Introduction
Suspensory fixation methods including endobutton, post and suture disc with hamstring grafts are commonly used in anterior cruciate ligament (ACL) reconstruction. But the phenomenon of bone tunnel enlargement has been usually found in cases with suspensory fixation and accelerated rehabilitation [10]. It was considered that tendon graft motion in the bone tunnel produced by long-distance fixation and cyclic loading led to failure of tendon-bone interface healing. It has been recognised by more and more authors that enhancing and accelerating healing of the tendon graft in the bone tunnel is of importance to prevent reconstruction failure during cyclic loading of the graft after surgery prior to conversion from mechanical to biological fixation [10]. Some substances that influence bone-tendon healing have been studied including cell factor, hyaluronan and osteoconductive calcium phosphate ceramics, but most of them only enhanced the bonding through an indirect connection which was unlike the normal ACL insertion. In this study, osteoinductive calcium phosphate biomaterials were used to enhance early bonding between tendon and bone. The purpose of this study was to research the healing pattern of tendon in a bone tunnel with suspensory fixation and to explore the influence of osteoinductive calcium phosphate ceramics (OICPC) on tendon-bone bonding.
Materials and methods
Study design
The study was approved by the Research Ethics Committee of our institution. Thirty New Zealand White adult rabbits were divided into the OICPC group and a control group. ACL reconstruction with semitendinosus tendon and suspensory fixation was carried out in all of these rabbits. In the OICPC group, OICPC particles were implanted in the bone tunnel. The animals were sacrificed at 4, 8 and 12 weeks postoperatively for serial histological observation and morphological analysis.
Reconstruction model
The animals were anaesthetised with 25 mg/kg thiopental sodium. Intramuscular cefazolin 80 mg/kg was administered preoperatively and on the first postoperative day for infection prophylaxis. The surgical field was sterilised with povidone-iodine. The hind limb knee joint was approached via a medial peripatellar arthrotomy. Semitendinosus tendon was collected and was folded into two bands as grafts. The ACL was excised and a bone tunnel, with a diameter of 2.5 mm, was made in the proximal tibia just anterior to the medial collateral ligament (MCL), ending at the original point of insertion of the ACL on the intercondylar tibial spine. A femoral tunnel of the same size was made from the midline of the intercondylar notch ending just superior to the origin of the lateral collateral ligament (LCL). Two end points of the graft were sutured with #2 non-absorbable sutures. The graft was passed through the tibial and femoral bone tunnels and 2.5 mg OICPC particles was implanted in each bone tunnel in the OICPC group. The sutures were knotted repeatedly and the big knot compressed the cortical bone at the outlet of the femoral tunnel. Then tibial end sutures were tightened in the same way. The patella was then repositioned; retinaculum and skin were sutured. Each rabbit was allowed unrestricted daily activities in its own cage.
OICPC material
The particle of OICPC was porous hydroxyapatite/tricalcium phosphate (HA/TCP) ceramics with a ratio of 70/30. After initial preparation, the HA/TCP powders foamed with diluted 10% H2O2. After drying, the porous green body was sintered at 1,200°C to obtain porous ceramic. The particles with 50 ~ 60% pores were obtained as a final product. The osteoinduction of the materials had been proved [23, 25].
Histological observation
Five animals were killed at 4, 8 and 12 weeks after operation in each group, respectively. The tibial specimens and the femoral specimens were fixed in a 10% buffered formalin solution immediately after harvesting from each limb. After the specimen was decalcified, it was cast in paraffin blocks. The specimens sectioned parallel to the longitudinal axis of the bone tunnel were stained with haematoxylin and eosin, Sirius red and Masson trichrome for light microscopy. According to Yamakado’s classification [22], interface tissue at the tendon-bone junction was graded morphologically in terms of separation between bone and tendon, interface without collagen fibre continuity, Sharpey’s fibre continuity (indirect connection) and direct type of insertion. Each group had ten specimens (five tibial and five femoral) in the observation time. If two types of interface tissue were found in the same section, then the specimen was assigned to a higher grade.
Morphological quantitative evaluation
With Masson trichrome sections, the amount of new bone formation over the grafted tendon was measured in a 1 mm2 frame fixed on an objective lens in a ×40 magnified optical field at the central part of the grafted tendon using the IMS/DNA colour image analysis system (Shenteng Technique Ltd. Co., Shanghai, People’s Republic of China) for histomorphometry. Five different randomly selected areas were investigated for measurement in each specimen. Student’s t test was employed for statistical analysis, and p values less than 0.05 were considered statistically significant.
