Abstract
In a prospective clinical study, 59 patients with anteromedial osteoarthritis of the knee (61 knee joints) underwent minimally invasive medial Oxford unicompartmental arthroplasty phase 3. Clinical and radiographic examinations of 56 knees were carried out at five (4–7) years. American Knee Society (AKS) scores improved from mean 45.5 (20–80) points (knee score) and 55 (15–100) points (function score) before surgery to 90 (30–100) points in both scores after surgery. The position of each implant was determined on screened radiographs using an image intensifier. The implant position was analysed according to the Oxford X-ray rating system. We evaluated nine measures, and there was no detectable correlation between implant position and clinical result. However, long-term studies are needed before it is possible to elaborate an evidence-based guideline on positioning.
Introduction
The Oxford medial unicompartmental knee replacement (Oxford® Unicompartmental Knee, Biomet, Bridgend, UK) has been used successfully for more than two decades to treat anteromedial arthritis of the knee [19]. Since 1998, the phase 3 instrumentation allows for a minimally invasive surgical approach. The advantages of this procedure are less postoperative pain, faster recovery, and better joint mobility in the long term [1, 18, 22].
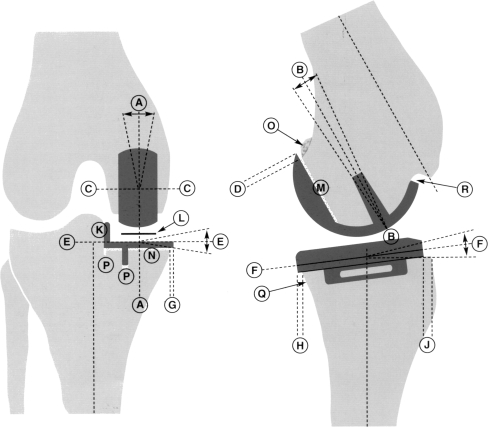
Despite the advanced instrumentation, medial Oxford unicompartmental knee arthroplasty (UKA) is still a demanding procedure with a substantial learning curve [5, 25, 31]. One essential surgical objective is accurate positioning of the components according to the guidelines proposed by the Oxford group (Fig. 1). A tolerance within defined limits is accepted due to the fully congruent mobile bearing design. Nonetheless, some authors have shown that a substantial proportion of implants put in even by experienced surgeons can be found to be outside the proposed limits of tolerance [16, 27]. We therefore performed a clinical and radiographic study to determine whether a deviant position of the implant had a negative influence on the postoperative clinical result.
Fig. 1.
Radiograph analysis in the Oxford Unicompartmental Knee Replacement Phase 3 Operating Manual
Materials and methods
In a prospective clinical and radiological study, 59 consecutive patients (61 knee joints) with anteromedial osteoarthritis of the knee joint were included. Patients were selected strictly according to the criteria proposed by the Oxford group [18]. Cemented Oxford UKA was performed by eight surgeons in 61 knees through a minimally invasive incision between September 2001 and August 2004. The mean follow-up was five (4–7) years. The American Knee Society (AKS) Score [8] was used for clinical evaluation pre- and postoperatively. The AKS developed a total knee rating system consisting of the three main parameters of pain, stability, and range of motion (ROM) [8]. One hundred points can be obtained by a well-aligned knee with no pain, 125° of motion, and negligible anteroposterior and mediolateral instability. Fifty points are allotted for pain, 25 for stability, and 25 for ROM. This rating system was used as it is simple but more exacting and more objective than other knee scores [8].
Patient-related information was gathered with the aid of a standardised questionnaire administered before surgery and at the time of the follow-up, which was completed before the clinical and radiological examination. Only screened radiographs were obtained in a standardised manner by one examiner using an image intensifier. The knee joint was manually positioned so that in the anteroposterior plane, the tibial prosthesis was precisely parallel to the X-ray beam, whereas for the lateral view, the femoral condyles were positioned exactly superimposed to each other. Positioning of the implants was evaluated as specified for radiographic analysis in the Oxford Unicompartmental Knee Replacement Phase 3 Operating Manual (Fig. 1). Each measurement was performed twice by two independent observers.
To evaluate the correlation between the positioning parameters and the clinical result, the AKS scores of joints that were within the accepted tolerance were compared with those that were not. To this end, the AKS scores of both groups were displayed in box plots showing the median, Q1, Q3, and whiskers defined by the 95th percentiles. In addition, the groups were compared statistically by the exact Mann−Whitney U test. A p value <0.05 was considered statistically significant, though it cannot be interpreted in a confirmatory sense due to multiple testing. The analysis was done using the statistical software SAS, Version 9.13 for Windows (Cary, NC, USA).
