Abstract
The primary objective of this study was to investigate the implications of pathological fractures on therapy outcome in patients with primary malignant bone tumours and to determine whether limb salvage can be safely performed. A retrospective analysis of 447 patients with primary malignant bone tumours, treated between 1985 and 2005, was performed. Multivariate Cox regression analysis was used to investigate the influence of pathological fractures and further independent variables on survival rate. In 52 of the 447 patients, the primary malignant bone tumour was complicated by a pathological fracture. These fractures were more common in malignant fibrous histiocytoma (MFH) of the bone and in the tumour stages IIa/b and III. Ablative surgery was performed in ten patients and limb salvage surgery in 42 patients. The mortality risk for patients with pathological fractures was significantly increased by a factor of 1.82 (p = 0.015), and overall duration of survival was significantly lower in the fracture group, with a median of 6.2 years (p < 0.00001). In univariate and multivariate analysis, fracture, higher tumour stages and resection margins remained a significant predictor of worse survival. Overall survival, rate of local recurrence and distant metastases were not affected by the type of surgical treatment selected; there was no difference between the patients who underwent limb salvage and those who underwent an amputation. Pathological fracture in patients with primary malignant bone tumours is a predictor of worse survival and significantly increases mortality risk. Reconstructive surgery did not influence the survival rate, showing that limb salvage therapy is safe when adequate resection margins are achieved.
Introduction
Pathological fractures from primary malignant bone tumours are devastating events for patients and remain a significant clinical concern. The incidence of pathological fractures has been reported at between 2% and 25% [1, 3, 14, 17]. They may substantially reduce patients’ functional independence and quality of life. Further, pathological fractures are reported to significantly reduce survival rate and worsen prognosis by potentially spreading tumour cells via the fracture haematoma into adjacent tissues or by development of joint involvement [1]. It has also been postulated that damage to the microcirculation may further facilitate metastases [1]. Misdiagnosis of a pathological fracture may result in inappropriate treatment, delaying diagnosis and potentially spreading the disease. Thus, prevention and delay of fracture are important treatment objectives. Surgical treatment options for pathological fracture in malignant bone sarcomas range from reconstructive surgery to amputation [20]. Limb salvage has become a popular alternative to amputation for the treatment of malignant bone tumours and can improve functional outcome [23–25]. Within this setting it is of pivotal interest whether limb salvage has any effect on the long-term survival of patients, and few studies have specifically compared the outcome of limb salvage versus amputation [1, 2, 5, 13, 16, 22, 27].
The literature is unclear about the implications of a pathological fracture for therapy outcome for patients with primary malignant bone tumours, and the impact on mortality risk is controversial [2, 13, 14, 16, 22, 24, 27].
The primary objective of this study was to identify the prognostic value of independent variables in patients with primary malignant bone tumours with regard to survival rate and to determine whether the incidence of pathological fracture is associated with a poor outcome. In addition we analysed whether limb salvage can be safely performed without compromising oncological outcome.
Patients and methods
A retrospective survey was performed on 447 patients with localised primary malignant bone tumours treated between 1985 and 2005 at the University Clinic of Orthopaedic Surgery, Heidelberg, which is a national referral centre for bone tumours. The data were derived from our internal tumour registry, in which clinical, radiological and histopathological information as well as details of treatment and outcome are recorded.
By performing univariate and multivariate analysis (Cox regression; SPSS 12.0; SPSS Inc., Chicago, USA) we investigated the influence of the following independent variables on survival rate: Pathological fracture (yes, no); tumour entity (osteosarcoma, chondrosarcoma, Ewing sarcoma/primitive neuroectodermal tumor [PNET], malignant fibrous histiocytoma [MFH], fibrosarcoma); clinical stage (Ia/Ib, IIa/IIb, III), localisation (upper extremity, lower extremity, pelvis/hip, others); operative treatment (limb-saving, ablative); resection margins (wide, marginal, intralesional); tumour regression after adjuvant therapy (no adjuvant therapy, good response, poor response, data not available); and development of distant metastases (yes, no). Patients with non-operative treatment were excluded from this study.
Tumour entity
Tumour entity was defined after standard histological investigation and suitable immunohistochemistry studies by two independent pathologists from separate institutions (the local pathologist and a reference pathologist).
Clinical stage
The clinical stage was defined according to the Musculoskeletal Tumour Society staging system (Enneking et al.) [11].
