Abstract
We measured bone density (BD) changes to assess adaptive bone remodelling five years after uncemented total hip arthroplasty with taper-design femoral component using quantitative computed-tomography-assisted osteodensitometry (qCT). Nineteen consecutive patients (21 hips) with degenerative joint disease were enrolled in the study. A press-fit cup and a tapered uncemented stem ceramic−ceramic pairing were used in all patients. Serial clinical, radiological and qCT osteodensitometry assessments were performed after the index operation and at the one, two and five year follow-ups. At the latest follow-up, the clinical outcome was rated satisfactory in all hips. The radiological assessment showed signs of osteointegration with stable fixation of all cups and stems. Overall, there was evidence of a BD loss at year five (p = 0.004). We estimate that BD loss was between 2.2% and 12.1% in comparison with baseline postoperative values. Progressive loss of BD in the metaphyseal region was observed in all hips. We found unremarkable BD changes of diaphyseal cortical BD throughout the five year follow-up period. qCT osteodensitometry technology allows differentiation of cortical and cancellous BD changes over time. Periprosthetic BD changes at the five year follow-up are suggestive of stable stem osteointegration with proximal femoral diaphysis load transfer and metaphyseal stress shielding.
Introduction
Bone density (BD) assessment using dual-energy X-ray absorptiometry (DXA) has been used to monitor bone remodelling changes after total hip arthroplasty (THA) [4, 8, 17, 20]. The major disadvantage of DXA is the inability to differentiate between cortical and cancellous bone. In previous studies, we presented an innovative in vivo method of quantitative computed-tomography (qCT)-assisted osteodensitometry that accurately differentiates cortical and cancellous BD changes around the femoral component after THA [12, 15]. The objective of this prospective one-cohort study was to assess femoral bone adaptive remodelling around an uncemented femoral component with a taper design and hydroxyapatite (HA) coating five years after the index operation. The hypotheses of this study were: (1) BD changes occur predominantly in the metaphyseal region of the femur; (2) loss of cancellous BD is greater than cortical BD.
Material and methods
Twenty-nine consecutive patients (31 hips) with degenerative joint disease and without deformity of the proximal femur were enrolled in this study. The criteria for exclusion were age <18 years and >80 years, refusal to consent, pregnancy, metabolic bone disease and previous failed THA. All hips were operated upon by one surgeon in one institution. The average patient age at the index operation was 58 (range 30 –81) years. There were 16 men and 13 women. The mean body mass index (BMI) was 28.4 (range 20−33). The patients received an uncemented THA with a taper-design femoral component coated with HA (Summit; DePuy International, Leeds, UK), and a press-fit titanium cup (Duraloc; DePuy) with ceramic−ceramic pairing (Biolox Delta, CeramTec, Plochingen, Germany). Postoperatively, patients commenced touch weight bearing for four to six weeks and full weight bearing thereafter. The clinical outcome was assessed using the Harris Hip Score (HHS) and the Oxford Hip Score (OHS). Serial anterior–posterior and lateral-view radiographs were taken postoperatively, six months, one, two and five years after the index operation. Preoperative radiological bone quality according to the classification of Dorr et al. [2] was rated A in all hips. CT scans were carried out on all patients postoperatively and one, two and five years after the index operation. The method used for qCT assessment has been already described in the one and two year follow-up reports [10, 11]. Statistical analysis was carried out using the “R” software package (Version 2.1.1, Vienna, Austria). For all analyses, we did one-way analysis of variance (ANOVA), using the Levene test to check for equal variance and the Shapiro-Wilks test to check normality. Of primary interest was determination of the average difference between postoperative, one year, two year and five year follow-up BD changes for both cancellous and cortical bone. With this in mind, we averaged the bone slice readings for each patient and carried out a paired t test analysis (on the difference in BD) for cortical and cancellous bone.