Results
The mean age of the 59 patients (30 women, 29 men) was 63 (45–78) years at the time of surgery. Mean body mass index (BMI) was 29 (20–42). Two of the 61 prostheses (59 patients) included in the study were revised: one for loosening of the tibial component and one for persistent pain. Three patients were not able to attend the follow-up examination due to distance. They were contacted by telephone and asked about the function of their arthroplasty with reference to the patient questionnaire. All three were satisfied with the results of the surgery and implant function. The remaining 56 joints were available for clinical and radiographic review. The mean follow-up period was five (4–7) years. The ROM increased from mean 115° (80–150) before surgery to 130° (100–155) after the operation. The AKS score (Fig. 2) improved from mean 45.5 (20–80) points for the knee score and 55 (15–100) points for the function score before surgery to 90 (30–100) points for each postoperatively. The tolerances of femoral and tibial components are shown in Table. 1.
Fig. 2.
Overall postoperative American Knee Society (AKS) score
Table 1.
Positioning of femoral and tibial component
| Feature | Outside tolerance (%) | Inside tolerance (%) | p value |
|---|---|---|---|
| Femoral component | |||
| Valgus/varus | 2 (4%) | 54 (96%) | 0.0591 |
| Extension/flexion | 18 (32%) | 38 (68%) | 0.9164 |
| Lateral/medial | 40 (71%) | 16 (29%) | 0.9856 |
| Posterior overhang | 19 (34%) | 37 (66%) | 0.2110 |
| Tibial component | |||
| Valgus/varus | 1 (2%) | 55 (98%) | 0.4464 |
| Slope | 7 (12%) | 49 (88%) | 0.9901 |
| Medial overhang | 25 (45%) | 31 (55%) | 0.6814 |
| Posterior overhang | 43 (77%) | 13 (23%) | 0.1303 |
| Anterior overhang | 22 (39%) | 34 (61%) | 0.2981 |
The intraobserver reliability showed a correlation with r = 0.952 (p = 0.001). The interobserver reliability was slightly higher, with a correlation coefficient of r = 0.973 (p = 0.001).
Positioning of femoral implants (Table 1)
Varus/valgus alignment (author guidelines: <10° varus to <10° valgus)
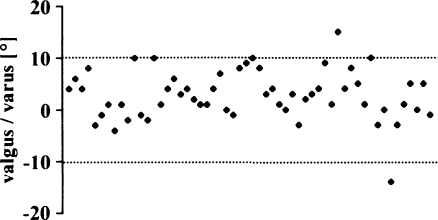
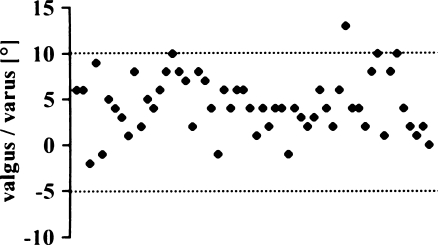
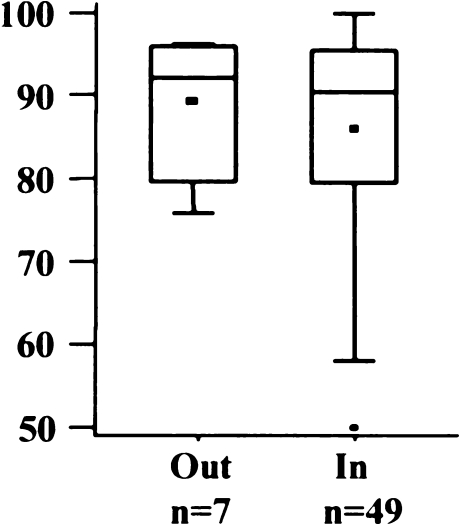
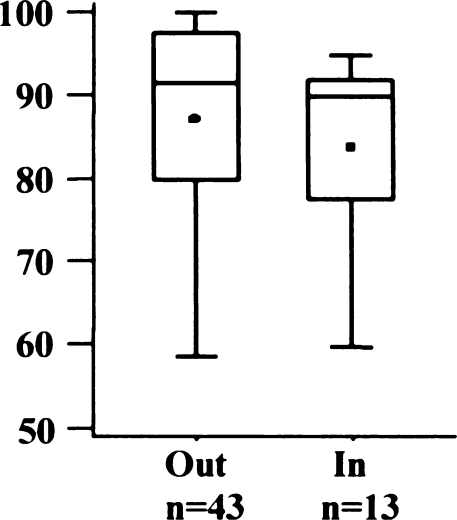
Only two implants were outside the proposed tolerance of 10° varus to 10° valgus (Fig. 3). Most implants were positioned in a slight varus [mean 2.8°; standard deviation (SD) 4.7°, range 14° valgus to 15° varus]. The AKS scores of the two knees outside the tolerance were inferior to the within-tolerance group (Fig. 4), but the difference was not statistically significant (p = 0.0591).