Resection margins
Resection margins were defined according to Enneking et al. as radical, wide, marginal and intralesional [11].
Standard therapy
The study population included only those patients who received standard therapy. In osteosarcoma and Ewing sarcoma this was defined as pre-operative chemotherapy, followed by tumour resection and post-operative chemotherapy.
For osteosarcoma, chemotherapy was administered according to the COSS protocol, for Ewing sarcoma according to the protocol of the EICESS groups [6, 7, 9, 28].
Tumour regression
Tumour regression in response to preoperative chemotherapy was determined histologically according to the six-grade scale of Salzer-Kuntschik et al., where grade 1 denotes no viable tumour cells; grade 2, solitary viable cells or one islet of less than 0.5 cm; grade 3, less than 10%; grade 4, 10–50%; grade 5, more than 50%; grade 6, no effect of chemotherapy. A good response was defined as less than 10% viable tumour cells in the surgical specimen, corresponding to response grades 1–3. Grades 4–6 were considered a poor response [22].
Statistical analysis
Overall survival was determined by Kaplan–Meier survival analysis and compared among groups with a log rank test [15]. Variables with a p value of <0.10 in the univariate analysis were entered as covariates into the stepwise Cox proportional-hazards regression model to identify multivariate predictors of survival rate [8]. The hazard ratio with 95% confidence interval was used to measure the strength of the relationship between each multivariate predictor and the corresponding outcome.
Results
The study population encompassed 447 patients (260 males and 187 females) with primary malignant bone tumours registered between 1985 and 2005 (Table 1). Fifty-two patients (11.6%) suffered a pathological fracture. The median age of the study population at time of tumour diagnosis was 27 years (range 2–84). The groups with and without a fracture were comparable with regard to gender and age at diagnosis (see Table 1). The prospective median duration of follow-up of the study population was 15 years.
Table 1.
Demographics and clinical features of the study population divided into fracture and non-fracture groups
| Parameter | Variable | Study population N = 447 | Non-fracture group n = 395 (88.4%) | Fracture group n = 52 (11.6%) | p value |
|---|---|---|---|---|---|
| Patients demographics | Median age at diagnosis, years (range) | 27.4 (2–84) | – | – | |
| Mean age at diagnosis, years (SD) | 33.4 (±19.8) | 32.8 (±19.1) | 37.7 (±24.2) | ||
| Male | 260 (58.2%) | 265 (67.1%) | 30 (57.7%) | 0.528 | |
| Female | 187 (41.8%) | 130 (32.9%) | 22 (42.3%) | ||
| Tumour type | Osteosarcoma | 190 (42.5%) | 168 (42.5%) | 22 (42.3%) | 0.524 |
| Chondrosarcoma | 130 (29.2%) | 117 (29.6%) | 13 (25.0%) | ||
| Ewing sarcoma/PNET | 79 (17.7%) | 70 (17.7%) | 9 (17.3%) | ||
| MFH | 41 (9.2%) | 33 (8.4%) | 8 (8.4%) | ||
| Fibrosarcoma | 7 (1.6%) | 7 (1.8%) | 0 | ||
| Tumour location | Upper extremity | 76 (17%) | 67 (17%) | 9 (17%) | 0.297 |
| Lower extremity | 266 (59.5%) | 231 (58.5%) | 35 (67.3%) | ||
| Pelvis/hip | 74 (16.6%) | 70 (17.7%) | 4 (7.7%) | ||
| Others | 31 (6.9%) | 27 (6.8%) | 4 (7.7%) | ||
| Tumour stage | Ia/b | 146 (32.7%) | 139 (35.9%) | 7 (13.5%) | 0.001 |
| IIa/b | 253 (56.6%) | 217 (56.1%) | 36 (69.2%) | ||
| III | 40 (8.9%) | 31 (8.0%) | 9 (17.3%) | ||
| Not available | 8 (1.8%) | – | – | ||
| Response to neoadjuvant chemotherapy | Good response | 127 (28.4%) | 112 (28.4%) | 15 (28.8%) | 0.336 |
| Poor response | 83 (18.6%) | 72 (18.3%) | 11 (21.2%) | ||
| No data available | 62 (13.9%) | 59 (14.8%) | 3 (5.8%) | ||
| Local recurrence | Local recurrence (total study population) | 39 (8.7%) | 33 (8.4%) | 6 (11.5%) | 0.35 |