Results
Histological findings
Four weeks
In the control group, gaps were found between graft and tunnel wall in the entrance of the tunnel. Amorphous fibrous tissue without penetrating collagen fibres was observed between graft tendon and bone. Separation between bone and tendon appeared in some specimens. In the tendon substance located within the tunnel, deficiency of fibroblast cells was observed. In the OICPC group, gaps were also found between graft and tunnel wall in the internal entrance of the tunnel. But a large amount of new bones formed in the deep portion of the tunnel where chondrocyte-like cells emerged. Penetrating fibres appeared between bone and tendon graft, but Sharpey’s fibres could be found in only two of ten specimens (Fig. 1a). Most of the specimans had four layers including a central necrosis portion of graft tendon substance without fibroblast cells, lateral portion of tendon graft with a small number of fibroblast cells, interface tissue with amorphous loose connective tissue and new bone region (Fig. 1b).
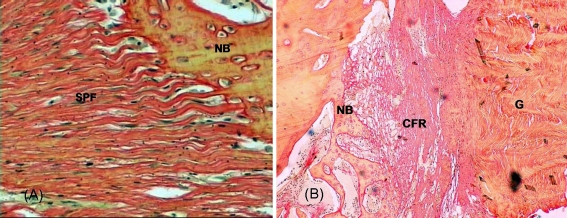
Fig. 1.
a Special penetrating fibres (SPF) which anchored on new bone (NB) base appeared in the OICPC group at 4 weeks. Sirius red, ×400. b Most of the specimens in the OICPC group had four layers: central necrosis portion of graft tendon substance without fibroblast cells, lateral portion of tendon graft with a small number of fibroblast cells, interface tissue with amorphous loose connective tissue and new bone (NB) region. CFR collagen fibres of interface tissue, G graft. Sirius red, ×40
Eight weeks
In the control group, the fibroblast cells appeared in the central portion of tendon substance and blood vessel buds were first found in interface tissue and the lateral portion of the graft. Penetrating fibres could not be seen in the interface tissue (Fig. 2a). The volume of new bone formation increased but was still less than that of the OICPC group, and the chondrocyte-like cells could not be seen. In the OICPC group, massive Sharpey’s fibres, which formed between tendon and new bone, were greater than those in the deep portion of the bone tunnel at 4 weeks (Fig. 2b). These fibres, which were derived from and were perpendicular to the new bone basal plate, appeared green in colour in the section stained with Masson trichrome and orange in colour in the section stained with Sirius red. A classic direct connection could not be seen in sections 8 weeks after surgery, but fibrocartilage piles were observed in new bone surface with special penetrating fibres, which differed from classic Sharpey’s fibres (Fig. 4a).
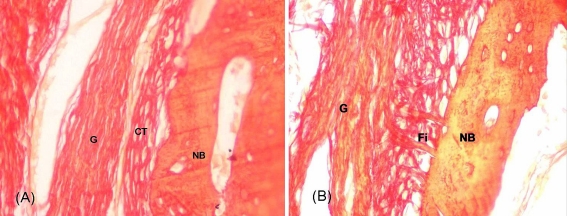
Fig. 2.
a Connective tissue (CT) and new bone (NB) formed at the bone-tendon interface without Sharpey’s fibres in the control group at 8 weeks. G graft. Sirius red, ×400. b Massive penetrating Sharpey’s fibres (Fi) formed between new bone (NB) and tendon graft (G) in the OICPC group at 8 weeks. Sirius red, ×400
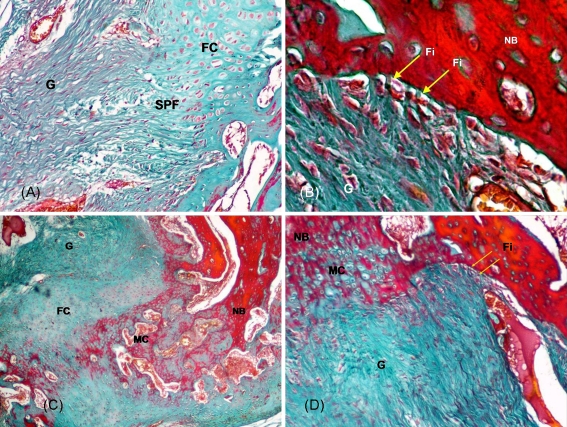
Fig. 4.