Flexion/extension angle (< 5° flexion to < 5° extension)
Fig. 3.
Varus/valgus alignment of femoral implant
Fig. 4.
American Knee Society (AKS) score regarding varus/valgus alignment of femoral implant
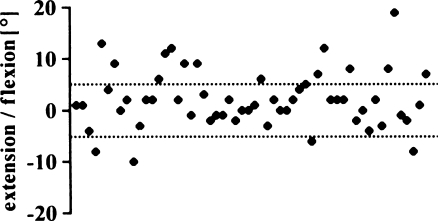
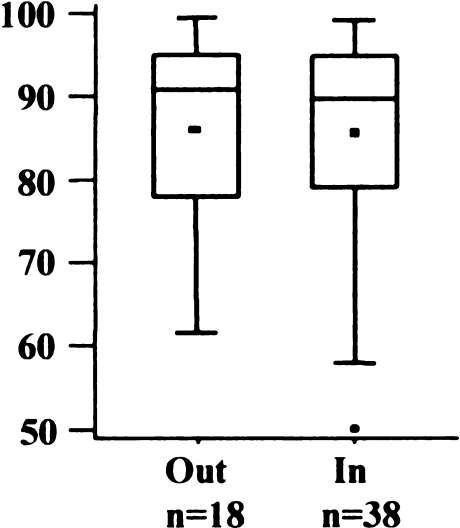
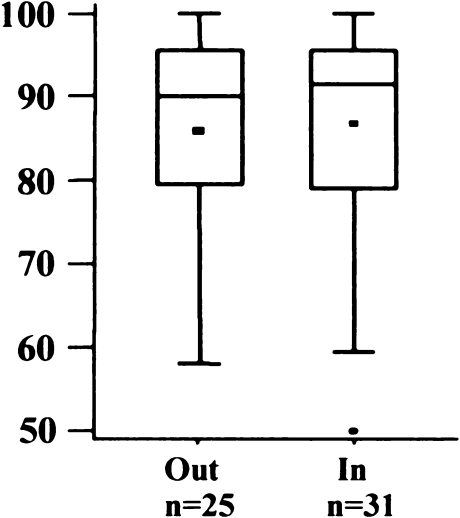
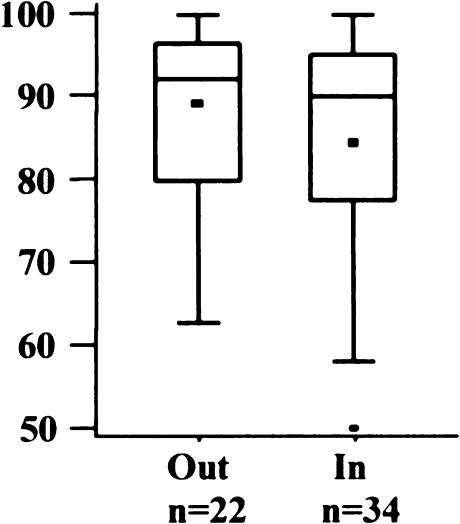
Eighteen knees (32%) were outside the recommended tolerance of 5° flexion to 5° extension (Fig. 5). The mean was 2.1° flexion (SD 5.5°; range 10° extension to 19° flexion). There was no significant difference between the AKS scores of both groups (Fig. 6) (p = 0.9164).
Medial/lateral position (position centrally)
Fig. 5.
Flexion/extension angle of femoral implant
Fig. 6.
