Abstract
The aim of this study was to evaluate the relationship between a low acromion index and osteoarthritis of the shoulder. Three patient groups were used: (I) instability, n = 53; (II) calcifying tendonitis, n = 109; and (III) osteoarthritis, n = 120. Standardised digital X-rays were evaluated from the true anteroposterior and axillary views. Joint space width at three levels in each plane and the size of humeral osteophytes were measured and osteoarthritis was graded according to Samilson. The acromion index was calculated according to Nyffeler et al. (J Bone Joint Surg Am 88:800–805, 2006) in the true anteroposterior view. There were two independent investigators. Interobserver reliability was excellent for all measurements in the anteroposterior (AP) projection but inferior in the axillary projections, especially in group III. The mean acromion index was 0.64 ± 0.07 in group I, 0.64 ± 0.08 in group II and 0.73 ± 0.12 in group III. The acromion index was not correlated with the joint space width nor with the size of the osteophytes or the Samilson grading in group III. The data of the study did not show a significant association between a low acromion index and typical signs of osteoarthritis at the shoulder. The theoretical concept of a small acromion index associated with the development of osteoarthritis of the shoulder is not supported.
Introduction
Osteoarthritis of the shoulder is a common disease with increasing incidence by age due to degenerative changes. The reason for the development of osteoarthritis can be secondary to infection, instability, osteonecrosis, trauma with direct or indirect impairment of the joint cartilage and certain metabolic (e.g. hyperuricaemia) and other diseases such as rheumatoid arthritis, sickle cell disease, haemophilia, and neurotrophic diseases such as syringomyelia, etc. [6, 13]. The underlying cause of the so-called primary osteoarthritis of the shoulder is still unknown. Genetic factors and individual features of connective tissue have recently become the focus of basic research [2, 11, 12, 21].
Mechanical factors such as individual variations of humeral torsion and glenoid version have been investigated without a clear relation to the development of osteoarthritis [22].
Recently, Nyffeler et al. introduced the acromion index as a new biomechanical value describing the lateral extension of the acromion above the humeral head [16]. The authors were able to show a significant relationship between a high acromion index and full-thickness rotator cuff tears. The authors postulated that the resultant force vector of the deltoid muscle with a large extension of the acromion resulting in a higher ascending force can lead to subacromial impingement and degenerative changes of the acromion. On the other hand, a smaller lateral extension of the acromion resulting in a higher compression force against the glenoid cavity can lead to osteoarthritis.
Neer described primary gleno-humeral osteoarthritis consisting of limitation of gleno-humeral movement, loss of joint space, humeral head enlargement due to osteophytes and the absence of rotator cuff tears [15].