| Limb salvage | 31 (9.0%) | 26 (8.6%) | 5 (11.9%) | 0.54 | |
| Ablative surgery | 8 (7.8%) | 7 (7.5%) | 1 (10%) | ||
| Distant metastasis | No distant metastasis (tumour stage I + II) | 320 (80.2%) | 286 (80.3%) | 34 (79.1%) | 0.489 |
| Distant metastasis (tumour stage I + II) | 79 (19.8%) | 70 (19.7%) | 9 (20.9%) | ||
| Type of surgery | Limb salvage | 344 (77.0%) | 302 (76.5%) | 42 (80.8%) | 0.496 |
| Ablative surgery | 103 (23.0%) | 93 (23.5%) | 10 (19.2%) | ||
| Resection margins | Wide | 355 (79.4%) | 317 (80.3%) | 38 (73.1%) | 0.360 |
| Marginal | 45 (10.1%) | 39 (9.9%) | 6 (11.5%) | ||
| Intralesional | 47 (10.5%) | 39 (9.9%) | 8 (15.4%) |
SD standard deviation, PNET primitive neuroectodermal tumour, MFH malignant fibrous histiocytoma
Values given as n (%) unless otherwise noted
The two groups (with/without fracture) displayed no statistically significant difference regarding the distribution of the tumour types, and the most frequent tumour was osteosarcoma, followed by chondrosarcoma (Table 1). The highest incidence of fracture was in patients with MFH (8/41), followed by osteosarcoma (22/190), Ewing sarcoma/PNET (9/79) and chondrosarcoma (13/130).
No significant difference regarding the tumour location could be shown between the two groups. The most frequently affected site was the lower extremity, followed by the upper extremity (Table 1). Patients with pathological fractures displayed significantly higher tumour stages (p = 0.001), with 69.2% (36/52) presenting stage IIa/b and 17.3% (9/52) stage III (Table 1). Accordingly, the rate of distant metastases at study entry corresponds to the tumour stages. Neoadjuvant chemotherapy was administered equally in both groups according to standard protocols. No statistically significant difference regarding response rate to neoadjuvant chemotherapy was found between the fracture group and the non-fracture group (p = 0.336) (Table 1). However, 127 patients of the study population were classified as good responders (grades 1–3). Although a tendency towards more local recurrence in the fracture group seemed to exist, the local recurrence rate was not statistically different between the two groups (p = 0.35). The incidence of distant metastases during the study for the patients without metastatic disease at diagnosis (tumour stages I and II) was comparable between the fracture and control groups (Table 1).
Limb-saving surgery was performed in 344 of the 447 patients and ablative surgery in 103 patients (Table 1). Surgical treatment and resection margins were comparable between the two groups (Table 1). No difference with respect to rate of local recurrence, rate of distant metastases and overall duration of survival was shown between the patients who underwent limb salvage and those who underwent amputation. The comparison of local recurrence between patients in the fracture group only, treated with ablative or limb saving surgery, revealed no statistically significant differences with local recurrence in 10% of the ablative group and 11.9% of the group treated with limb saving surgery.
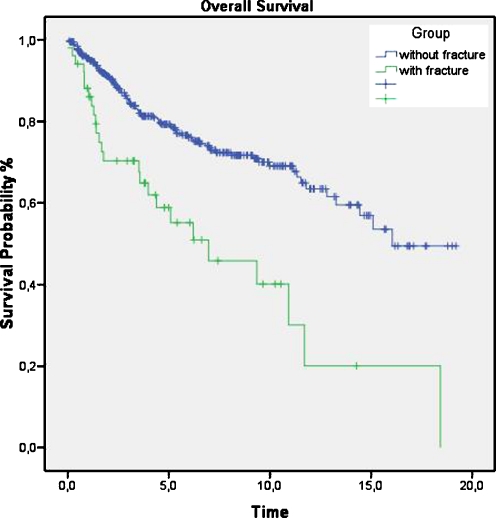
The numbers of patients alive versus deceased at the end of the study period are shown in Table 2. The survival rate of patients in the non-fracture group (76.7%) was significantly higher than that in the fracture group (50%). Overall survival was significantly lower in the fracture group, with a median of 6.2 years compared to 16 years for patients without pathological fracture (p < 0.00001). The one-, five- and ten-year overall survival rates were 86%, 54% and 39%, respectively, for patients with pathological fracture and 96%, 78% and 69% for patients without pathological fracture (Fig. 1).