American Knee Society (AKS) score regarding flexion/extension angle of femoral implant
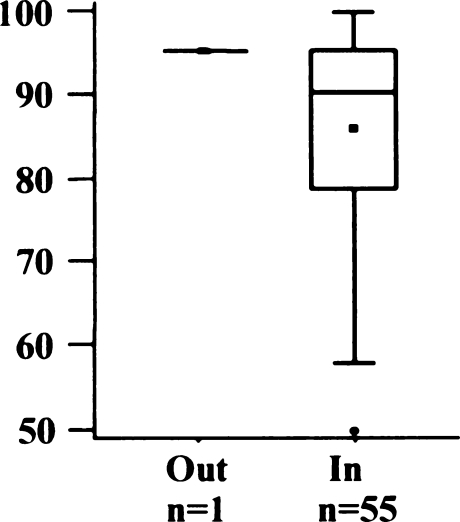
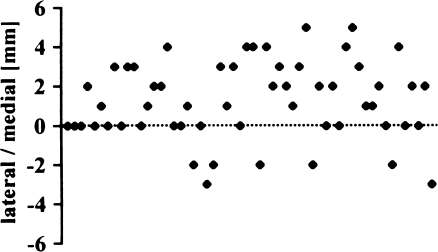
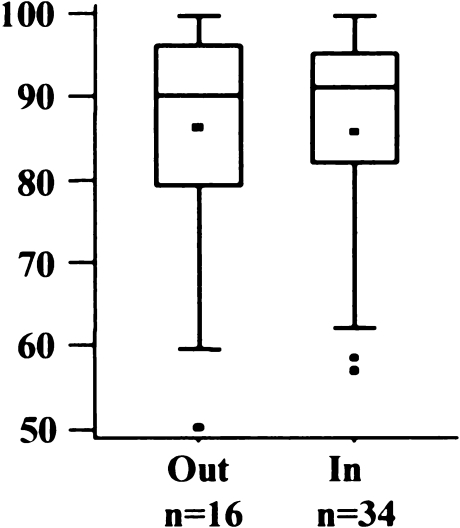
Only 16 knees (29%) were exactly centred in the medial/lateral position (Fig. 7). Most implants were placed slightly medial, with a mean of 1.2 mm (SD 2.0 mm; range 3 mm lateral to 5 mm medial). AKS scores did not differ in the two groups (Fig. 8) (p = 0.9856).
Posterior overhang (exact fit to <2 mm overhang)
Fig. 7.
Medial/lateral position of femoral implant
Fig. 8.
American Knee Society (AKS) score regarding medial/lateral position of femoral implant
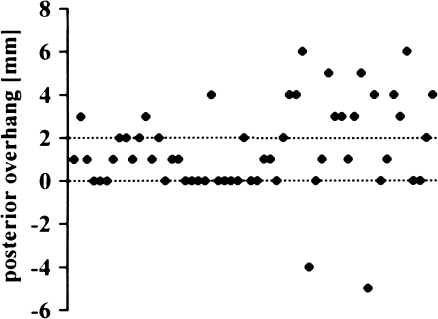
Sixty-six percent of the implants (n = 37) were within the tolerance of 0 −2 mm posterior overhang (Fig. 9) (mean 1.2 mm; SD 2.3 mm; range –5 to 6 mm). Two implants were too short; 17 knees had an overhang of 3 mm or more. The AKS scores did not differ between the two groups (Fig. 10) (p = 0.2110).
Fig. 9.
Posterior overhang of femoral implant
Fig. 10.
American Knee Scoiety (AKS) score regarding posterior overhang of femoral implant
Positioning of tibial implants (Table 1)
Varus/valgus alignment (<10° varus to <5° valgus)
In all but one case, the recommended position of 5° valgus to 10° varus was attained (Fig. 11). The mean position was 4.4° varus (SD 3.2°; range 2° valgus to 13° varus).
Slope (+7° to −5°)
Fig. 11.
Varus/valgus alignment of tibial implant
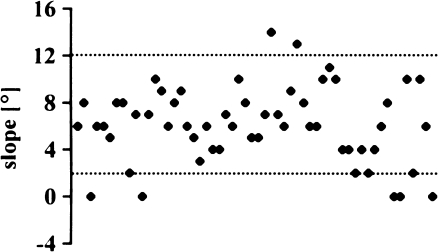
Eighty-eight percent (49 knees) were within the proposed slope of 2° to 12° (Fig. 12); for all knees, the mean was 6.1° (SD 3.2°; range 0° to 14°). The AKS scores of the seven knees outside the tolerance did not differ (Fig. 13) (p = 0.9901).
