Abstract
The purpose of this study was to observe early lesions of rat epiphyseal plates and metaphysis caused by T-2 toxin and T-2 toxin combined with a low nutrition diet to determine possible pathogenic factors of Kashin-Beck disease (KBD). Ninety Wistar rats were divided into three groups. Group A was fed with a normal diet as control; group B was fed with a normal diet and T-2 toxin; and group C was fed with a low nutrition diet and T-2 toxin. The left knee specimens were collected, fixed in formaldehyde solution, stained by hematoxylin and eosin and Masson. After two weeks, the epiphyseal plate showed necrosis of chondrocytes in groups B and C. After four weeks, more obvious chondrocyte necrosis appeared. The positive rate of Lamellar necrosis in group C was significantly higher than that in groups B and A (P < 0.01). Metaphyseal trabecular bone showed sparse disorder and disruption in group C. T-2 toxin combined with a low nutrition diet could lead to more serious chondrocyte necrosis in the epiphyseal plate and disturb metaphyseal trabecular bone formation.
Introduction
Kashin-Beck disease (KBD) is an endemic, chronic, degenerative osteoarthropathy, which develops mainly in children five to 15 years of age. It seems that the basic pathological change is the development of multiple areas of cell necrosis in the growth plates [1, 2]. The epiphysis shows focal or irregular premature closure. Limited motion and enlargement of peripheral joints, deformities and dwarfism are present on clinical examination [3]. Although KBD has been studied for more than 100 years, we still cannot definite its aetiology. The current hypotheses about the aetiology of KBD include mycotoxins in stored grain [4–6], trace elements deficiency in nutrition (mainly selenium and iodine) [7, 8] and high level of humic acid in drinking water [9, 10].
T-2 toxin is a potent member of the trichothecene mycotoxin family produced by moulds, particularly the Fusarium species. It is characterised by a substituted epoxy trichothecene ring structure mainly through contaminated food and causes harm to humans and animals. According to epidemiological studies, mycotoxins are significantly associated with the risk of KBD. In Chinese KBD areas, microbiological examinations have shown that wheat crops were contaminated by Fusarium Link ex Fr. spp [11]. Cereal samples in high incidence areas of KBD were found to be more seriously contaminated with trichotecenes (T-2 toxins) when compared to those in low incidence areas [12]. A significant difference in the levels of Trichothecium roseum (Pers) Link ex gray, Dreschlera Ito, Alternaria Nees ex Fr. [13] and Ulocladium sp. [4] were observed between affected and non-affected families in Tibet.
The Aba region of Sichuan Province is an endemic area of KBD. It is located on the Tibetan plateau, which is a high altitude, high humidity and low temperature region [14, 15]. Most people in the KBD affected areas live at altitudes between 2500 and 3500 m. Our investigation discovered that local grain storage was easily contaminated by the mildew. High altitude and the extremely severe weather conditions affect the species of grain planted. The natural environment and people’s customs are similar to those in Tibet. The local resident diet structure is unitary, barley content was more than 90%, and they have low protein, fat, and vitamin intake. This dietary pattern can easily induce malnutrition [16]. We speculate mycotoxins and low nutrition diet may have a certain role in the occurrence of KBD in the Aba affected area.
In this study, we used T-2 toxin combined with two different diets (normal and low-nutrition) to feed rats and observe the epiphyseal plate and metaphysis histological changes. This was done in order to clarify the effect of T-2 toxin and low nutrition diet on the development of growth plate and modification of collagen in these tissues through animal experiments. Furthermore, we provide experimental evidence to further study the relation between T-2 toxin, low nutrition and KBD.
Materials and methods
Experimental animals and grouping
Experimental animals were purchased in Sichuan University Huaxi Animal Experimental Centre. There were 90 Wistar rats, half male and half female, with weight between 60 and 70 g. The rats were randomly divided into three groups, each group had thirty rats. Group A was fed with a normal diet as control; group B was fed with a normal diet and T-2 toxin; and group C was fed with a low nutrition diet and T-2 toxin. All rats were supplied with distilled water freely accessible at all times. The experiment was approved by the Animal Ethics Committee, Sichuan University West China school of Medicine.
Diet and administration of T-2 toxin
Two kinds of diets were prepared, as shown in Table 1. There was no fish meal, bone meal, corn oil or soybean flour in the low nutrition diet. The content of low selenium yeast was increased. The content of protein, lipids, multivitamins, and selenium in the low nutrition diet was lower than that in the normal diet. T2 toxin was purchased from Trilogy Company (USA) at a purity of 99%; Crystalline T-2 toxin was dissolved in absolute ethanol and diluted with 0.9% normal saline. The T-2 toxin involved intragastric administration five days per week for four weeks at a 1 mg/kg wd dose level.