Table 2.
Survival data
| Parameter | Variable | Study population N = 447 | Non-fracture group n = 395 | Fracture group n = 52 | p value |
|---|---|---|---|---|---|
| Survival status at end of study | Alive | 329 (73.6%) | 303 (76.7%) | 26 (50.0%) | <0.001 |
| Deceased | 118 (26.4%) | 92 (23.3%) | 26 (50.0%) | ||
| Overall survival in years | Mean (95% CI) | 12.7 (11.7–13.6) | 13.4 (12.4–14.4) | 7.9 (5.5–10.4) | <0.0001 (log rank test) |
| Median (95% CI) | 15.1 (12.5–17.7) | 16.1 | 6.2 (2.9–9.5) |
CI confidence interval
Fig. 1.
Cumulative survival for patients with and those without a pathological fracture. The one-, five- and ten-year overall survival rates were 86%, 54% and 39%, respectively, for patients with pathological fracture and 96%, 78% and 69% for patients without pathological fracture
The univariate analysis revealed that the independent variables pathological fracture, higher tumour stage and inadequate resection margins were associated with poor survival. These variables were therefore selected for multivariate analysis, which demonstrated that the occurrence of pathological fractures increased the mortality risk by a factor of 1.82 (p = 0.015) compared to patients without pathological fractures (Table 3). Moreover, patients with higher tumour stages at the time of diagnosis had a greater risk of death: two-fold for stage IIa/b (p = 0.004, HR 2.1) and six-fold for stage III (p < 0.0001, HR 6.46) compared to patients with stage Ia/b. Additionally, inadequate resection margins were a prognostic factor for poor survival compared to wide margins. When resection margins were marginal the mortality risk increased by a factor of 1.9 (p = 0.02) and for intralesional resection margins the figure was 2.8 (p < 0.0001, HR 2.83). In contrast, survival was positively associated with good response to neoadjuvant chemotherapy (p = 0.015, HR 0.68).
Table 3.
Results of univariate and multivariate Cox regression analysis. Variables with a p value of <0.10 in the univariate analysis were entered as covariates into the stepwise Cox proportional-hazards regression model to identify multivariate predictors of survival
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| p value | Hazard ratio | 95% confidence interval (CI) | p value | |
| Pathological fracture | 0.000001 | 1.82 | 1.13–2.95 | 0.015 |
| Tumour stage IIa/b | 0.060 | 2.1 | 1.27–3.49 | 0.004 |
| Tumour stage III | 0.0001 | 6.46 | 3.33–12.54 | 0.0001 |
| Marginal resection margins | 0.017 | 1.9 | 1.11–3.26 | 0.02 |
| Intralesional resection margins | 0.0001 | 2.83 | 1.73–4.64 | 0.0001 |
| Good response | 0.068 | 0.68 | 0.30–0.88 | 0.015 |
Discussion
Pathological fractures in patients with primary malignant bone tumours sharply decrease quality of life and remain a significant clinical concern. The implications and prognostic value of pathological fractures on mortality rate and local recurrence, as well as the appropriate treatment, remain important issues for discussion. The identification of prognostic factors is important in order to improve therapy outcome and more effectively tailor surgical treatment to the individual patient.
A total of 447 patients with primary malignant bone tumours were included in our study with a prospective median follow-up time of 15 years. The fracture group and the control group were comparable regarding age and gender and received similar treatment according to standardised criteria (Table 1).
The total incidence of pathological fractures in our series was 11.6% (52 patients); the rate differed significantly depending on the tumour entity, as previously reported by others [1, 5, 10, 17, 18, 25, 29]. In our study pathological fractures occurred most frequently in patients with MFH, followed by osteosarcoma, Ewing sarcoma/PNET and chondrosarcoma.
Moreover, no statistical difference regarding the tumour location could be shown when comparing the two groups. The lower extremity was predominantly affected in both groups, followed by the upper extremity, in accordance with the literature [1, 5, 24, 29].
For most patients, the development of a symptomatic pathological fracture is a devastating event that heralds the end stage of their disease. The literature is contradictory regarding the implications of pathological fractures for the survival rate.