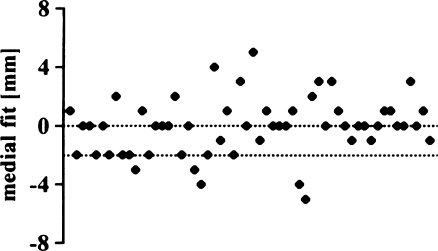
Medial overhang (exact fit to <2 mm overhang)
Fig. 12.
Slope of tibial implant
Fig. 13.
American Knee Society (AKS) score regarding slope of tibial implant
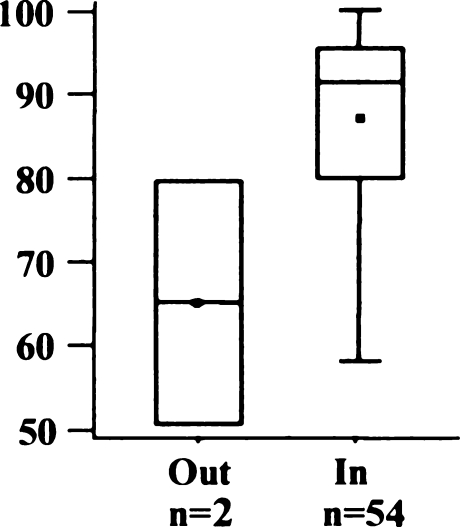
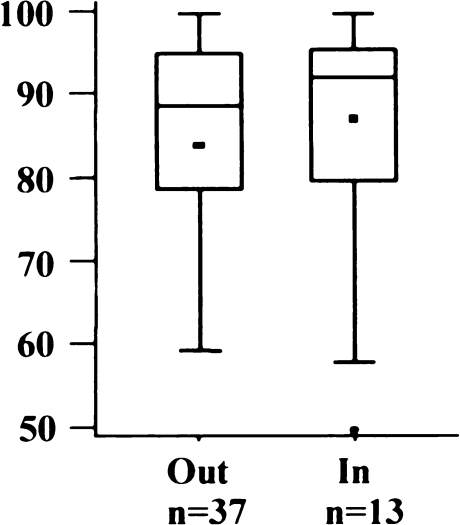
The proposed medial fit (0 to 2 mm) was achieved in 31 knees (55%) (Fig. 14). Six implants were overhanging 3 mm or more, 14 prostheses were at least 2 mm too short. Mean medial fit was –0.1 mm (SD 2.0 mm; range –5 to 5 mm). Differences between AKS-Scores were not significant in both groups (Fig. 15) (p = 0.6814).
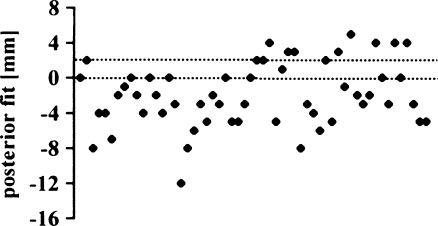
Posterior overhang (exact fit to <2 mm overhang)
Fig. 14.
Medial overhang of tibial implant
Fig. 15.
American Knee Society (AKS) score regarding medial overhang of tibial implant
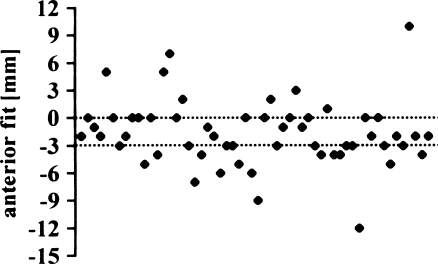
The posterior fit appeared to be the least reliable measure in our series (Fig. 16). Only 23% of the tibial components (13 knees) were within the recommended tolerance of 0−2 mm overhang (mean –2.0 mm; SD 3.6 mm; range −12 to 5 mm). AKS scores were even better in the group outside the proposed tolerance (Fig. 17), but there was no significant difference (p = 0.1303).
Anterior overhang (exact fit to <3 mm shorter)
Fig. 16.
Posterior overhang of tibial implant
Fig. 17.
American Knee Society (AKS) score regarding posterior overhang of tibial implant
In this series, 34 knees (61%) were within the tolerance (Fig. 18); mean underhang was 1.7 mm (SD 3.6 mm; range 12 mm underhang to 10 mm overhang). AKS scores were slightly better for knees out of the tolerance (Fig. 19) but were not statistically significant (p = 0.2981).
