Abstract
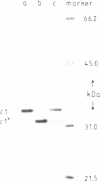
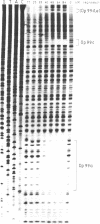
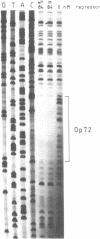
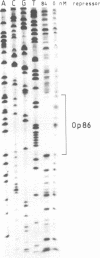
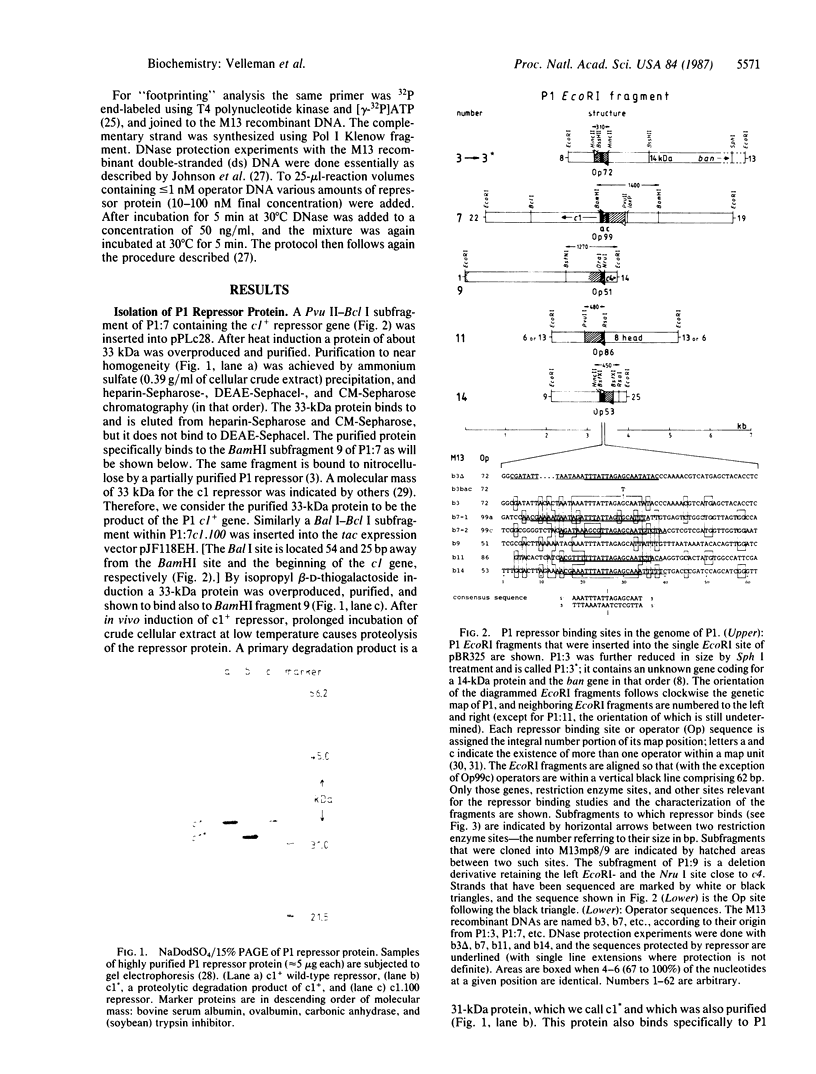
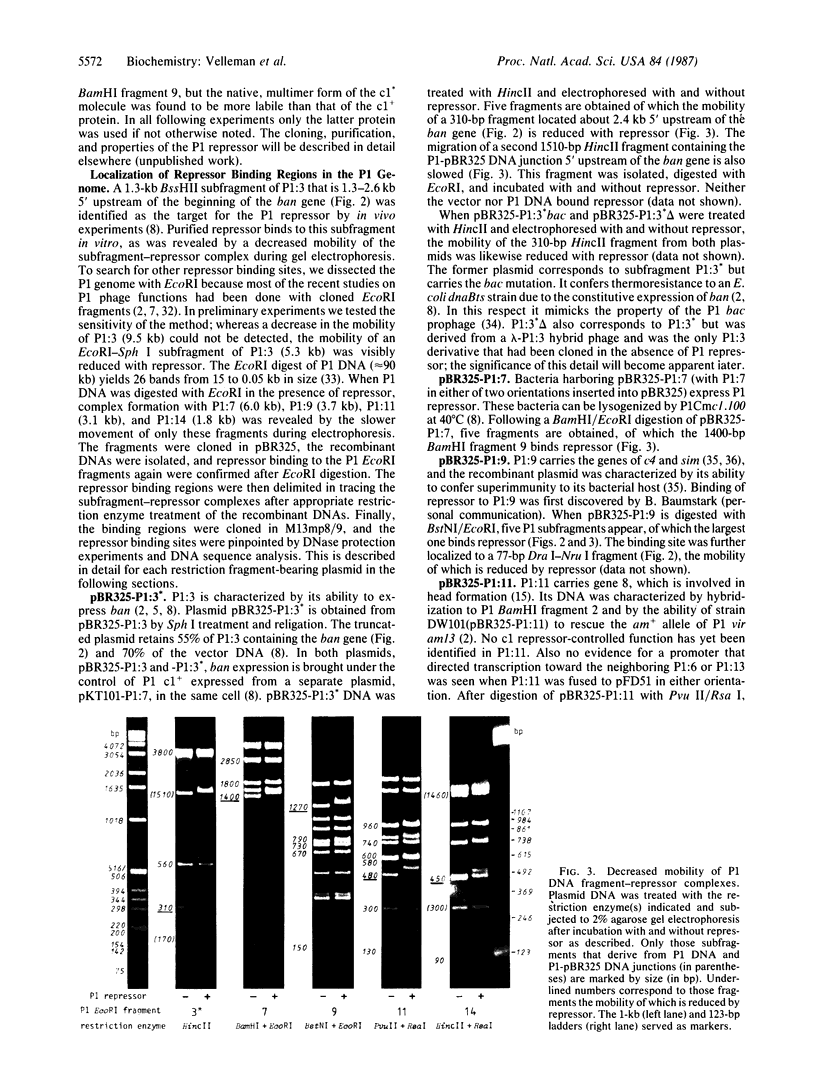
After digestion of bacteriophage P1 DNA with EcoRI in the presence of P1 repressor, 6 repressor binding sites were identified in 5 of 26 EcoRI fragments. Binding sites were localized by the decreased mobility of DNA fragment-repressor complexes during electrophoresis and by DNase protection ("footprinting") analysis. The repressor binding sites, or operators, comprise a 17-base-pair-long consensus sequence lacking symmetrical elements. Three operators can be related to known genes, whereas the function of the others is still unknown. The mutant P1 bac, rendering ban expression constitutive, is identified as an operator-constitutive mutation of the ban operon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin S., Sternberg N., Yarmolinsky M. Miniplasmids of bacteriophage P1. I. Stringent plasmid replication does not require elements that regulate the lytic cycle. J Mol Biol. 1978 Apr 5;120(2):297–309. doi: 10.1016/0022-2836(78)90069-4. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Timmis K. N. Host: vector systems for gene cloning in Pseudomonas. Curr Top Microbiol Immunol. 1982;96:47–67. doi: 10.1007/978-3-642-68315-2_4. [DOI] [PubMed] [Google Scholar]
- Baumstark B. R., Scott J. R. The c1 repressor of bacteriophage P1. I. Isolation of the c1 protein and determination of the P1 DNA region to which it binds. J Mol Biol. 1980 Jul 15;140(4):471–480. doi: 10.1016/0022-2836(80)90266-1. [DOI] [PubMed] [Google Scholar]
- Baumstark B. R., Scott J. R. The c4 gene of phage P1. Virology. 1987 Feb;156(2):197–203. doi: 10.1016/0042-6822(87)90398-9. [DOI] [PubMed] [Google Scholar]