a Fibrocartilage (FC) piles were observed in new bone surface with special penetrating fibres (SPF) in the OICPC group at 8 weeks. G graft. Masson, ×100. b Indirect type of connection with classic penetrating Sharpey’s fibres (Fi, yellow arrows) appeared in the OICPC group at 12 weeks. NB new bone, G graft. Masson, ×400. c Direct type of connection including fibrocartilage (FC) and mineralised cartilage (MC) zones appeared in the OICPC group at 12 weeks. G graft, NB new bone. Masson, ×40. d A special transection appearance was observed at 12 weeks in one specimen with OICPC, in which the direct connection and the indirect connection could be seen in the same section. Simultaneously a new type of connection only with calcified cartilage and without fibrocartilage was found on the opposite side of the classic direct type of connection. NB new bone, MC mineralised cartilage, Fi Sharpey’s fibres (yellow arrows), G graft. Masson, ×100
Twelve weeks
In the control group, the amount of new bone formation was much greater than that at 4 weeks. Sharpey’s fibres were observed in some sections, but a direct connection and fibrocartilage piles could not be seen (Fig. 3a). In the OICPC group, an indirect connection was observed in almost all specimens (Fig. 3b) and a classic direct connection composed of four zones including tendon, fibrocartilage, calcified cartilage and new bone basal plate was noted in two specimens (Fig. 3c). A special transection appearance was observed at 12 weeks in one specimen with OICPC, in which a direct connection and an indirect connection could be seen in the same section. Simultaneously a new type of connection without fibrocartilage was found in the opposite side of the classic direct type of connection (Fig. 4b–d). Penetrating fibres were located on both sides of cartilage structures in the tunnel.
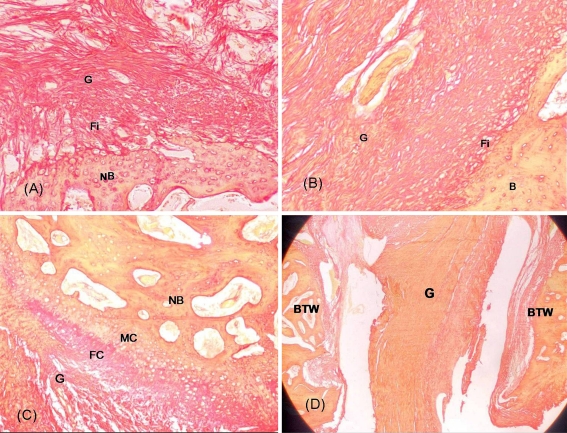
Fig. 3.
a Penetrating Sharpey’s fibres (Fi) appeared at 12 weeks in the control group. G graft, NB new bone. Sirius red, ×100. b Indirect type of connection with continuous penetrating Sharpey’s fibres appeared in the OICPC group at 12 weeks. G graft, Fi Sharpey’s fibres, B bone. Sirius red, ×100. c Direct type of connection including fibrocartilage (FC) and mineralised cartilage (MC) zones appeared in the OICPC group at 12 weeks. NB new bone, G graft. Sirius red, ×100. d In the internal entrance of the tunnel, obvious separation between tendon graft and bone tunnel wall (BTW) was observed in most specimens whether it was from the control group or the OICPC group. G graft. Sirius red, ×10
In the entrance of the tunnel, obvious separation between tendon graft and bone tunnel wall was observed in most specimens whether it was from the control group or the OICPC group (Fig. 3d). Tendon-bone healing was found only in the deep portion of the bone tunnel.
Yamakado’s classification of tendon-bone junction
The results of morphological grading of interface tissue at the tendon-bone junction are shown in Table 1.
Table 1.