Table 1.
Food composition given to the Wistar rats
| Feed ingredients (%) | Normal diet (%) | Low-nutrition diet (%) |
|---|---|---|
| Flour | 20 | 12 |
| Wheat | 7.2 | 28 |
| Corn | 25.6 | 35 |
| Wheat bran | 4 | 4 |
| Fish meal | 6 | 0 |
| Bone meal | 2.8 | 0 |
| Herb flour | 8 | 0 |
| Common Salt | 1 | 1 |
| Dried yeast | 2 | 20 |
| Soybean flour | 22 | 0 |
| Corn oil | 1 | 0 |
| Lysine | 0.2 | 0 |
| Methionine | 0.18 | 0 |
| Multivitamins | 0.02 | 0 |
Sample preparation
We collected information including the external hair lustre, activity, size and weight of the rats. After one, two and four weeks, we took out ten rats from each group randomly, and the left knee including the distal femur and the proximal tibia was harvested. The specimens were fixed in formaldehyde solution (8%) and decalcified in a solution of ammonium chloride (70 g), formic acid (50 ml) and hydrochloric acid (14 ml) in water (1000 ml). The specimens then were rinsed in ethanol and processed for paraffin embedding. The histological sections were cut and stained with haematoxylin and eosin and Masson.
Methods of evaluation
According to the standards of necrosis category previously described [17], the necrosis categories included focus necrosis, lamellar necrosis, penetration necrosis and zonal necrosis. Focus necrosis (FN) involved two to three cell columns with two to three cell necroses in each column at one cell zone. Lamellar necrosis (LN) involved more than two focus necrosis in one cell zone. Penetration necrosis (PN) involved a focus necrosis penetration cell zone. Zonal necrosis (ZN) had whole cell zone necrosis. The necrosis showed cell vacuolated changes, pyknosis and lysis of nuclei, as well as loss of cell morphology.
Masson staining of collagen was divided into three staining results. Positive staining in grade I was light blue, in grade II it was blue, and in grade III it was dark blue. Deep staining means high collagen content.
Statistical analyses
Statistical analyses were performed independently by a non-clinical research assistant and an outside party to ensure objectivity, using SPSS version 11.5 software. Weights from control and treated groups were compared using the Kruskal-Wallis test based on mean ranks. Results were considered statistically significant if the p-value was less than 0.05 for continuous variables. Satistical analyses for the positive rate of cell necrosis were conducted with the chi-square test.
Results
Rat diet analysis
The normal rats’ diet and low nutrition diet were detected by the Centre for Disease Control and Prevention of Sichuan. In a low nutrition diet, the protein content was 15.23%, the calcium content was 0.27%, the selenium content was less than 0.10 mg/kg, and the ferrous content was 100.10 mg/kg. These nutritional elements were lower than in the normal diet. The results of rat diet analysis are shown in Table 2.
Table 2.
Rat diet analysis results
| Item | Normal diet | Low nutrition diet |
|---|---|---|
| Protein (g/100 g) | 18.64 | 15.23 |
| Calcium (g/100 g) | 0.68 | 0.27 |
| Selenium (mg/kg) | 0.14 | <0.10 |
| Iron (mg/kg) | 208 | 100.10 |
Observation of general condition
The initial weights of groups A, B and C were, respectively, 67.2 ± 2.8 g, 68.3 ± 3.1 g and 67.9 ± 2.0 g. There was no statistically significant difference between groups (P > 0.05). In group A, there was no rat death, all rat’s had brightly coloured pelts; activities were usual, weight gain was fast with a weekly increase of 15–20 g, and the weight was 148.1 ± 19.2 g at the fourth week. In group B, weight gain was slow, with a weekly increase of 12–15 g, and the weight was 124.6 ± 12.2 g at the fourth week. In group C, weight was gained slowly, the weekly increase was 9–12 g, and the weight was 108.6 ± 10.2 g at the fourth week. At the fourth week, in groups B and C, each group had two rats that died. The weight gain was the greatest in group A (P < 0.05), group B was second (P < 0.05), and group C rats had the lowest weight gain (P < 0.05). The observation of general condition is presented in Table 3.
Table 3.