- Baumstark B. R., Stovall S. R., Ashkar S. Interaction of the P1c1 repressor with P1 DNA: localization of repressor binding sites near the c1 gene. Virology. 1987 Feb;156(2):404–413. doi: 10.1016/0042-6822(87)90420-x. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bächi B., Arber W. Physical mapping of BglII, BamHI, EcoRI, HindIII and PstI restriction fragments of bacteriophage P1 DNA. Mol Gen Genet. 1977 Jun 24;153(3):311–324. doi: 10.1007/BF00431596. [DOI] [PubMed] [Google Scholar]
- D'Ari R., Jaffé-Brachet A., Touati-Schwartz D., Yarmolinsky M. B. A dnaB analog specified by bacteriophage P1. J Mol Biol. 1975 May 25;94(3):341–366. doi: 10.1016/0022-2836(75)90207-7. [DOI] [PubMed] [Google Scholar]
- Danner D. B. Recovery of DNA fragments from gels by transfer to DEAE-paper in an electrophoresis chamber. Anal Biochem. 1982 Sep 1;125(1):139–142. doi: 10.1016/0003-2697(82)90394-3. [DOI] [PubMed] [Google Scholar]
- Devlin B. H., Baumstark B. R., Scott J. R. Superimmunity: characterization of a new gene in the immunity region of P1. Virology. 1982 Jul 30;120(2):360–375. doi: 10.1016/0042-6822(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther E., Bagdasarian M., Schuster H. Cloning of the dnaB gene of Escherichia coli: the dnaB gene of groPB534 and groPB612 and the replication of phage lambda. Mol Gen Genet. 1984;193(2):225–230. doi: 10.1007/BF00330672. [DOI] [PubMed] [Google Scholar]
- Heilmann H., Reeve J. N., Pühler A. Identification of the repressor and repressor bypass (antirepressor) polypeptides of bacteriophage P1 synthesized in infected minicells. Mol Gen Genet. 1980 Apr;178(1):149–154. doi: 10.1007/BF00267223. [DOI] [PubMed] [Google Scholar]
- Heisig A., Severin I., Seefluth A. K., Schuster H. Regulation of the ban gene containing operon of prophage P1. Mol Gen Genet. 1987 Mar;206(3):368–376. doi: 10.1007/BF00428873. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Wulff D. L., Rosenberg M. Bacteriophage lambda protein cII binds promoters on the opposite face of the DNA helix from RNA polymerase. Nature. 1983 Aug 25;304(5928):703–708. doi: 10.1038/304703a0. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Meyer B. J., Ptashne M. Interactions between DNA-bound repressors govern regulation by the lambda phage repressor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5061–5065. doi: 10.1073/pnas.76.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mural R. J., Chesney R. H., Vapnek D., Kropf M. M., Scott J. R. Isolation and characterization of cloned fragments of bacteriophage P1 DNA. Virology. 1979 Mar;93(2):387–397. doi: 10.1016/0042-6822(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Rak B., von Reutern M. Insertion element IS5 contains a third gene. EMBO J. 1984 Apr;3(4):807–811. doi: 10.1002/j.1460-2075.1984.tb01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. Genetic studies on bacteriophage P1. Virology. 1968 Dec;36(4):564–574. doi: 10.1016/0042-6822(68)90188-8. [DOI] [PubMed] [Google Scholar]
- Sternberg N. A characterization of bacteriophage P1 DNA fragments cloned in a lambda vector. Virology. 1979 Jul 15;96(1):129–142. doi: 10.1016/0042-6822(79)90179-x. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Austin S., Hamilton D., Yarmolinsky M. Analysis of bacteriophage P1 immunity by using lambda-P1 recombinants constructed in vitro. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5594–5598. doi: 10.1073/pnas.75.11.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Hoess R. The molecular genetics of bacteriophage P1. Annu Rev Genet. 1983;17:123–154. doi: 10.1146/annurev.ge.17.120183.001011. [DOI] [PubMed] [Google Scholar]
- Touati-Schwartz D. A dnaB analog ban, specified by bacteriophage P1: genetic and physiological evidence for functional analogy and interactions between the two products. Mol Gen Genet. 1979 Jul 13;174(2):173–188. doi: 10.1007/BF00268354. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Jr, Walker J. T. Genetic studies of coliphage P1. III. Extended genetic map. J Virol. 1976 Oct;20(1):177–187. doi: 10.1128/jvi.20.1.177-187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]