Yamakado interface morphological grade
| 4 weeks | 8 weeks | 12 weeks | ||||
|---|---|---|---|---|---|---|
| Controls | OICPC | Controls | OICPC | Controls | OICPC | |
| Direct type | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 2/10 |
| Indirect type | 0/10 | 2/10 | 0/10 | 7/10 | 2/10 | 7/10 |
| Interface | 4/10 | 8/10 | 8/10 | 3/10 | 7/10 | 1/10 |
| Separation | 6/10 | 0/10 | 2/10 | 0/10 | 1/10 | 0/10 |
Evaluation of area of new bone over grafted tendon
In the sections stained with Masson trichrome, the area of new bone over the grafted tendon in a 1 mm2 frame on slides in the OICPC group at 4, 8 and 12 weeks was 0.26 ± 0.13, 0.40 ± 0.11 and 0.47 ± 0.11 mm2, respectively, while that in the control group was 0.16 ± 0.05, 0.23 ± 0.04 and 0.32 ± 0.08. The area of new bone in the OICPC group was significantly greater than that in the control group.
Discussion
The normal insertion site of tendon or ligament to bone is a highly specialised tissue that functions to diminish stress concentration at the soft tissue-hard tissue interface. There are direct and indirect types of insertion, which are distinguishable by their distinct histological structure. The direct insertion is composed of four distinct zones: tendon, unmineralised fibrocartilage, mineralised fibrocartilage and bone. The indirect insertion contains collagen fibres that blend with periosteal collagen, which are, in turn, anchored to underlying bone without an intervening zone of fibrocartilage. The indirect insertion contains Sharpey’s fibres, which refers to the collagen fibres that are direct connections from ligament or tendon into bone. An ideal healing pattern of tendon in the bone tunnel is the direct type of insertion such as normal ACL attachment sites, but the healing pattern and healing time are influenced by many elements. The healing time of tendon in the bone tunnel (hamstring grafts) is much slower than that of bone-bone joint (patellar tendon graft). On the other hand, most authors’ studies showed an indirect insertion between tendon and bone tunnel with suspensory fixation [8]. Kawakami et al. used a rabbit model to study tendon healing in an extra-articular bone tunnel. It appeared that a mature indirect insertion with Sharpey-like collagen fibres formed at 8 weeks [6]. Tomita et al. reported that the flexor tendon graft was anchored to the intra-articular bone tunnel wall with newly formed collagen fibres resembling Sharpey's fibres by 12 weeks in dog models with suspensory fixation [18]. In our study, we also found an indirect insertion in an intra-articular bone tunnel at 12 weeks in the control group without direct type of insertion. Robert et al. performed tendon-bone insertion biopsies in 12 cases with ACL reconstruction using four strands of semitendinosus and gracilis tendons fastened by a transfixion fixation. According to these histological results in patients, the time to obtain a mature indirect anchorage at the top of the tunnel was 10–12 months, which is much longer than that reported in animal models (6–24 weeks) [14]. Because simple suspensory fixation might not completely endure cyclic loading before secure bone-tendon healing formed, more and more authors have recognised the importance of enhancing and accelerating healing of tendon graft in a bone tunnel.
Most of the experimental work about tendon-bone healing in a bone tunnel focused on the function of the periosteum and cell factors including transforming growth factor (TGF)-β, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), blockage of matrix metalloproteinases, hyaluronan and especially bone morphogenetic protein (BMP) [2, 7, 9, 21]. In recent decades, it has been proved that BMP has an important role not only in tendon repair but also in tendon-bone healing [1, 4, 11, 12, 15]. Rodeo et al. showed that tendon healing in a bone tunnel could be enhanced by recombinant human BMP-2 (rhBMP-2). Histological analysis showed earlier and more abundant bone ingrowth into the tendon-bone interface, and the biomechanical testing showed an earlier increase in attachment strength. But after 8 weeks, the direct type of insertion could not be found [15]. Mihelic et al. showed that rhBMP-7 promoted complete tendon graft integration into the newly formed surrounding trabecular bone in the ACL reconstruction model, but a direct connection also could not be found 6 weeks after surgery [12]. Martinek et al. reported the results of BMP-2 gene transfer to improve the integration of semitendinosus tendon grafts at the tendon-bone interface [11]. A direct insertion could be seen at 8 weeks postoperatively, but the authors did not further probe the developmental mechanism of direct insertion.