Results
Three patients moved overseas before the last follow-up; four had a complete five year clinical and radiological follow-up but could not attend CT sessions for imaging of the femur. One patient who inadvertently commenced bisphosphonate therapy was excluded from the study. Therefore, 21 hips (19 patients) had a complete five year clinical, radiological and qCT follow-up. There were no serious complications requiring revision surgery. The mean preoperative HHS was rated 38 (range 22–70) points and was rated 95 (range 85–100) points at the five year follow up. The mean preoperative OHS was rated 42 (range 35–58) points and was rated 19 (range 21–12) points at the five year follow-up. All patients were able to walk without limping at follow-up. No patient reported thigh pain requiring analgesia, and no patient reported squeaking or any other noise from the ceramic hip joint. The radiological assessment showed stable cup and stem fixation with good osteointegration in all hips. There was no evidence of any radiolucent lines, osteolytic lesions or postoperative subsidence. Results of qCT osteodensitometry analysis are depicted in Tables 1 and 2 and Figs. 1, 2 and 3. The analysis of the surface area of each scan slice showed no statistical difference between postoperative, one, two and five year scans, confirming adequate precision for evaluation of the same bone area.
Table 1.
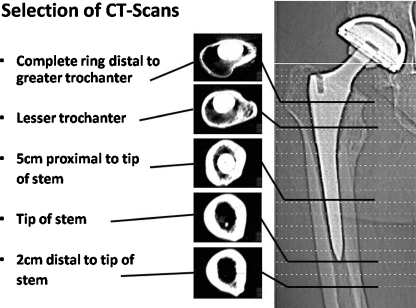
Postoperative baseline cortical bone density (BD) values [mean ± standard deviation (SD)] and follow-up BD values in the five regions of interest (ROI) (1 = greater trochanter region; 5 = 2 cm below the tip of the stem)
| ROI | Postoperative | 1 year | 2 years | 5 years | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 1 | 861.4 | 101.6 | 683.3 | 112.5 | 711.4 | 134.5 | 520.3 | 110.1 |
| 2 | 1,003.4 | 106.6 | 925.2 | 140.8 | 913.6 | 261.7 | 848.1 | 163.1 |
| 3 | 1,273.1 | 45.6 | 1219.2 | 53.9 | 1,263.1 | 40.3 | 1,229.4 | 65.4 |
| 4 | 1,296.1 | 47.8 | 1271.9 | 49.5 | 1,303.2 | 40.9 | 1,273.3 | 70.0 |
| 5 | 1,296.1 | 51.7 | 1280.8 | 48.7 | 1,309.9 | 40.2 | 1,272.7 | 69.4 |
Table 2.
Postoperative baseline cancellous bone density (BD) values [mean ± standard deviation (SD)] and follow-up BD values in the five regions of interest (ROI) (1 = greater trochanter region; 5 = 2 cm below the tip of the stem)
| ROI | Postoperative | 1 year | 2 years | 5 years | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 1 | 208.7 | 70.5 | 139.1 | 83.5 | 164.5 | 66.1 | 155.3 | 88.2 |
| 2 | 336.6 | 48.4 | 282.7 | 71.7 | 322.3 | 86.2 | 261.0 | 79.2 |
| 3 | 511.7 | 114.0 | 426.5 | 97.8 | 551.3 | 116.0 | 543.7 | 115.7 |
| 4 | 255.7 | 63.2 | 225.4 | 62.1 | 320.6 | 82.5 | 276.5 | 100.4 |
| 5 | 186.0 | 42.3 | 153.1 | 37.7 | 199.0 | 50.3 | 219.4 | 67.9 |
Fig. 1.
Five computed tomography scans (regions of interest) selected for quantitative osteodensitometry
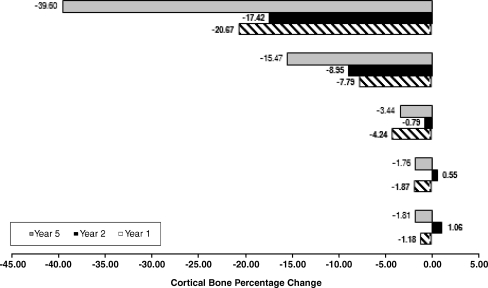
Fig. 2.