The aim of this study was to evaluate the relationship between the acromion index and degenerative changes of the glenohumeral joint leading to osteoarthritis at the shoulder.
Materials and methods
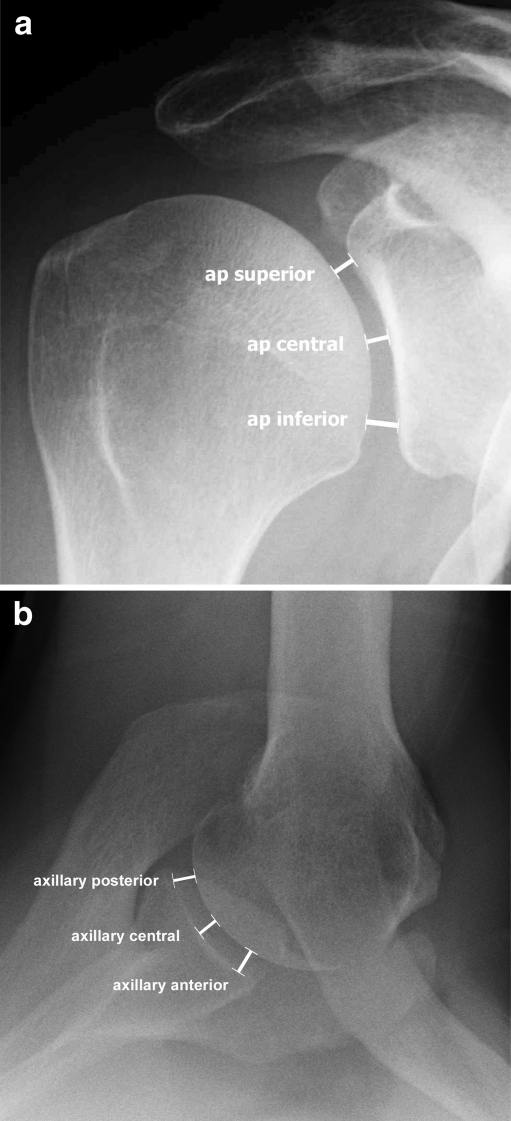
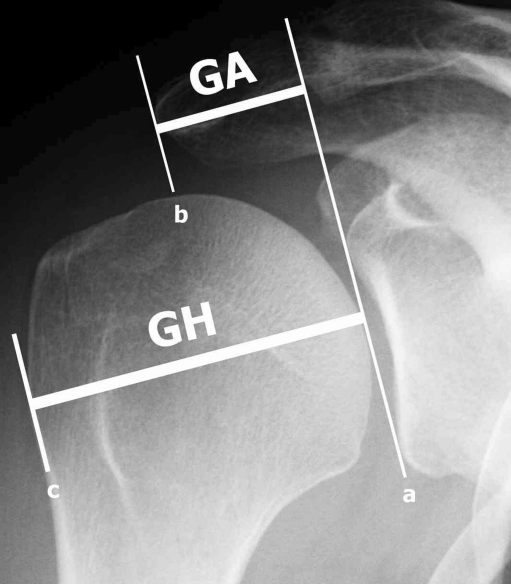
We performed a retrospective analysis of standardised X-rays at our institution from 2002 to 2008. All patients gave consent for the anonymous use of their data for this study and the study was approved by an IRB. Joint space width measurements were performed at three levels in the true anteroposterior view (superior, central and inferior level) (Fig. 1a) and at three levels in the axillary view (anterior, central and posterior) (Fig. 1b) using digital X-rays with a resolution of 0.01 mm. The measurements were made perpendicular to the tangent to the humeral joint surface at the described levels using the digital measurement tool in the highest available magnification on the screen. The acromion index (AI) was calculated as described by Nyffeler et al. by dividing the distance from the glenoid plane to the most lateral aspect of the acromion (marked GA in Fig. 2) by the distance from the glenoid plane to the most lateral aspect of the proximal humeral head (marked GH in Fig. 2) ) in the true anteroposterior view (Fig. 2) [16]. The true anteroposterior views were taken in neutral rotation of the forearm with the arm at the side of the body (no abduction). This is the most reliable position as it can easily be controlled by the technician and all the patients were able to maintain that position. Inaccuracy of measurements due to changes of the parameters by different positions of the arm are therefore avoided [16].
Fig. 1.
True anteroposterior (AP) X-ray (a) and axillary view (b) of a right shoulder illustrating the levels of joint space width measurement. AP superior, AP central and AP inferior measurements were made in the coronal plane and axillary anterior, axillary central and axillary posterior in the transverse plane. Measurements are made perpendicular to a tangent to the articular surface of the humerus at the different levels
Fig. 2.
True anteroposterior (AP) X-ray of a right shoulder. Line a is drawn at the glenoid level connecting the superior and inferior osseous margins of the glenoid cavity. Line b is parallel to line a at the level of the lateral margin of the acromion. Line c is parallel to lines a and b at the level of the most lateral part of the proximal part of the humerus. The distance GA between lines a and b is divided by the distance GH between lines a and c resulting in the acromion index, which characterises the lateral extension of the acromion above the humeral head
The size of the caudal humeral osteophyte was measured in the true anteroposterior view with neutral rotation of the arm and the patient was graded for the severity of osteoarthritis according to Samilson and Prieto (grade I: osteophyte <3 mm; grade II: 3–7 mm; grade III: >7 mm) [20]. The measurements were performed by two independent investigators (first and second author) (Fig. 1; Table 1).
Table 1.