Changes of hair, activity, faeces and body weight in each group
| Week | Index of observation | Group A | Group B | Group C |
|---|---|---|---|---|
| First week | Hair lacklustre (n) | 0 | 0 | 0 |
| Less activity (n) | 0 | 0 | 0 | |
| Loose stools (n) | 0 | 0 | 0 | |
| Weight (g) | 82±5.4 | 81±4.2 | 79 ± 4.1 | |
| Second week | Hair lacklustre (n) | 0 | 0 | 0 |
| Less activity (n) | 0 | 0 | 1 | |
| Loose stools (n) | 0 | 0 | 1 | |
| Weight (g) | 99.8 ± 4.1 | 97 ± 5.2 | 94 ± 3.6* | |
| Fourth week | Hair lacklustre (n) | 0 | 5 | 6 |
| Less activity (n) | 0 | 1 | 4 | |
| Loose stools (n) | 0 | 1 | 1 | |
| Weight (g) | 148.1±19.2 | 124.6±12.2* | 108.6±10.2** |
Weight values given as mean ± standard deviation (SD)
*P < 0.05 vs group A,
** P < 0.05 vs group A and group B
Morphology of epiphyseal plate
At the first and second weeks, longitudinal sections of epiphyseal plate thickness showed no significant differences in each group. At the fourth week, group C had slightly uneven epiphyseal plate thickness, with slightly irregular shape. We did not find obvious epiphyseal plate deformation or disappearance.
Observation of epiphyseal plate histology
The observation of rat epiphyseal plate pathology is shown in Table 4 and Fig. 1. There was no epiphyseal plate chondrocyte necrosis in the resting zone, proliferative zone, or the hypertrophic zone in group A at the first, second, and fourth weeks. Epiphyseal cartilage cells in group A were rich, cell columns were neatly arranged in the proliferative zone, nuclei was deeply stained, there was clear cytoplasm, and the cell outlines were clear. Chondrocytes in the hypertrophic zone were arranged closely.
Table 4.
Results of rat epiphyseal plate pathohistology observation
| Groups | Week | Resting cell zone necrosis | Proliferative cell zone necrosis | Hypertrophic cell zone necrosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FN | LN | PN | ZN | FN | LN | PN | ZN | FN | LN | PN | ZN | ||
| A | First | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Second | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fourth | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| B | First | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Second | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | |
| Fourth | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | |
| C | First | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Second | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | |
| Fourth | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 3 | 0 | 0 | |
FN focus necrosis, LN lamellar necrosis, PN penetration necrosis, ZN zonal necrosis
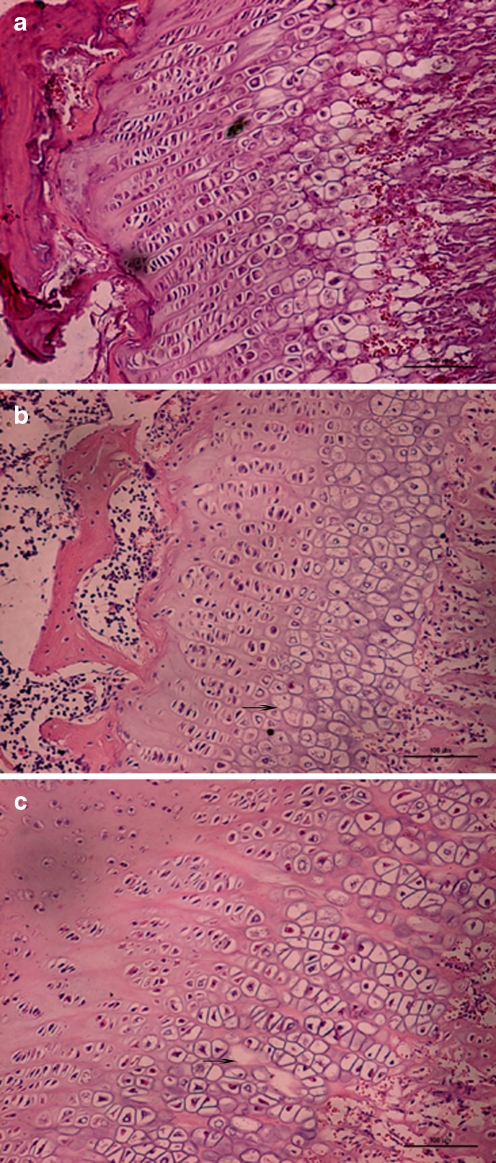
Fig. 1.