Cortical done density changes in the five regions of interest at the 1-year, 2-year and 5-year follow-up
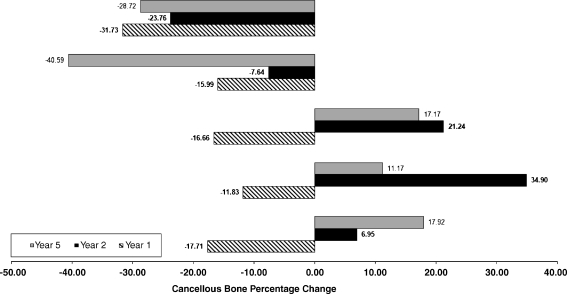
Fig. 3.
Cancellous bone density changes in the five regions of interest at the 1-year, 2-year, and 5-year follow-up
We found no significant correlation between factors such as: (1) gender, (2) BMI, (3) side and (4) size of the femoral component and extent of BD changes at the latest follow-up. From operation to year five, there was no significant difference in proportional loss of density between cortical and cancellous bone (p 0.44, 0.23 and 0.50). However, there was a significant difference in the proximal portion of the femur (greater and lesser trochanter regions) (p 0.03 and 0.0008). We estimate that the loss in BD was between 0.6% and 13.8% greater for cancellous than for cortical bone at the level of the greater trochanter and between 4.6% and 17.8% greater for cancellous than for cortical bone at the level of the lesser trochanter. Overall, there was strong evidence of a loss of BD at year five (p value = 0.004). We estimate that the loss of periprosthetic bone density was between 2.2% and 12.1%.
Cortical bone
There was strong evidence that BD was higher immediately after the operation than after five years (p 0.004). We estimated that mean BD was between 20.1 mg/CaHA/ml and 120.5 mg/CaHA/ml higher immediately after surgery than five years after the index operation [95% confidence interval (CI)]. BD loss was progressive in the most proximal portion of the femur. We observed unremarkable BD changes in the diaphyseal region.
Cancellous bone
There was strong evidence that BD was higher immediately after the operation than after one and five years (p 0.001). We estimated that mean BD was between 50.5 mg/CaHA/ml and 86.4 mg/CaHA/ml higher immediately after surgery than five years after the index operation (95% CI). At the five year follow-up, we observed remarkable BD loss in the most proximal region (greater trochanter and calcar); however, the loss appeared unchanged since the one year follow-up. In contrast, loss of BD in the lesser trochanter region appeared progressive. Interestingly, we observed BD increase in the diaphyseal regions.
Analysis for high BD baseline versus BD changes at follow-up
We separated the patients into two groups based on whether they were above or below the midpoint between the maximum and minimum BD baseline values (averaged across the five ROIs). We looked at BD loss after one and five years for cortical and cancellous bone. In all cases, there were no significant differences. Changes of femoral cortical and cancellous BD of the nonoperated site were unremarkable.
Discussion
The aim of this prospective qCT osteodensitometry study was to measure BD changes and assess adaptive remodelling around a femoral component with taper design and HA coating. At the five year follow-up, we observed a moderate decrease of both cortical and cancellous BD in the metaphysis. BD loss in the metaphysis was greater than cortical BD loss. We observed small changes of cortical BD in the diaphyseal regions. In contrast, cancellous BD increased in the diaphyseal regions. BD changes at the five year follow-up are suggestive of proximal femoral diaphysis load transfer with osteointegration and metaphyseal stress shielding.
BD changes adjacent to an implant have a multifactorial aetiology. Whereas age-related changes occur, focal osteolysis and stress shielding are the most important causal factors of periprosthetic bone loss [3]. Stress shielding is a phenomenon that describes the tendency of bone to atrophy in the absence of an adequate mechanical stimulus based on the principles known as Wolff’s law [12]. The extent of stress shielding depends on the size, design and elastic module of an implant and its biomechanical interactions with cortical and cancellous bone structures [6, 13, 17].