Measured values in mm for the distances GA and GH (see Fig. 2) and the calculated acromion index (AI) with standard deviation for groups I, II and III
| Group | GH | GA | AI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Female | Male | All | Female | Male | All | Female | Male | |||||||
| Group I (instability) | 45.77±4.57 | 41.38±3.23 | 47.12±3.14 | 29.45±4.32 | 24.39±3.48 | 31.34±3.54 | 0.64±0.07 | 0.60±0.07 | 0.65±0.07 | ||||||
| Group II (calcifying tendonitis) | 44.52±3.56 | 42.39±2.51 | 46.68±2.58 | 28.56±3.72 | 27.55±3.38 | 30.09±3.64 | 0.64±0.08 | 0.63±0.08 | 0.61±0.08 | ||||||
| Group III (osteoarthritis) | 39.94±4.32 | 37.48±3.70 | 42.10±3.64 | 29.04±4.28 | 27.34±3.66 | 30.53±4.26 | 0.73±0.12 | 0.70±0.12 | 0.73±0.12 | ||||||
GA is the distance from the glenoid plane to the most lateral aspect of the acromion; GH is the distance from the glenoid plane to the most lateral aspect of the proximal humeral head
We investigated three groups of patients with different diagnoses: group I patients with instability, group II patients with calcifying tendonitis and group III patients with existing osteoarthritis. Inclusion criteria for group I (n = 53) were instability of the shoulder with not more than two dislocations overall and no history of more than two months without bony injury and no previous surgery. Inclusion criteria for group II (n = 109) were presence of calcifying tendonitis of the supraspinatus tendon, no history of trauma or instability, no previous surgery and an intact rotator cuff. Inclusion criteria for group III (n = 120) were primary osteoarthritis of the shoulder, intact rotator cuff, no trauma, no previous surgery, no glenoid erosion.
As there is no accepted connection between calcifying tendonitis of the shoulder or instability at an early stage and the presence of osteoarthritis, groups I and II served as a control (non-arthritic patients) for group III with existing osteoarthritis of the shoulder. This compromise was necessary to obtain control patients rather than use healthy unaffected shoulders because the latter are not available with X-rays and obtaining X-rays for scientific purposes only is not justified in view of the radiation exposure.
Statistical analysis was performed using SPSS software package (version 13.0; SPSS Inc., Chicago, Illinois). Data included descriptive statistics, U-test according to Mann and Whitney for non-parametric independent variables, bivariate correlation analysis (Spearman), partial correlation analysis, and intraclass correlation coefficient. Level of significance was set at p < 0.05.
Results
Group I
The mean age of the patients in group I was 31.62 ± 11.80 years and 19 of 53 were female. There was no significant difference between male and female patients in terms of age (31.37 ± 10.65 vs. 32.06 ± 13.92). There were no differences for gender and side. The interobserver reliability was excellent in the AP projection (r = 0.883–0.871) and in the axillary projection (r = 0.875–0.862), and also for the acromion index (r = 0.898).
The mean joint space widths were 3.77 mm ± 0.95 superior AP, 3.11 mm ± 0.71 central AP, 3.37 mm ± 0.80 inferior AP, 3.92 mm ± 1.08 anterior axillary, 3.92 mm ± 0.78 central axillary and 4.79 mm ± 1.19 posterior axillary.
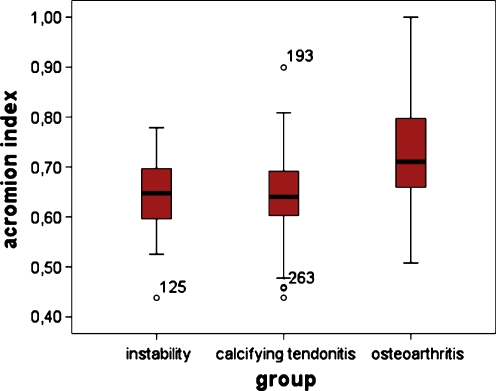
The mean GA distance was 29.45 mm ± 4.32 and mean GH was 45.77 mm ± 4.57, resulting in a mean acromion index of 0.64 ± 0.07 (Fig. 3).
Fig. 3.
Mean values with standard deviation of the acromion index for group I (instability), group II (calcifying tendonitis) and group III (osteoarthritis). The difference between groups I and II and group III is statistically significant (p = 0.001)
Group II
The mean age of the patients in group II was 48.2 ± 8.01 years, and 63 of 109 were female. No significant difference was found between male and female patients (48.60 ± 7.25 vs. 47.92 ± 8.58). There were no differences for gender and side. The interobserver reliability was excellent in the AP projection (r = 0.870–0.866) and in the axillary projection (r = 0.847–0.811) and for the acromion index (r = 0.899).