Although calcium phosphate biomaterials possessing excellent osteoconductivity and biocompatibility have been extensively used clinically as bone substitute for decades, few studies focused on bone-tendon healing with calcium phosphate biomaterials. Ishikawa et al. [5] injected a gel-formed composite of collagen and powdered hydroxyapatite (C-HAp) into the bone tunnel to enhance healing between tendon and bone in an extra-articular animal model. The study showed that new bone formation and Sharpey’s fibres emerging in the C-HAp group was much more than that in the control group before 8 weeks, but no Sharpey’s fibres were observed [3]. Tien et al. performed suspensory fixation ACL reconstruction in a rabbit model with special solubility calcium phosphate cement (CPC) to fill the gap between tendon and bone tunnel. The results showed that at 1 or 2 weeks, the mean maximal tensile strength of the union in the CPC group was much greater than that in the control group with statistical significance. The histological examination showed an early, diffuse and massive bone ingrowth with the use of CPC. By contrast, in the control group of rabbits only a thin layer of new bone and a mass fibrous tissue could be seen at the interface [17]. The authors stated that CPC could reinforce the fixation of the tendon attachment to bone and augment the overall effectiveness of tendon healing to bone, but the mechanism of augmentation was not analysed in depth and the difference in strength might have been attributed to a packing interaction when CPC solidified. Mutsuzaki et al. studied the mechanism of tendon graft hybridising with calcium phosphate and concluded that it could enhance bonding at the tendon-bone interface. They showed that both low-crystalline apatite and collagen at the tendon surface could guide osteoblasts to form new bone while they were resorbed by osteoclasts. An increase in calcium ion levels released from dicalcium phosphate dihydrate (DCPD) or low-crystalline apatite at the tendon surface might play an important role in cell adhesion [13]. All of the studies showed that osteoconductive calcium phosphate biomaterials could augment bonding of the tendon-bone interface in the bone tunnel. It was discovered that the study group showed greater new bone and earlier occurrence or maturity of Sharpey’s fibres compared to the control group. However, in all of these experiments, only an indirect attachment with Sharpey’s fibres could be found at the interface without a direct type of attachment.
Previous reports have shown that porous calcium phosphate ceramics could produce not only osteoconductivity but also osteoinductivity in vivo [23, 25]. Zhang et al. reported that calcium phosphate ceramics implanted in non-osseous tissue could concentrate endogenous BMP on the surface of the internal pores of the materials. The in vivo experiment showed less new bone formed after implantation of the materials in the muscles of dogs, in which the concentration of native BMPs on the calcium phosphate ceramics was inhibited by a monoclonal antibody of bovine bone morphogenetic protein (McAb-BMP). By contrast, much new bone formed in the samples not inhibited by McAb-BMP [24]. Concentration of the intrinsic biological signal (such as BMP) to the surface of porous calcium phosphate ceramics induced the mesenchymal cells to differentiate into preosteogenic cells [3]. The three-dimensional porous structure and the formation of bone-like apatite interface provided a suitable environment for osteogenesis [3].
In our study, enhancement of the development of new bone and Sharpey’s fibres could be seen at 4, 8 and 12 weeks in the group with calcium phosphate biomaterials. Especially at 12 weeks, occurrence of a direct type of attachment with classic four-zone structure hinted that OICPC could augment the healing of tendon to bone. Meanwhile, earlier formation of new bone and Sharpey’s fibres and occurrence of a direct attachment could be observed in the study group compared to the control group. Thus it was assumed that OICPC might not only improve formation of new bone, but also promote aggregation of fibroblasts or facilitate multi-directional differentiation of mesenchymal cells, which made a collagen fibre matrix. Subsequently, it might provide favourable circumstances for tendon healing. In addition, a large amount of new bone was formed in the early phase of the healing process as a sufficient anchoring base for Sharpey’s fibres. According to fact that a direct attachment only appeared in the OICPC group combined with the research results reported by Martinek et al. [11], it might also be expected that there was some internal relationship between osteoinductivity of the local interface and the direct attachment. In this study, there was a special histological appearance which contained cartilage piles. It was also assumed that the osteoinductivity of the local interface led to the development of a direct attachment through the process of endochondral ossification. However, this deduction need to be confirmed by further research. Although the materials implanted in the bone tunnel produced osteoinductivity, the process of osteogenesis could not be seen in the internal portion of the tendon or interval space of tendon bundles. The possible reason might be that the process of necrosis occurred in the substance of the tendon and cells invaded from the circumjacent bone wall. This could be supported by the histological appearance in which necrosis with deficiency of cells in graft substance in the bone tunnel was observed at 4 weeks in the OICPC group. In addition, it also might be relevant to material type and implantation technique.