Taper-design stems aim to achieve metaphyseal fixation with proximal load transfer to limit stress shielding. Finite-element analyses showed that the extent of stress shielding is directly related to stem stiffness, with lower E-modulus materials such as titanium reducing proximal stress shielding [5, 18]. HA-coated implants show good clinical and radiological outcomes with moderate BD loss and improved implant fixation when compared to porous-coated implants [14, 17]. DXA studies have reported proximal bone resorption around uncemented stems, which can range from 20% to 50% [7, 9, 19]. A recent DEXA study reported periprosthetic BD loss of up to 12.9% in cemented stems five years after surgery [1]. When using DXA, structural differences of cortical and cancellous bone are not taken into consideration when measuring BD after THA [8, 17]. Using sectional CT imaging, a separate analysis of cortical and cancellous bone structures can be achieved [12]. The CT-assisted osteodensitometry technology used in this study is accurate and reproducible [16]. We found BD changes ranging from −39.5% to +1.1% in cortical bone and from −40.6% to +34.9% in cancellous bone. We estimated that the mean loss of periprosthetic BD was between 2.2% and 12.1%. The reported figures compare favourably with other osteodensitometry studies on uncemented femoral components [7, 14, 16].
In comparison to the two year follow-up data of our previous study [10, 11], we observed progressive decrease of cortical BD in the metaphyseal area and decrease of cancellous BD around the lesser trochanter region. Paradoxically, BD of cancellous bone increased in the diaphyseal area. This is related to the slow but progressive replacement of cortical bone by cancellous bone in this region.
In summary, the taper-design stem assessed in this study has shown satisfactory clinical and radiological results. BD changes observed five years after THA with the Summit stem compare favourably with BD changes reported in other studies using anatomical-design and taper-design stems [7, 15]. In conjunction with finite-element analysis, qCT allows generation of accurate patient-specific meshes on which to model implants and their effect on bone remodelling [18]. This technology can be useful in predicting bone remodelling and quality of implant fixation using prostheses with different design and/or biomaterials [12]. In the future, this tool could be used for preclinical validation of new implants before their widespread introduction into clinical practice.
Acknowledgments
The authors thank Mr. Garnet Tregonning, Dr. Jacob Munro, Dr. Lyndon Bradley, Dr. Godwyn Choy, Dr. Andrew Graydon, Dr. Melissa Rossaak and Dr. Keryn Reilly for their assistance during the clinical follow-ups.
Footnotes
This study was supported by an educational grant from the Stevenson Charitable Trust, New Zealand, and was partially funded by DePuy Int., Leeds, UK
This study was approved by the local Ethical Board Review Committee
References
- 1.Dorr Digas G, , Faugere Kaerrholm J . Five-year DEXA study of 88 hips with cemented femoral stem. . Int Orthop. 2008 doi: 10.1007/s00264-008-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14:231–242. doi: 10.1016/8756-3282(93)90146-2. [DOI] [PubMed] [Google Scholar]
- 3.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg (Br) 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons CE, Davies AJ, Amis AA, Olearnik H, Parker BC, Scott JE. Periprosthetic bone mineral density changes with femoral components of differing design philosophy. Int Orthop. 2001;25:89–92. doi: 10.1007/s002640100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huiskes R, Weinans H, Dalstra M. Adaptive bone remodelling and biomechanical design considerations for noncemented total hip arthroplasty. Orthopedics. 1989;12:1255–1267. doi: 10.3928/0147-7447-19890901-15. [DOI] [PubMed] [Google Scholar]