The mean joint space widths were 3.40 mm ± 1.05 superior AP, 2.88 mm ± 0.78 central AP, 3.26 mm ± 0.78 inferior AP, 3.95 mm ± 0.83 anterior axillary, 3.34 mm ± 0.84 central axillary and 4.05 mm ± 0.85 posterior axillary.
The mean GA distance was 28.56 mm ± 3.72 and mean GH was 44.52 mm ± 3.56, resulting in a mean acromion index of 0.64 ± 0.08 (Table 1).
Group III
The mean age of the patients in group III was 66.43 ± 9.74 years and 56 of 120 were female. Male patients were significantly younger (63.3 ± 9.2 vs. 70.0 ± 9.17, p = 0.001). There were no differences for gender and side. The interobserver reliability was excellent in the AP projection (r = 0.830–0.826) but inferior in the axillary projection (r = 0.621–0.732) and the acromion index (r = 0.761).
The mean joint space widths were 2.00 mm ± 1.40 superior AP, 1.46 mm ± 1.08 central AP, 1.48 mm ± 0.80 inferior AP, 3.01 mm ± 2.22 anterior axillary, 1.08 mm ± 1.12 central axillary and 1.17 mm ± 1.04 posterior axillary.
Seven patients were graded as Samilson 1, three patients as Samilson 2 and 110 patients as Samilson 3. The mean osteophyte size was 14.14 mm ± 6.19.
Male patients had significantly larger osteophytes (15.52 mm ± 6.33) than female (12.66 mm ± 5.69) (p = 0.012). This was not reflected in the Samilson classification.
Age of the patient showed a significant negative correlation with the joint space for all measurements (r = −0.401–0.791, p < 0.001), size of osteophytes (r = −0.719, p < 0.001) and the Samilson stage (r = −0.743, p < 0.001).
The mean GA distance was 29.04 mm ± 4.28 and mean GH was 39.94 mm ± 4.32, resulting in a mean acromion index of 0.73 ± 0.12 (Table 1).
There was no significant difference in the acromion index between groups I and II (p = 0.973) but there was for group I or II and group III (also true for the pooled data of groups I and II as control and group III) (p = 0.001).
The acromion index neither correlated with the joint space width in groups I–III nor with the size of osteophytes or Samilson stage in group III.
Discussion
Our data illustrate a good reliability of measurements of the radiological parameters (acromion index and joint space width) at the shoulder.
For practical reasons it should not be necessary to take joint space measurements at three levels in two planes. This becomes more important in cases of osteoarthritis as this disease frequently is accompanied by the development of caudal humeral osteophytes and dorsal subluxation of the humeral head with dorsal glenoid erosion. This significantly influences the joint space measurement as the almost spherical humeral head compared to the greater radius of the glenoid (radiological mismatch) together with dorsal decentering of the head simulates a falsely high value for the anterior joint space measurements in the transverse plane. This mechanism explains the inferior reliability at the axillary projections in group III which complicates measurement due to the loss of the normal appearance of the joint.
In contrast to the method used in our study with measurement at three distinct levels in two planes the smallest joint space width in any projection or the joint space width at the centre of the anteroposterior view in a non-arthritic shoulder should be regarded as the most reliable measurement in clinical settings [8].
The mean acromion index of 0.64 was almost identical in groups I and II which served as a control for the osteoarthritis group. Regarding the configuration of the acromion, the glenoid and the humeral head in relation to each other, both groups can be considered to represent the normal population, because there is no data that connects the underlying diseases of both groups to variations in bony anatomy as described here. Nyffeler et al. found exactly the same mean acromion index of 0.64 in their control group of normal subjects [16].
In contrast to Nyffeler et al., we found an increased instead of a decreased acromion index in group III with osteoarthritis in our study. Comparing the osteoarthritis groups of both studies there is no big difference in age (63.7 vs. 66.43 years) but in gender distribution (19 males out of 47 vs. 64 males out of 120 in our study). But we did not observe a correlation for age or gender to the acromion index in our study (males were found to have higher values of the distances GA and GH, which means bigger shoulders but no different acromion indices on average).