The tendon healing pattern in the bone tunnel was influenced by diverse modules, and the direct type of bone-tendon junction was related to fixation modality and biomechanical characteristics of the graft. Weiler et al., in a sheep model of ACL reconstruction with Achilles tendon autografts and absorbable interference screws, found that a fibrocartilage connection between tendon and bone appeared at 12 weeks after surgery. At the tunnel entrance site, a wide regular ligamentous insertion site was seen after 24 weeks. This insertion showed regular patterns such as the direct type of insertion of a normal ligament with a dense basophilic transition zone consisting of mineralised cartilage. This study strongly indicated that anatomical interference fixation is beneficial for tendon-to-bone incorporation by leading to the development of a direct type of ligament insertion [20]. Yamakado et al., using an extra-articular tendon-to-bone canine model with suspensory fixation, showed that patterns of tendon to bone healing in different portions of the bone tunnel were related to stress distribution. Tensile stress enhances the healing process of tendon-bone junctions, compressive stress promotes chondroid formation and shear load has little or no effect on regeneration of the tendon-bone junction [22].
In our suspensory fixation study, we found a special cross-sectional histological phenomenon with classic and atypical direct connection in face-to-face position at both sides of the tendon in the deep portion of the bone tunnel at 12 weeks in the OICPC group. This might be induced by stress distribution and osteoinductivity concomitantly. The atypical direct attachment, which has not been reported in other studies, showed that only a mineralised cartilage zone connected bone and tendon without a fibrocartilage zone. This might been associated with osteoblast-fibroblast interactions at the graft-bone junction [19]. However, the precise mechanism of the formation of an atypical direct attachment could not be explained and needs further research.
In our study, the process of bone healing mostly occurred in the deep portion of the bone tunnel in both groups. Incomplete and abortive bone healing could mainly be observed in the internal entrance portion close to the joint surface, which was similar to the results of Rodeo et al.’s study [16]. The reason why a fibrous joint between the tendon and bone did not form at the internal entrance of the bone tunnel might result from the suspensory fixation which led the tendon graft to swing and stretch in the internal entrance and led synovial fluid to invade into the internal entrance portion of the bone tunnel. When compared with interference fixation, suspensory fixation might lead to much later occurrence of bone healing in the articular portion of the tunnel than that in the deep portion of the tunnel.
The results of this study indicate that OICPC improves the histological healing process of the tendon in the bone tunnel with suspensory fixation, but the mechanism of enhancement of healing in the early phase and the significance of osteoinductivity in the healing process still remains to be elucidated.
Conflict of interest statement Each author certifies that he or she has no commercial associations that might pose a conflict of interest in connection with the submitted article.
Footnotes
Hao Shen, Gang Qiao and Hongbin Cao all contributed equally to this work.
References
- 1.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop. 2007;31:783–789. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demirag B, Sarisozen B, Ozer O, Kaplan T, Ozturk C. Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. J Bone Joint Surg Am. 2005;87:2401–2410. doi: 10.2106/JBJS.D.01952. [DOI] [PubMed] [Google Scholar]
- 3.Duan YR, Wang CY, Chen JY, Zhang XD (2001) Bone-like apatite formation on calcium phosphate ceramics in dynamic SBF. 5th Asian Symposium on Biomedical Materials, P23, 9–12 Dec
- 4.Hoffmann A, Gross G. Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop. 2007;31:791–797. doi: 10.1007/s00264-007-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa H, Koshino T, Takeuchi R, Saito T. Effects of collagen gel mixed with hydroxyapatite powder on interface between newly formed bone and grafted achilles tendon in rabbit femoral bone tunnel. Biomaterials. 2001;22:1689–1694. doi: 10.1016/S0142-9612(00)00331-8. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami H, Shino K, Hamada M, et al. Graft healing in a bone tunnel: bone-attached graft with screw fixation versus bone-free graft with extra-articular suture fixation. Knee Surg Sports Traumatol Arthrosc. 2004;12:384–390. doi: 10.1007/s00167-003-0484-2. [DOI] [PubMed] [Google Scholar]