- 6.Laine HJ, Puolakka TJ, Moilanen T, Pajamaki KJ, Wirta J, Lehto MU. The effects of cementless femoral stem shape and proximal surface texture on ‘fit-and-fill’ characteristics and on bone remodeling. Int Orthop. 2000;24:184–190. doi: 10.1007/s002640000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leali A, Fetto JF. Preservation of femoral bone mass after total hip replacements with a lateral flare stem. Int Orthop. 2004;28:151–154. doi: 10.1007/s00264-004-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martini F, Sell S, Kremling E, Kusswetter W. Determination of periprosthetic bone density with the DEXA method after implantation of custom-made uncemented femoral stems. Int Orthop. 1996;20:218–221. doi: 10.1007/s002640050067. [DOI] [PubMed] [Google Scholar]
- 9.Niinimaki T, Junila J, Jalovaara P. A proximal fixed anatomic femoral stem reduces stress shielding. Int Orthop. 2001;25:85–88. doi: 10.1007/s002640100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandit S, Graydon A, Bradley WC, Munro J, Pitto RP. CT-osteodensitometry in modern uncemented taper-design stem with hydroxyapatite coating. Australia-NZ J of Surg. 2006;76:778–781. doi: 10.1111/j.1445-2197.2006.03866.x. [DOI] [Google Scholar]
- 11.Pitto RP, Bhargava A, Pandit S, Walker C, Munro JT. Quantitative CT-assisted osteodensitometry of femoral adaptive bone remodelling after uncemented total hip arthroplasty. Int Orthop. 2008;32:589–595. doi: 10.1007/s00264-007-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitto RP, Mueller L, Reilly K, Schmidt R, Munro J. Quantitative computer assisted osteodensitometry in total hip arthroplasty. Int Orthop. 2007;31:431–438. doi: 10.1007/s00264-006-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenthall L, Bobyn JD, Tanzer M. Bone densitometry: influence of prosthetic design and hydroxyapatite coating on regional adaptive bone remodelling. Int Orthop. 1999;23:325–329. doi: 10.1007/s002640050383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Sotelo J, Lewallen DG, Harmsen WS, Harrington J, Cabanela ME. Comparison of wear and osteolysis in hip replacement using two different coatings of the femoral stem. Int Orthop. 2004;28:206–210. doi: 10.1007/s00264-004-0558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt R, Nowak TE, Mueller L, Pitto RP. Osteodensitometry after total hip replacement with uncemented taper-design stem. Int Orthop. 2004;28:74–77. doi: 10.1007/s00264-003-0519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt R, Pitto RP, Kress A, Ehremann C, Nowak TE, Reulbach U, Forst R, Muller L. Inter- and Intraobserver assessment of periacetabular osteodensitometry after cemented and uncemented total hip arthroplasty using computed tomography. Arch Ortho Trauma Surg. 2005;125:291–297. doi: 10.1007/s00402-005-0812-8. [DOI] [PubMed] [Google Scholar]
- 17.Scott DF, Jaffe WL. Host-bone response to porous-coated cobalt-chrome and hydroxyapatite-coated titanium femoral components in hip arthroplasty. Dual-energy x-ray absorptiometry analysis of paired bilateral cases at 5 to 7 years. J Arthroplasty. 1996;11:429–437. doi: 10.1016/S0883-5403(96)80033-7. [DOI] [PubMed] [Google Scholar]
- 18.Shim V, Pitto RP, Streicher RM, Hunter PJ, Anderson IA. The use of sparse CT datasets for auto-generating FE models of the femur and pelvis. J of Biomechanics. 2007;40:26–35. doi: 10.1016/j.jbiomech.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Weber D, Pomeroy DL, Brown R, Schaper LA, Badenhausen WE, Jr, Smith W, Curry JI, Suthers KE. Proximally porous coated femoral stem in total hip replacement−5- to 13-year follow-up report. Int Orthop. 2000;24:97–100. doi: 10.1007/s002640000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerahn B, Lausten GS, Kanstrup IL. Prospective comparison of differences in bone mineral density adjacent to two biomechanically different types of cementless femoral stems. Int Orthop. 2004;28:146–150. doi: 10.1007/s00264-003-0534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]