Epiphyseal plate showing cell necrosis (arrows) stained by haematoxylin and eosin (magnification ×200). a Normal control group (fourth week) rat epiphyseal cartilage cells, cell columns were arranged regularly, nucleus clear, transparent cytoplasm. b Normal feed + T-2 toxin group (fourth week), cell columns with disorders, mast cell layer condensation nuclei can be seen with pyknosis and lysis of nuclei, cells appear with vacuolated changes. c Low nutrition feed + T-2 toxin group (fourth week), cell columns with disorders, sparse, patchy cell-free zone appears. Lamellar necrosis in hypertrophic zone
There was no epiphyseal plate chondrocyte necrosis in group B in the first week. There were two cases out of ten of focal necrosis in hypertrophy in group B in the second week. There were five cases out of eight of focal necrosis in the fourth week. Arrangement of proliferative cell columns was irregular, cell columns presented shorter and sparser compared with that in the control group.
There was no epiphyseal plate chondrocyte necrosis in group C in the first week; there were four cases out of ten of focal necrosis in group C in the second week. At the fourth week, five cases showed lamellar necrosis in the hypertrophic or proliferative zones. Three cases showed only focal necrosis. Arrangement of proliferative cell columns was irregular, cell columns showed varying lengths and were sparse. The positive rate of cell necrosis was not significantly higher than that in group B (P > 0.05). The positive rate of lamellar necrosis was 62.5%, which was significantly higher than that in groups B or A (P <0.01). Metaphyseal trabecular bone arranged sparsely, with disorder in group C at the fourth week.
A cartilage matrix fills in the gap between proliferative cell columns, which is the longitudinal arrangement of collagen fibres, and Masson staining of collagen was blue. In group A the dyeing was deep. In groups B and C, the dyeing was pale, was relatively sparse, and collagen staining disappeared in the necrosis zone. T-2 toxin may affect the epiphyseal cartilage cell synthesis, secretion of collagen components. The observation results are shown in Table 5.
Table 5.
Epiphyseal plate histology Masson staining observation in each group
| Group | First week | Second week | Fourth week |
|---|---|---|---|
| A | Grade III, 10 cases | Grade III, 10 cases | Grade III, 10 cases |
| B | Grade III, 10 cases | Grade III, 4 cases | Grade II, 8 cases |
| Grade III, 6 cases | |||
| C | Grade III, 10 cases | Grade III, 3 cases | Grade III, 5 cases |
| Grade II, 7 cases | Grade I, 3 case |
Discussion
In order to classify the aetiology of KBD, many investigations have been performed from different perspectives. Epidemiological studies showed that the risk factors for KBD included low socio-economic status, trace element deficiency, fungal contamination, low dietary antioxidants, protein-calorie malnutrition, physical environment (microtrauma and cold) and other factors [18, 19]. However, no single factor could explain the pathogenesis of KBD. Multifactorial aetiology should be considered in the study of KBD [18]. Although it is not known whether animals living in KBD endemic regions suffer from this disease, many authors have tried to reveal the mechanism of KBD through animal experiments. Alteration of the growth plate in a KBD animal model can be reproduced but, up to now, the macroscopic deformation of the bone and joint cannot. Yang et al. used Fusarium-contaminated grain to feed chickens; in the fifth week, the proliferative and transition zone cells appeared with large-area cell necrosis. This type of organisation was similar to the changes of cartilage deep necrosis in KBD, and Yang et al. believe the T-2 toxin is the aetiology of KBD [20], but this view is not widely been accepted. Xiong found T-2 toxin can induce the articular cartilage of deep zonal necrosis or patchy necrosis in miniature pigs, but the epiphyseal plate showed lesser damage [12]. There was no consensus about the result when T-2 toxin was used alone in an animal experiment.
In this study, we found T-2 toxin retarded the growth of rats. The combination of T-2 toxin and low nutrition diet had a more serious effect. At the fourth week, the epiphyseal plate revealed different necrosis changes in the experimental groups; the T-2 toxin combined with low nutrition diet group showed lamellar necrosis in the hypertrophic and proliferative zones. Furthermore, two experimental groups showed short cell columns, which were sparse and interrupted in the proliferative zone. As a mechanism in the pathogenesis, T-2 toxin or its metabolites are believed to enhance the production of oxygen radicals to such an extent exceeding physiological limits that the normal body radical scavengers are overwhelmed [21]. Excessive oxygen radicals may stimulate lipid peroxidation, causing damage to cell membranes and DNA, resulting in cell injury [22, 23]. Moreover, T-2 toxin can bind to the eukaryotic 60 S ribosomal subunit causing inhibition of protein, DNA and RNA synthesis [24]. Through the metaphyseal rich, open capillary network, T-2 toxin can inhibit the proliferation of cartilage cells, and reduce synthesis of proteoglycan and collagen [22, 23]. In a normal diet, some antioxidant materials, including vitamin C, vitamin E, and selenium can inhibit or relieve cytotoxicity of T-2 toxin [25, 26]. Lack of these nutritional elements will cause the cell to be injured easily. On the other hand, low-protein diet will lead to cell division, proliferation and self-repair capacity decline.