The acromion index is influenced by the joint space, which means a reduced joint space as found in osteoarthritis decreases the distance GH but not GA. If we take the data of our control groups I and II we can assume a normal joint space of 3 mm. Correcting the distance GH for complete loss of 3 mm joint space would result in an acromion index of 0.68 ± 0.11. Walch et al. have defined partial loss of joint space of 2–5 mm in their study, although there is no clear description of the distribution of the values [22]. Petersson et al. have described a normal joint space of about 4–5 mm [18, 19]. Correcting the distance GH in our study for an assumed total loss of 5 mm of joint space would result in an acromion index of 0.64 ± 0.10, which is still clearly above the value of 0.60 described by Nyffeler et al. Whether there are regional differences regarding the study population or other reasons that could explain the differences remains uncertain and needs further investigation.
We are not aware of any study that measured the joint space in osteoarthritis and compared it to a control group as we have done in our study, and we consider our data to be original and reliable as described above. A post-hoc power analysis of the data for the acromion index shows a power of 1.0 (with α > 0.05).
Apreleva et al. have shown that in an experimental set-up the glenohumeral joint reaction force increases with abduction angles to 90° with varying forces according to the ratio of forces in the supraspinatus and deltoid muscle [1]. The authors showed a clear functional relationship between the supraspinatus and deltoid muscle for abduction and that the muscles can compensate for malfunction of the other. With the application of these findings to the concept of an increased joint reaction force with a low acromion index, one could speculate that the theoretically unfavourable moment arm of the deltoid is compensated by less muscle activation of the supraspinatus in early abduction or, for example, counteraction of the latissimus dorsi and pectoralis muscle by those individuals leading to an unchanged resultant joint reaction force overall.
Other authors have shown that the distribution of forces at the shoulder form part of a complex functional system with different findings in different clinical settings and in all three planes with regard to forces and stability, and it is difficult to predict certain values [5, 7, 9, 10, 14, 17].
The experimental determination of true joint reaction forces is also difficult and there is a discrepancy for theoretically determined and in vivo measured values [3, 4, 23, 24].
The theoretical influence of the acromion index on joint reaction forces only acts in the frontal plane (until the onset of the horizontal pull of the middle deltoid muscle at 90° of abduction) but not in the sagittal and transverse planes. As all-day activities of the patients are mostly combined movements of the shoulder in all planes, the effect of a biomechanically unfavourable low acromion index may not be strong enough to affect the entire system in terms of development of osteoarthritis.
Further experimental studies with investigation of the functional relationship of the rotator cuff, the scapulothoracic and thoracohumeral, and the deltoid muscles in different biomechanical conditions could clarify this.
Conclusion
The data of this study did not show a significant association between a low acromion index and osteoarthritis at the shoulder. The theoretical concept of a small acromion index resulting in increased contact pressure to the glenohumeral joint leading to the development of osteoarthritis of the shoulder is not supported. Further research is necessary to identify other pathogenetic mechanisms of omarthrosis.
Acknowledgments
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Apreleva M, Parsons IMt, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9:409–417. doi: 10.1067/mse.2000.106321. [DOI] [PubMed] [Google Scholar]
- 2.Aspden RM. Osteoarthritis: a problem of growth not decay? Rheumatology. 2008;47:1452–1460. doi: 10.1093/rheumatology/ken199. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann G, Graichen F, Bender A, Kaab M, Rohlmann A, Westerhoff P. In vivo glenohumeral contact forces—measurements in the first patient 7 months postoperatively. J Biomech. 2007;40:2139–2149. doi: 10.1016/j.jbiomech.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann G, Graichen F, Rohlmann A, Westerhoff P, Bender A, Gabel U, Heinlein B. Loads acting on orthopaedic implants. Measurements and practical applications. Orthopade. 2007;36:194–202. doi: 10.1007/s00132-007-1055-x. [DOI] [PubMed] [Google Scholar]