Fig. 18.
Anterior overhang of tibial implant
Fig. 19.
American Knee Society (AKS) score regarding anterior overhang of tibial implant
Discussion
The success of total knee arthroplasty is based on a variety of factors. Perfect component alignment is one of the most important determinants of good clinical outcome and longevity of the device [3, 4, 11, 14, 21, 26, 29], whereas malalignment of >3° in the anteroposterior direction results in high failure rates, loosening, and revision [3]. The same mechanism seems to apply for fixed-bearing UKA [10, 12, 30]. In Oxford UKA, the fully congruent design with a mobile bearing apparently is less susceptible to malpositioning, and a wider variation of implant positions is acceptable [7, 17, 18, 20]. Apart from implant design, this is mainly due to the fact that the Oxford UKA is based on ligament balance, and the limb alignment is realigned to the predisease state. A range of accepted implant positions has been proposed by the designers of the implant [17, 18, 20]. Positions within these limits should not affect the clinical outcome or the longevity of the arthroplasty [19, 20].
Clinical results obtained in our consecutive series of 56 minimally invasive implantations of Oxford medial unicompartmental knee replacements were good (mean AKS 90 points). Other independent centres reported slightly inferior scores (AKS 71−91 points) [9, 13, 23]. However, our results are not quite on a par with the excellent results in the designer series studied in Oxford [20, 24]. This might be due to different patient populations and other factors, such as patient selection and surgical experience with this specific implant [25].
In our series, the radiographic study of implant positioning showed that a considerable proportion of implants was not within the recommended limits for several positioning criteria. However, we found no correlation of clinical results and implant positioning between the two groups in our series. Values outside the recommended limits therefore do not necessarily seem to be associated with inferior function in the short term. Nonetheless, we should discuss several positioning items in closer detail: The recommended varus/valgus position of the femoral component was achieved with the phase 3 instrumentation in almost all cases (96%); only two implants were found to be outside the defined range of tolerance. This factor did not influence the clinical result, as the femoral component was still centred over the tibial implant as a result of the fully congruent implant design. The flexion/extension angle of the femoral component seems to cause no problems. However, care should be taken that sufficient bone is removed from the anterior and posterior aspect of the femur during the operation to avoid any impingement of the insert in full extension and deep flexion. An essential point is the medial position of the femoral component of the prosthesis. A deviation can cause the inlay to slide medially, irritating the soft tissues and eventually causing spinning and dislocation.
Posterior overhang of the femoral implant seems to be forgiving, as in 17 of our patients, an overhang of 3 mm and more could be observed without clinical relevance. In case of undersizing or retained osteophytes, the femoral component is too short and bone or cartilage is standing proud (Fig. 20). This may result in restricted flexion, as posterior impingement of the meniscus may occur at about 110° of flexion. In two patients, bone stood proud to the femoral component posteriorly, but neither patient had restricted flexion. One complained of pain in maximum flexion, the other had no symptoms at all and was very well satisfied with the outcome of the operation. However, overhang of bone and cartilage may lead to increased wear of the polyethylene inlay in the long term.
Fig. 20.