Our results confirmed that T-2 toxin could induce chondrocytes necrosis, reducing the content of collagen in the growth plate. This effect is relatively slight in a normal diet. Low nutrition aggravates the damage of chondrocytes, and disturb metaphyseal trabecular bone formation. The damage caused by T-2 toxin may occur in various organs and tissues such as spleen, bone marrow, thymus, kidney. In our study, we only observed the performance of rat epiphyseal plates and metaphysis; the time of observation was relatively short. The further development of these lesions and biochemical changes and the relationship with Kashin-Beck disease needs further study. Multinutritional elements, which play a crucial role in the lesions of epiphyseal plate and metaphysis, will be studied in our next experiment.
Acknowledgements
The authors wish to express their gratitude to Dr Xiu-qun Li and other teachers from the Tissue Engineering Lab, West China Hospital, Sichuan University, for providing experimental sites and technical assistance.
Footnotes
A key project in The Eleventh Five-Year National Science and Technology Pillar Program, P.R. China (2007BA125B04).
The first two authors contributed equally to this work.
Contributor Information
Yun-fen Yao, Email: bb_yaoyunfeng@126.com.
Fu-xing Pei, Email: Peifuxing@VIP.163.com.
References
- 1.Pasteels JL, Liu FD, Hinsenkamp M, Rooze M, Mathieu F, Perlmutter N. Histology of Kashin-Beck lesions. Int Orthop. 2001;25(3):151–153. doi: 10.1007/s002640000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mo DX. Pathology and selenium deficiency in Kashin-Beck Disease. In: Combs GF, Spallholz JE, Levander OA, Oldfield JE, editors. Selenium in biology and medicine, 3rd symposium. New York: Van Nostrand Reinhold; 1987. pp. 924–933. [Google Scholar]
- 3.Xiong G. Diagnostic, clinical and radiological characteristics of Kashin-Beck disease in Shaanxi Province, PR China. Int Orthop. 2001;25:147–150. doi: 10.1007/s002640100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasseur C, Suetens C, Michel V, Mathieu F, Begaux F, Nolard N, Haubruge E. A 4-year study of the mycological aspects of Kashin-Beck disease in Tibet. Int Orthop. 2001;25:154–158. doi: 10.1007/s002640000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai FY, Chen YD, Chen QT. Investigation on the contamination fungi of grain in Tian-Shui Kashin-Beck disease areas. Chin J Cont Endem Dis. 1990;5:33–34. [Google Scholar]
- 6.Haubruge E, Chasseur C, Debouck C, Begaux F, Suetens C, Mathieu F, Michel V, Gaspar C, Rooze M, Hinsenkamp M, Gillet P, Nollard N, Lognay G. The prevalence of mycotoxins in Kashin-Beck disease. Int Orthop. 2001;25(3):159–162. doi: 10.1007/s002640100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno-Reyes R, et al. Kashin-Beck osteoarthropathy in rural Tibet in relation to selenium and iodine status. N Engl J Med. 1998;3339:1112–1120. doi: 10.1056/NEJM199810153391604. [DOI] [PubMed] [Google Scholar]
- 8.Zhang WH, Neve J, Xu JP, Vanderpas J, Wang ZL. Selenium, iodine and fungal contamination in Yulin District (People’s Republic of China) endemic for Kashin-Beck disease. Int Orthop. 2001;25:188–190. doi: 10.1007/s002640100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grange M, Mathieu F, Begaux F, Durand MC. Kashin-Beck disease and drinking water in Central Tibet. Int Orthop. 2001;25(3):167–169. doi: 10.1007/s002640000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Niu C, Bodo M, Gabriel E, Notohm H, Wolf E, Muller PK. Fulvic acid supplementation and selenium deficiency disturb the structural integrity of mouse skeletal tissue. Biochem J. 1993;289:829–883. doi: 10.1042/bj2890829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Y, Yoshizawa T, Yang J-S, Zhang S-Y, Zhang B-J. A survey of occurrence of Fusarium mycotoxins in corn and wheat samples from Shanxi and Shanxi Provinces, China. Mycotoxin Res. 1992;8:85–91. doi: 10.1007/BF03192221. [DOI] [PubMed] [Google Scholar]