- 7.Kohno T, Ishibashi Y, Tsuda E, Kusumi T, Tanaka M, Toh S. Immunohistochemical demonstration of growth factors at the tendon-bone interface in anterior cruciate ligament reconstruction using a rabbit model. J Orthop Sci. 2007;12:67–73. doi: 10.1007/s00776-006-1088-8. [DOI] [PubMed] [Google Scholar]
- 8.Koski JA, Ibarra C, Rodeo SA. Tissue-engineered ligament: cells, matrix, and growth factors. Orthop Clin North Am. 2000;31:437–452. doi: 10.1016/S0030-5898(05)70162-0. [DOI] [PubMed] [Google Scholar]
- 9.Ma CB, Kawamura S, Deng XH, Ying L, Schneidkraut J, Hays P, Rodeo SA. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am J Sports Med. 2007;35:597–604. doi: 10.1177/0363546506296312. [DOI] [PubMed] [Google Scholar]
- 10.Martin SD, Martin TL, Brown CH. Anterior cruciate ligament graft fixation. Orthop Clin North Am. 2002;33:685–696. doi: 10.1016/S0030-5898(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 11.Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, Huard J. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002;84-A:1123–1131. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Mihelic R, Pecina M, Jelic M, et al. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619–1625. doi: 10.1177/0363546504263703. [DOI] [PubMed] [Google Scholar]
- 13.Mutsuzaki H, Sakane M, Ito A, Nakajima H, Hattori S, Miyanaga Y, Tanaka J, Ochiai N. The interaction between osteoclast-like cells and osteoblasts mediated by nanophase calcium phosphate-hybridized tendons. Biomaterials. 2005;26:1027–1034. doi: 10.1016/j.biomaterials.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Robert H, Es-Sayeh J, Heymann D, Passuti N, Eloit S, Vaneenoge E. Hamstring insertion site healing after anterior cruciate ligament reconstruction in patients with symptomatic hardware or repeat rupture: a histologic study in 12 patients. Arthroscopy. 2003;19:948–954. doi: 10.1016/j.arthro.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 16.Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34:1790–1800. doi: 10.1177/0363546506290059. [DOI] [PubMed] [Google Scholar]
- 17.Tien YC, Chih TT, Lin JH, Ju CP, Lin SD. Augmentation of tendon-bone healing by the use of calcium-phosphate cement. J Bone Joint Surg Br. 2004;86:1072–1076. doi: 10.1302/0301-620X.86B7.14578. [DOI] [PubMed] [Google Scholar]
- 18.Tomita F, Yasuda K, Mikami S, Sakai T, Yamazaki S, Tohyama H. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:461–476. doi: 10.1053/jars.2001.24059. [DOI] [PubMed] [Google Scholar]
- 19.Wang IE, Shan J, Choi R, Oh S, Kepler CK, Chen FH, Lu HH. Role of osteoblast-fibroblast interactions in the formation of the ligament-to-bone interface. J Orthop Res. 2007;25:1609–1620. doi: 10.1002/jor.20475. [DOI] [PubMed] [Google Scholar]
- 20.Weiler A, Hoffmann RF, Bail HJ, Rehm O, Südkamp NP. Tendon healing in a bone tunnel. Part II: histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18:124–135. doi: 10.1053/jars.2002.30657. [DOI] [PubMed] [Google Scholar]
- 21.Yagishita K, Sekiya I, Sakaguchi Y, Shinomiya K, Muneta T. The effect of hyaluronan on tendon healing in rabbits. Arthroscopy. 2005;21:1330–1336. doi: 10.1016/j.arthro.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Yamakado K, Kitaoka K, Yamada H, Hashiba K, Nakamura R, Tomita K. The influence of mechanical stress on graft healing in a bone tunnel. Arthroscopy. 2002;18:82–90. doi: 10.1053/jars.2002.25966. [DOI] [PubMed] [Google Scholar]
- 23.Yuan HP, Yang Z, Bruij JD, et al. Material-dependent bone induction by calcium phosphate ceramics: a 2.5-year study in dog. Biomaterials. 2001;22:2617–2623. doi: 10.1016/S0142-9612(00)00450-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XD (2001) The concentration and role of native BMPs in porous calcium phosphate ceramics (PL2). 5th Asian Symposium on Biomedical Materials, P17, 9–12 Dec
- 25.Zhang XD, Yuan HP, Groot K (2000) Calcium phosphate biomaterials with intrinsic osteoinductivity, notebook: workshop1#, biomaterials with intrinsic osteoinductivity, The 6th World Biomaterials Congress, 15–20 May, Hawaii