Hoffmann et al. studied 593 patients with Ewing sarcoma and found no significant impact of pathological fracture on survival rate; this was confirmed by Pochananugool et al. [13, 19]. Lee et al. did not find any influence on oncological outcome in 227 patients with chondrosarcoma, 46 of whom suffered a pathological fracture [16].
In contrast, the majority of recent studies have found the occurrence of a pathological fracture in bony sarcomas to be associated with lower survival rate [5, 10, 12, 21, 24, 29]. Zeifang et al. reported a lower survival rate in patients with primary malignant bone disease complicated by a fracture, and this was confirmed by Ebeid et al. [10, 29]. In a recent study by Bramer et al., pathological fractures significantly reduced the survival rate in patients with osteosarcoma and chondrosarcoma, but not in those with Ewing sarcoma [5].
Our data show a significantly lower overall duration of survival in the fracture group, with a median of 6.2 years compared to 16 years in the non-fracture group (p < 0.00001). The one-, five- and ten-year overall survival rates were also significantly lower for patients with pathological fracture (Fig. 1).
A variety of independent variables (e.g. tumour size and tumour volume) are known to influence the survival of patients with malignant bone tumours. Thus, the increased mortality risk could be biased by accumulation of these prognostically unfavourable factors. We addressed this by performing univariate and multivariate analysis. Pathological fracture remained a predictor of worse survival, independent of other variables and raised the mortality risk significantly by a factor of 1.82 (p = 0.015) (Table 3). Further, the results of univariate and multivariate analyses of potential prognostic factors demonstrate that, apart from fracture, higher tumour stage and inadequate resection margins are also associated with decreased overall survival (Table 3). In contrast, a good response to neoadjuvant chemotherapy was positively associated with survival (p = 0.015, HR 0.68).
Even though it has been shown that pathological fractures increase the mortality rate, the basis for this correlation remains unclear. The theory that pathological fractures worsen prognosis by spreading the tumour via the fracture haematoma leading to an increased rate of local recurrence and distant metastases has been discussed controversially [1, 2, 5, 24].
In our study, no statistical difference could be shown regarding the rate of local recurrence between the two groups (Table 1), although a tendency towards a higher occurrence in the fracture group seemed to exist. The incidence of distant metastases during the study for the patients without metastatic disease at diagnosis (tumour stage I and II) was not affected by the occurrence of pathological fracture and comparable between the two groups (Table 1). The fact that the rate of local recurrence and distant metastases were not significantly higher in the fracture group runs counter to the theory of micro-metastasis and seems to indicate that it is probably not the dissemination of tumour cells that impairs survival. More likely, the fracture denotes the aggressive nature of the tumour. In accordance with this, patients with pathological fractures displayed significantly higher tumour stages, underlining the theory that pathological fractures occur more often in patients with more advanced disease and are an expression of higher aggressiveness and malignancy. However, in multivariate analysis pathological fracture remained an independent predictor of worse survival and significantly raised the mortality risk. Thus, further studies are needed to examine the basis for this correlation.
The rates of local recurrence and distant metastases are of pivotal interest in clinical decision-making regarding surgical approach. There has been intense debate regarding limb salvage versus immediate and aggressive removal of the tumour, including early amputation to halt fracture-induced disease progression.
However, few non-randomised studies have specifically compared the outcomes of limb salvage and amputation with regard to local recurrence and survival rate in patients with primary malignant bone tumours who suffer a pathological fracture [1, 2, 4, 5, 10, 21, 24–26, 29].
In 1996, Abudu et al. reviewed the surgical treatment and oncological outcome in 40 patients with a pathological fracture in localised osteosarcoma [1]. The overall survival rate did not differ significantly between the patients who underwent limb salvage and those who had an amputation. The rate of local recurrence was higher in patients managed with limb salvage. However, this correlation disappeared after correction for surgical margin, leading to the conclusion that if wide margins cannot be achieved, the risk of local recurrence following limb salvage is substantial.
In another single-centre review, the cases of 18 of 187 patients with osteosarcoma complicated by a pathological fracture were evaluated. The difference in local tumour control and survival between the patients who had limb salvage and those with amputation was not significant [25]. A multi-centre case-matched study by the same authors did not reveal any difference regarding local recurrence and survival rate between resection and amputation, as confirmed by Bacci et al. [4, 24]. Bramer et al. also evaluated the influence of pathological fracture on rates of local recurrence and survival in patients with bony sarcomas. No difference was found between resection and amputation as long as adequate margins were obtained [5].