- 12.Xiong Yongmin. Toxic effect of T-2 toxin on articular cartilage in Chinese experimental mini-pig. Chin J Endem Dis. 1997;12:4–6. [Google Scholar]
- 13.Chasseur C, Suetens C, Nolard N, Begaux F, Haubruge E. Fungal contamination in barley and Kashin-Beck disease in Tibet. Lancet. 1997;350:1074. doi: 10.1016/S0140-6736(05)70453-0. [DOI] [PubMed] [Google Scholar]
- 14.Wu YH, Andreas L, Jin ZD, Wang SM, Gerhard HS, Richard WB, Xia WL. Holocene climate development on the central Tibetan Plateau: A sedimentary record from Cuoe Lake. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;234:328–340. doi: 10.1016/j.palaeo.2005.09.017. [DOI] [Google Scholar]
- 15.Bai A, Zhai P, Liu X. Climatology and trends of wet spells in China. Theor Appl Climatol. 2007;88:139–148. doi: 10.1007/s00704-006-0235-7. [DOI] [Google Scholar]
- 16.Dang S, Yan H, Yamamoto S, Wang X, Zeng L. Poor nutritional status of younger Tibetan children living at high altitudes. Eur J Clin Nutr. 2004;58:938–946. doi: 10.1038/sj.ejcn.1601915. [DOI] [PubMed] [Google Scholar]
- 17.Mo DX. KBD cartilage necrosis histopathology and clinical significance. Chin J Endemicol. 1982;1(2):116–120. [Google Scholar]
- 18.Hinsenkamp M, Mathieu F, Claus W, Collard F, Maertelaer V. Effects of physical environment on the evolution of Kashin-Beck disease in Tibet. Int Orthop. 2009;33:1085–1088. doi: 10.1007/s00264-009-0738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suetens C, Moreno-Reyes R, Chasseur C, Mathieu F, Begaux F, Haubruge E, Durand MC, Nève J, Vanderpas J. Epidemiological support for a multifactorial aetiology of Kashin-Beck disease in Tibet. Int Orthop. 2001;25(3):180–187. doi: 10.1007/s002640100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JB, Sun DJ, Wang G. The observation on the culture of Fusarium isolated from the cereal produced in KBD areas causing cartilage necrosis of growth plate in broiler chickens. Chin J End Dis Prev. 1993;8(6):325–326. [Google Scholar]
- 21.Rizzo A, Atroshi R, Hirvi T, et al. Protective effect of antioxidants against free radical–mediated lipid peroxidation induced by DON or T-2 toxin. J Vet Med. 1994;A41:81–90. doi: 10.1111/j.1439-0442.1994.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 22.Vila B, Jaradat ZV, Marquardt RR, Frohlich AA. Effect of T-2 toxin on in vivo lipid peroxidation and vitamin E status in mice. Food Chem Toxicol. 2002;40:479–486. doi: 10.1016/S0278-6915(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 23.Minervini F, Fornelli F, Lucivero G, Romano C, Visconti A. T-2 toxin immunotoxicity on human B and T lymphoid cell lines. Toxicology. 2005;210:81–91. doi: 10.1016/j.tox.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Eriksen GS, Pettersson H. Toxicological evaluation of trichothecenes in animal feed. Anim Feed Sci Technol. 2004;114:205–239. doi: 10.1016/j.anifeedsci.2003.08.008. [DOI] [Google Scholar]
- 25.Shokri F, Heidari M, Gharagozloo S, Ghazi Khansari M. In vitro inhibitory effects of antioxidants on cytotoxicity of T-2 toxin. Toxicology. 2000;146:171–176. doi: 10.1016/S0300-483X(00)00172-4. [DOI] [PubMed] [Google Scholar]
- 26.Jaradat ZW, Borja V, Marquardt RR. Adverse effects of T-2 toxin on chicken lymphocytes blastogenesis and its protection with vitamin E. Toxicology. 2006;225:90–96. doi: 10.1016/j.tox.2006.05.005. [DOI] [PubMed] [Google Scholar]