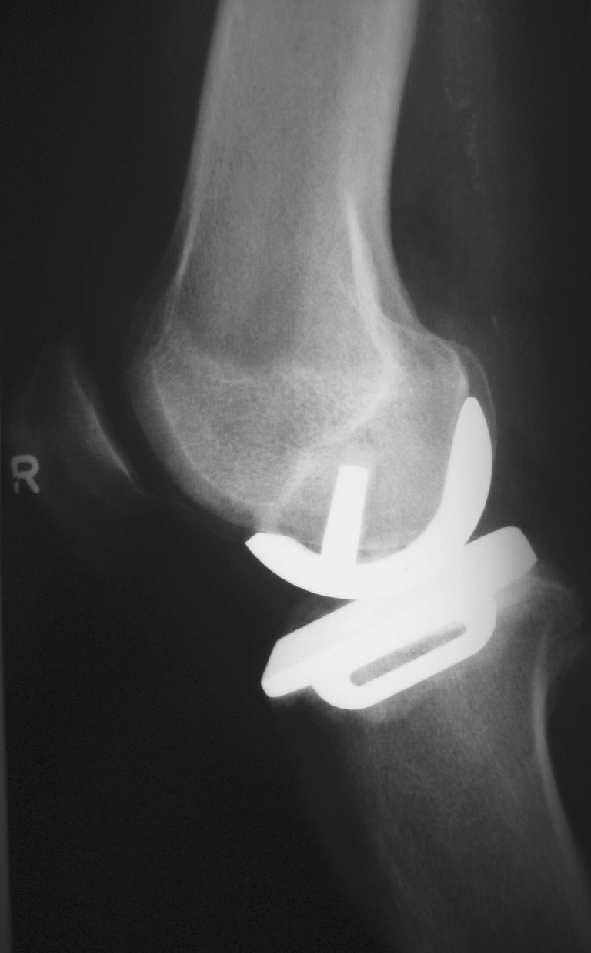
Undersizing of the femoral component
Positioning of the tibial implant involves pitfalls, but our data show that there are less sources of error in comparison to the femoral implant. Nonetheless, it seems essential to set the slope precisely, as this is key for normal kinematics of the joint with intact ligaments. Major deviations may influence ligament tension throughout the ROM and may lead to limited ROM or damaged ligaments. Although we found a considerable rate of implant positions outside the proposed tolerance, we found no correlation between implant position and clinical result in terms of the AKS score. However, this finding applies only to deviations reported in our patient population after a relatively short follow-up period of four to seven years. In the long term, deviant implant positions (i.e., if impingement occurs) may lead to increased polyethylene wear and may result in implant failure [6, 15, 27, 28, 32, 33]. Therefore, long-term studies are necessary to define evidence-based tolerance limits for implant positions. More sophisticated image guidance systems might help to improve implant positioning in the future [2].
References
- 1.Aldinger PR, Clarius M, Murray DW, et al. Medial unicompartmental knee replacement using the “Oxford Uni” meniscal bearing knee. Orthopade. 2004;33(11):1277–1283. doi: 10.1007/s00132-004-0712-6. [DOI] [PubMed] [Google Scholar]
- 2.Aldinger PR, Gill HS, Schlegel U, et al. Is computer navigation a useful tool in unicompartmental knee arthroplasty? A pilot cadaver study. Orthopade. 2005;34(11):1096–1102. doi: 10.1007/s00132-005-0883-9. [DOI] [PubMed] [Google Scholar]
- 3.Bargren JH, Blaha JD, Freeman MA. Alignment in total knee arthroplasty. Correlated biomechanical and clinical observations. Clin Orthop Relat Res. 1983;173:178–183. [PubMed] [Google Scholar]
- 4.Benjamin J. Component alignment in total knee arthroplasty. Instr Course Lect. 2006;55:405–412. [PubMed] [Google Scholar]
- 5.Aufdenkampe AK, Arscott DB, Clarius M, Hauck C, Seeger JB, et al. Pulsed lavage reduces the incidence of radiolucent lines under the tibial tray of Oxford unicompartmental knee arthroplasty : Pulsed lavage versus syringe lavage. Int Orthop. 2009 doi: 10.1007/s00264-009-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher N, Agarwal M, Reuben SF, et al. Sporting and physical activity following Oxford medial unicompartmental knee arthroplasty. Knee. 2006;13(4):296–300. doi: 10.1016/j.knee.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Goodfellow J, O’Connor J, Murray DW. The Oxford meniscal unicompartmental knee. J Knee Surg. 2002;15(4):240–246. [PubMed] [Google Scholar]
- 8.Insall JN, Dorr LD, Scott RD, et al. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 9.Jahromi I, Walton NP, Dobson PJ, et al. Patient-perceived outcome measures following unicompartmental knee arthroplasty with mini-incision. Int Orthop. 2004;28(5):286–289. doi: 10.1007/s00264-004-0573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeer PJ, Keene GC, Gill P. Unicompartmental knee arthroplasty: an intermediate report of survivorship after the introduction of a new system with analysis of failures. Knee. 2004;11(5):369–374. doi: 10.1016/j.knee.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Jeffcote B, Shakespeare D. Varus/valgus alignment of the tibial component in total knee arthroplasty. Knee. 2003;10(3):243–247. doi: 10.1016/S0968-0160(02)00101-1. [DOI] [PubMed] [Google Scholar]
- 12.Kasodekar VB, Yeo SJ, Othman S. Clinical outcome of unicompartmental knee arthroplasty and influence of alignment on prosthesis survival rate. Singapore Med J. 2006;47(9):796–802. [PubMed] [Google Scholar]
