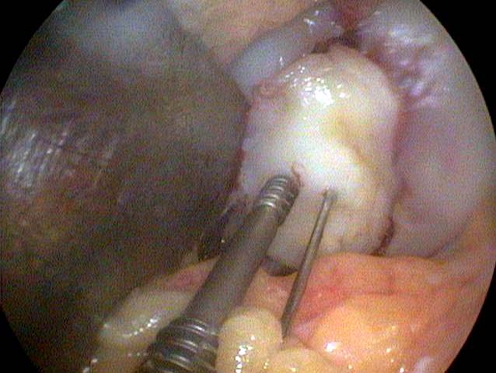
Fig. 4.
Reduction and fixation of the osteochondral fragment with Herbert screws
Cell viability
Chondrocytes were isolated by sequential protease digestion of the tissue (1 h in 0.2% pronase), followed by overnight digestion in 0.025% collagenase-p in DMEM/F-12 medium (GIBCO BRL, Life Technologies, Grand Islands, NY, USA) supplemented with 5% serum and 50 ug gentamycin/ml. The released cells were submerged in a solution of PBS containing Calcein –AM (2, 7 uM) and ethidium homodimer 1 (5.0 Um) (Live/Dead and Viability/Cytotoxic Kits, Molecular Probes, OR, USA) for 1 h at 4°C and rinsed twice in PBS for 20 min each. “Live” cells that are metabolically active and have an intact membrane and fluoresce green, indicative of intracellular esterase activity. Conversely, “dead” cells, inactive metabolically and having a compromised plasma membrane, and fluoresce red, indicating ethidium homodimer -1 bound to DNA [14]. A blinded observer who generally works with fluorescence microscopy (CPG) manually counted dead and live cells, and a total average viability was determined. A minimum of 100 cells were counted.
Results
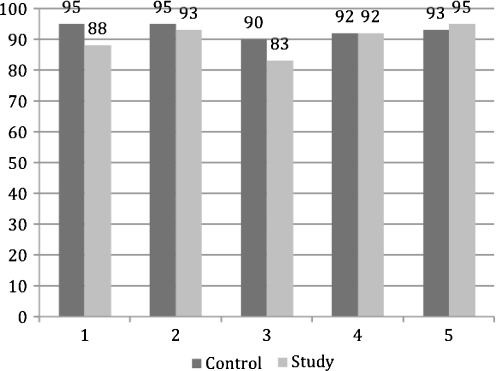
Arthroscopic osteochondral loose fragments had irregularity and softening of the cartilage in all five cases. All fragments were dislocated and loose within the defect (grade IV ICRS). Percent of viable cells was similar in both the control and study groups. The average percentage of viable cells in the study group was 88% (CI 95% 14–94) (Fig. 5). The control group showed 92% viable cells (CI 95% 16–96) (Graph 1).
Fig. 5.
Live-dead assay from cartilage tissue taken from study detached osteochondral fragment (green cells: live cells—red cells: dead cells)
Graph 1.
Percent viability in the control and study tissues from each of the five cases
Discussion
The primary question of this investigation was whether chondrocytes from osteochondral loose body fragments are viable before the reduction and fixation is performed. This study shows that chondrocytes from osteochondral loose body fragments do retain viability before reattachment.
Cartilage nutrition is normally maintained by both the subchondral bone and the synovial fluid. Survival of the chondrocytes from the osteochondral loose body fragments suggests that the synovial fluid provides enough nutrition to the cartilage while it is deprived of the subchondral bone nutrition [15]. Previous reports about the natural evolution of the OCD fragments are controversial. Some authors argue that there is a definitive loss of viability and matrix degeneration in these fragments while others deny these changes. Most likely, these differences were because of different study designs, animal models and joint disease location [5]. Aurich et al. [16] analysed dissected cartilage knee fragments from 12 patients with clinical diagnosis of OCD. They showed that these fragments preserved high viability. In contrast to our study, none of these OCD fragments were reduced and fixed. Gianini et al. [9] showed the capacity of chondrocytes, harvested from detached osteochondral fragments from the talus, to serve as a source of viable cells for the ACI procedure. In contrast to our study, this was performed in the talus. Different biological behaviour has been shown between knee and talus cartilage. Also, none of the osteochondral knee fragments were reduced and fixed [17].
To our knowledge this is the first study to analyse the viability of osteochondral loose body fragments of the knee in patients with OCD. A limitation of this study is that histological analysis was not performed. Thus, we were unable to determine if there was cartilage degeneration of the osteochondral loose body fragments. Also, as the samples were digested we could not determine if viability remained the same in all layers of the cartilage. Cartilage layers have different metabolic and biochemical functions. Probably, greater viability should be expected in the superficial layer as this was constantly moist from the synovial fluid. Future experiments with tissue explants will provide more information about the viability in different layers of the osteochondral loose body fragments.