- 5.Favre P, Jacob HA, Gerber C. Changes in shoulder muscle function with humeral position: a graphical description. J Shoulder Elbow Surg. 2009;18:114–121. doi: 10.1016/j.jse.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Habermeyer P, Engel G. Endoprothetik. In: Habermeyer P, editor. Schulterchirurgie, Elsevier. München, Jena: Urban & Fischer; 2005. pp. 497–553. [Google Scholar]
- 7.Karlsson D, Peterson B. Towards a model for force predictions in the human shoulder. J Biomech. 1992;25:189–199. doi: 10.1016/0021-9290(92)90275-6. [DOI] [PubMed] [Google Scholar]
- 8.Kircher J, Morhard M, Magosch P, Ebinger N, Lichtenberg S, Habermeyer P. How much are radiological parameters related to clinical symptoms and function in osteoarthritis of the shoulder? Int Orthop. 2009 doi: 10.1007/s00264-009-0846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konrad GG, Jolly JT, Labriola JE, McMahon PJ, Debski RE. Thoracohumeral muscle activity alters glenohumeral joint biomechanics during active abduction. J Orthop Res. 2006;24:748–756. doi: 10.1002/jor.20062. [DOI] [PubMed] [Google Scholar]
- 10.Labriola JE, Lee TQ, Debski RE, McMahon PJ. Stability and instability of the glenohumeral joint: the role of shoulder muscles. J Shoulder Elbow Surg. 2005;14:32S–38S. doi: 10.1016/j.jse.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Loughlin J. The genetic epidemiology of human primary osteoarthritis: current status. Expert Rev Mol Med. 2005;7:1–12. doi: 10.1017/S1462399405009257. [DOI] [PubMed] [Google Scholar]
- 12.Mary B, Goldbring SRG. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 13.Matsen F, Rockwood CA, Wirth M, Lippitt S, Parsons M. Glenohumeral arthritis and its management. In: Rockwood C, Matsen F, Wirth M, Lippitt S, editors. The shoulder. Philadelphia: Saunders; 2004. pp. 879–1007. [Google Scholar]
- 14.McMahon PJ, Debski RE, Thompson WO, Warner JJ, Fu FH, Woo SL. Shoulder muscle forces and tendon excursions during glenohumeral abduction in the scapular plane. J Shoulder Elbow Surg. 1995;4:199–208. doi: 10.1016/S1058-2746(05)80052-7. [DOI] [PubMed] [Google Scholar]
- 15.Neer CS., 2nd Degenerative lesions of the proximal humeral articular surface. Clin Orthop. 1961;20:116–125. [PubMed] [Google Scholar]
- 16.Nyffeler RW, Werner CM, Sukthankar A, Schmid MR, Gerber C. Association of a large lateral extension of the acromion with rotator cuff tears. J Bone Joint Surg Am. 2006;88:800–805. doi: 10.2106/JBJS.D.03042. [DOI] [PubMed] [Google Scholar]
- 17.Parsons IM, Apreleva M, Fu FH, Woo SLY. The effect of rotator cuff tears on reaction forces at the glenohumeral joint. J Orthop Res. 2002;20:439–446. doi: 10.1016/S0736-0266(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 18.Petersson CJ. Degeneration of the gleno-humeral joint: an anatomical study. Acta Orthop. 1983;54:277–283. doi: 10.3109/17453678308996570. [DOI] [PubMed] [Google Scholar]
- 19.Petersson CJ, Redlund-Johnell I. Joint space in normal gleno-humeral radiographs. Acta Orthop. 1983;54:274–276. doi: 10.3109/17453678308996569. [DOI] [PubMed] [Google Scholar]
- 20.Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65:456–460. [PubMed] [Google Scholar]
- 21.Valdes AM, Spector TD. The contribution of genes to osteoarthritis. Med Clin North Am. 2009;93:45–66. doi: 10.1016/j.mcna.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Walch G, Boulahia A, Boileau P, Kempf JF. Primary glenohumeral osteoarthritis: clinical and radiographic classification. The Aequalis Group. Acta Orthop Belg. 1998;64(Suppl 2):46–52. [PubMed] [Google Scholar]
- 23.Westerhoff P, Graichen F, Bender A, Rohlmann A, Bergmann G. An instrumented implant for in vivo measurement of contact forces and contact moments in the shoulder joint. Med Eng Phys. 2009;31:207–213. doi: 10.1016/j.medengphy.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Westerhoff P, Rohlmann A, Bender A, Graichen F, Bergmann G. In vivo shoulder joint forces at isolated motions. J Biomech. 2008;41:S144. doi: 10.1016/S0021-9290(08)70144-1. [DOI] [Google Scholar]