Our results with regard to the relationship between the type of operative management and overall survival were similar to the results in these studies. Altogether, 344 of the 447 patients in the study population were treated with limb salvage and 103 with ablative surgery. No differences in surgical treatment were found between the groups with and without pathological fracture (p = 0.496).
We found no difference with respect to overall survival, rate of local recurrence and distant metastases between the patients with a pathological fracture who underwent limb-saving surgery and those who underwent amputation.
It has to be taken into account that the achievement of adequate margins of resection is probably of higher importance than solely the occurrence of a fracture when selecting the surgical approach. In our study population, the proportions of patients with adequate and inadequate surgical margins were comparable between the two groups (Table 1). When complete tumour resection is anatomically possible and adequate margins can be obtained, limb-saving surgery does not seem to have a negative influence, i.e. the rate of local recurrence and distant metastases are not higher, and the duration of survival is not lower.
Conclusions
In summary, it can be concluded that patients with primary malignant bone sarcomas who sustain a pathological fracture have shorter overall survival than patients without a fracture. In our study the rate of local recurrence and distant metastases was not affected by pathological fracture, leading to the conclusion that a pathological fracture is not always a contraindication to limb salvage, and the performance of a limb-salvage procedure in carefully selected patients does not significantly increase the risk of local recurrence, distant metastases or death.
Neoadjuvant chemotherapy is known to substantially improve therapy outcome in patients with primary malignant bone tumours. In our study the fracture and non-fracture groups did not demonstrate any significant difference in their response to neoadjuvant chemotherapy (p = 0.336; Table 2), even though the patients in the fracture group presented with higher tumour stages. This leads to the conclusion that standard protocols of neoadjuvant chemotherapy remain valid and should be applied in patients with pathological fractures and higher tumour stages. In conclusion, we recommend that patients with a pathological fracture from a malignant bone tumour should be treated by neoadjuvant chemotherapy after non-operative stabilisation of the fracture (e.g. splint or external fixator) in combination with appropriate analgesia. Then, resection of the tumour should be performed with adequate margins, which may entail performing an extended wide resection in some cases.
Acknowledgments
Transparency statement / conflict of interests The manuscript submitted does not contain information about medical devices or drugs.No funds were received in support of this study. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Contributor Information
Babak Moradi, Email: Babak.Moradi@med.uni-heidelberg.de.
Anita Zahlten-Hinguranage, Email: anita.zahlten-h@nct-heidelberg.de.
Burkhard Lehner, Email: Burkhard.Lehner@med.uni-heidelberg.de.
Felix Zeifang, Phone: +49-6221-9650, FAX: +49-6221-969289, Email: Felix.Zeifang@med.uni-heidelberg.de.
References
- 1.Abudu A, Sferopoulos NK, Tillman RM, Carter SR, Grimer RJ. The surgical treatment and outcome of pathological fractures in localised osteosarcoma. J Bone Jt Surg Br. 1996;78:694–698. [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Longhi A, et al. Nonmetastatic osteosarcoma of the extremity with pathologic fracture at presentation: local and systemic control by amputation or limb salvage after preoperative chemotherapy. Acta Orthop Scand. 2003;74:449–454. doi: 10.1080/00016470310017776. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G. Pathologic fracture in osteosarcoma. J Bone Jt Surg Am. 2003;85A:1848–1849. doi: 10.2106/00004623-200309000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Bramer JAM, Abudub AA, Grimerb RJ, Carterb SR, Tillman RM (2007) Do pathological fractures influence survival and local recurrence rate in bony sarcomas? Eur J Cancer 1944–1951 [DOI] [PubMed]
- 6.Bramwell VH, Steward WP, Nooij M, et al. Neoadjuvant chemotherapy with doxorubicin and cisplatin in malignant fibrous histiocytoma of bone: a European Osteosarcoma Intergroup study. J Clin Oncol. 1999;17:3260–3269. doi: 10.1200/JCO.1999.17.10.3260. [DOI] [PubMed] [Google Scholar]