Conclusion
The results of this study suggest that cartilage from osteochondral loose body fragments is viable. Preservation with reduction and fixation of these fragments should be attempted whenever possible.
References
- 1.Makino A, Muscolo DL, Puigdevall M, Costa-Paz M, Ayerza MA. Arthroscopic fixation of osteochondritis dissecans of the knee: clinical, magnetic resonance imaging, and arthroscopic follow-up. Am J Sports Med. 2005;33(10):1499–1504. doi: 10.1177/0363546505274717. [DOI] [PubMed] [Google Scholar]
- 2.Kivistö R, Pasanen L, Leppilahti J, Jalovaara P. Arthroscopic repair of osteochondritis dissecans of the femoral condyles with metal staple fixation: a report of 28 cases. Knee Surg Sports Traumatol Arthrosc. 2002;10:305–309. doi: 10.1007/s00167-002-0294-y. [DOI] [PubMed] [Google Scholar]
- 3.Dines JS, Fealy S, Potter HG, Warren RF. Outcomes of osteochondral lesions of the knee repaired with a bioabsorbable device. Arthroscopy. 2008;24(1):62–68. doi: 10.1016/j.arthro.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Pascual-Garrido C, Friel NA, Kirk SS, McNickle AG, Bach BR, Jr, Bush-Joseph CA, Verma NN, Cole BJ. Midterm results of surgical treatment for adult osteochondritis dissecans of the knee. Am J Sports Med. 2009;37(Suppl 1):125S–130S. doi: 10.1177/0363546509350833. [DOI] [PubMed] [Google Scholar]
- 5.Michael JW, Wurth A, Eysel P, König DP. Long-term results after operative treatment of osteochondritis dissecans of the knee joint—30 year results. Int Orthop. 2008;32(2):217–221. doi: 10.1007/s00264-006-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca F, Balacó I. Fixation with autogenous osteochondral grafts for the treatment of osteochondritis dissecans (stages III and IV) Int Orthop. 2009;33(1):139–144. doi: 10.1007/s00264-007-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuroki K, Cook JL, Tomlinson JL, Kreeeger JM. In vitro characterization of chondrocytes isolated from naturally occurring osteochondrosis lesions of the humeral head of dogs. Am J Vet Res. 2001;63(2):186–193. doi: 10.2460/ajvr.2002.63.186. [DOI] [PubMed] [Google Scholar]
- 8.Touten Y, Adachi N, Deie M, Tanak N, Ochi M. Histologic evaluation of osteochondral loose bodies and repaired tissues after fixation. Arthroscopy. 2007;23(2):188–196. doi: 10.1016/j.arthro.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Giannini BR, Grigolo B, Franceschi L, Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle. Osteoarthritis Cartilage. 2005;13(7):601–607. doi: 10.1016/j.joca.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8(2):333–345. doi: 10.2174/138945007779940025. [DOI] [PubMed] [Google Scholar]
- 11.Kühn K, D'Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12(1):1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Pascual Garrido C, Hakimiyan AA, Rappoport L, Oegema TR, Wimmer MA, Chubinskaya S. Anti-apoptotic treatments prevent cartilage degradation after acute trauma to human ankle cartilage. Osteoarthritis Cartilage. 2009;17(9):1244–1251. doi: 10.1016/j.joca.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smet A, Fisher DR, Burnstein MI, Lange RH. Value of MR imaging in staging osteochondral lesions of the talus (osteochondritis dissecans): results in 14 patients. AJR Am J Roentgenol. 1990;154(3):555–558. doi: 10.2214/ajr.154.3.2106221. [DOI] [PubMed] [Google Scholar]
- 14.Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Inmunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-M. [DOI] [PubMed] [Google Scholar]
- 15.Lee D, Salih V, Stockton E, Stanton J, Bentley G. Effect of normal synovial fluid on the metabolism of articular chondrocytes in vitro. Clin Orthop Relat Res. 1997;342:228–238. doi: 10.1097/00003086-199709000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Aurich M, Anders J, Trommer T, Liesaus E, Seifert M, Schömburg J, Rolauffs B, Wagner A, Mollenhauer J. Histological and cell biological characterization of dissected cartilage fragments in human osteochondritis dissecans of the femoral condyle. Ach Orthop Trauma Surg. 2006;126(9):606–614. doi: 10.1007/s00402-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 17.Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis Cartilage. 2005;13(2):93–103. doi: 10.1016/j.joca.2004.11.006. [DOI] [PubMed] [Google Scholar]