- 7.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 8.Cox DR. Regression models and life-tables. J R Stat Soc Series B. 1972;34:187–202. [Google Scholar]
- 9.Craft A, Cotterill S, Malcolm A, et al. Ifosfamide-containing chemotherapy in Ewing’s sarcoma: the Second United Kingdom Children’s Cancer Study Group and the Medical Research Council Ewing’s Tumor Study. J Clin Oncol. 1998;16:3628–3633. doi: 10.1200/JCO.1998.16.11.3628. [DOI] [PubMed] [Google Scholar]
- 10.Ebeid W, Amin S, Abdelmegid A. Limb salvage management of pathologic fractures of primary malignant bone tumors. Cancer Control. 2005;12:57–61. doi: 10.1177/107327480501200107. [DOI] [PubMed] [Google Scholar]
- 11.Enneking WF, Spanier SS, Goodman MA. Current concepts review. The surgical staging of musculoskeletal sarcoma. J Bone Jt Surg Am. 1980;62:1027–1030. [PubMed] [Google Scholar]
- 12.Glasser DB, Lane JM, Huvos AG, Marcove RC, Rosen G. Survival, prognosis, and therapeutic response in osteogenic sarcoma: the Memorial Hospital experience. Cancer. 1992;69:698–708. doi: 10.1002/1097-0142(19920201)69:3<698::AID-CNCR2820690317>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann C, Jabar S, Ahrens S, et al. Prognosis in Ewing sarcoma patients with initial pathological fractures of the primary tumor site. Klin Padiatr. 1995;207:151–157. doi: 10.1055/s-2008-1046532. [DOI] [PubMed] [Google Scholar]
- 14.Jaffe N, Spears R, Eftekhari F, Robertson R, Cangir A, Takaue Y, Carrasco H, Wallace S, Ayala A, Raymond K, et al. Pathologic fracture in osteosarcoma. Impact of chemotherapy on primary tumor and survival. Cancer. 1987;59:701–709. doi: 10.1002/1097-0142(19870215)59:4<701::AID-CNCR2820590407>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 16.Lee FY, Mankin HJ, Fondren G, et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Jt Surg Am. 1999;81:326–338. doi: 10.2106/00004623-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Mulder JO, Schutte HE, Kroon HM, Taconis WK (1993) Intraosseous osteosarcoma: conventional type. In Kroon HM (ed) Radiologic atlas of bone tumors. Elsevier Science, Amsterdam, pp 51–55
- 18.Natarajan MV, Govardhan RH, Williams S, Raja Gopal TS. Limb salvage surgery for pathological fractures in osteosarcoma. Int Orthop. 2000;24(3):170–172. doi: 10.1007/s002640000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pochanugool L, Subhadharaphandou T, Dhanachai M, Hathirat P, Sangthawan D, Pirabul R, Onsanit S, Pornpipatpong N. Prognostic factors among 130 patients with osteosarcoma. Clin Orthop Relat Res. 1997;345:206–214. doi: 10.1097/00003086-199712000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Rozeman LB, Cleton-Jansen AM, Hogendoorn PC. Pathology of primary malignant bone and cartilage tumours. Int Orthop. 2006;30(6):437–444. doi: 10.1007/s00264-006-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110(8):1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 22.Salzer-Kuntschik M, Brand G, Delling G. Determination of the degree of morphological regression following chemotherapy in malignant bone tumors. Pathologe. 1983;4(3):135–141. [PubMed] [Google Scholar]
- 23.Sarahrudi K, Hora K, Heinz T, Millington S, Vécsei V. Treatment results of pathological fractures of the long bones: a retrospective analysis of 88 patients. Int Orthop. 2006;30(6):519–524. doi: 10.1007/s00264-006-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Jt Surg Am. 2002;84A:49–57. [PubMed] [Google Scholar]
- 25.Scully SP, Temple HT, O’Keefe RJ, Mankin HJ, Gebhardt M. The surgical treatment of patients with osteosarcoma who sustain a pathologic fracture. Clin Orthop. 1996;324:227–232. doi: 10.1097/00003086-199603000-00028. [DOI] [PubMed] [Google Scholar]
- 26.Simon MA, Aschilman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Jt Surg Am. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 27.Simon MA. Current concepts review. Limb salvage in osteosarcoma. J Bone Jt Surg Am. 1988;70:307–310. [PubMed] [Google Scholar]
- 28.Souhami RL, Craft AW, Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet. 1997;350:911–917. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 29.Zeifang F, Sabo D, Ewerbeck V. Pathological fracture in primary malignant bone tumors. Chirurg. 2000;71:1121–1125. doi: 10.1007/s001040051188. [DOI] [PubMed] [Google Scholar]