- 13.Keys GW, Ul-Abiddin Z, Toh EM. Analysis of first forty Oxford medial unicompartmental knee replacement from a small district hospital in UK. Knee. 2004;11(5):375–377. doi: 10.1016/j.knee.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Lam LO, Shakespeare D. Varus/valgus alignment of the femoral component in total knee arthroplasty. Knee. 2003;10(3):237–241. doi: 10.1016/S0968-0160(02)00098-4. [DOI] [PubMed] [Google Scholar]
- 15.Luscombe KL, Lim J, Jones PW, et al. Minimally invasive Oxford medial unicompartmental knee arthroplasty: a note of caution! Int Orthop. 2006;3183:321–324. doi: 10.1007/s00264-006-0202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller PE, Pellengahr C, Witt M, et al. Influence of minimally invasive surgery on implant positioning and the functional outcome for medial unicompartmental knee arthroplasty. J Arthroplasty. 2004;19(3):296–301. doi: 10.1016/j.arth.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Murray DW. Mobile bearing unicompartmental knee replacement. Orthopedics. 2005;28(9):985–987. doi: 10.3928/0147-7447-20050901-35. [DOI] [PubMed] [Google Scholar]
- 18.Murray DW. Unicompartmental knee replacement: now or never? Orthopedics. 2000;23(9):979–980. doi: 10.3928/0147-7447-20000901-29. [DOI] [PubMed] [Google Scholar]
- 19.Murray DW, Goodfellow JW, O'Connor JJ. The Oxford medial unicompartmental arthroplasty: a ten-year survival study. J Bone Joint Surg Br. 1998;80(6):983–989. doi: 10.1302/0301-620X.80B6.8177. [DOI] [PubMed] [Google Scholar]
- 20.Pandit H, Jenkins C, Barker K, et al. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br. 2006;88(1):54–60. doi: 10.1302/0301-620X.88B1.17114. [DOI] [PubMed] [Google Scholar]
- 21.Petersen TL, Engh GA. Radiographic assessment of knee alignment after total knee arthroplasty. J Arthroplasty. 1988;3(1):67–72. doi: 10.1016/S0883-5403(88)80054-8. [DOI] [PubMed] [Google Scholar]
- 22.Price AJ, Webb J, Topf H, et al. Rapid recovery after oxford unicompartmental arthroplasty through a short incision. J Arthroplasty. 2001;16(8):970–976. doi: 10.1054/arth.2001.25552. [DOI] [PubMed] [Google Scholar]
- 23.Rajasekhar C, Das S, Smith A. Unicompartmental knee arthroplasty. 2- to 12-year results in a community hospital. J Bone Joint Surg Br. 2004;86(7):983–985. doi: 10.1302/0301-620X.86B7.15157. [DOI] [PubMed] [Google Scholar]
- 24.Rees JL, Price AJ, Beard DJ, et al. Minimally invasive Oxford unicompartmental knee arthroplasty: functional results at 1 year and the effect of surgical inexperience. Knee. 2004;11(5):363–367. doi: 10.1016/j.knee.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Robertsson O, Knutson K, Lewold S, et al. The routine of surgical management reduces failure after unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2001;83(1):45–49. doi: 10.1302/0301-620X.83B1.10871. [DOI] [PubMed] [Google Scholar]
- 26.Saragaglia D, Estour G, Nemer C, et al. Revision of 33 unicompartmental knee prostheses using total knee arthroplasty: strategy and results. Int Orthop. 2009;33(4):969–974. doi: 10.1007/s00264-008-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shakespeare D, Ledger M, Kinzel V. Accuracy of implantation of components in the Oxford knee using the minimally invasive approach. Knee. 2005;12(6):405–409. doi: 10.1016/j.knee.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Shakespeare D, Ledger M, Kinzel V. The influence of the tibial sagittal cut on component position in the Oxford knee. Knee. 2005;12(3):169–176. doi: 10.1016/j.knee.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Smith JL, Jr, Tullos HS, Davidson JP. Alignment of total knee arthroplasty. J Arthroplasty. 1989;4(Suppl):S55–S61. doi: 10.1016/S0883-5403(89)80008-7. [DOI] [PubMed] [Google Scholar]
- 30.Squire MW, Callaghan JJ, Goetz DD, et al. Unicompartmental knee replacement. A minimum 15 year followup study. Clin Orthop Relat Res. 1999;367:61–72. doi: 10.1097/00003086-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Svard UC, Price AJ. Oxford medial unicompartmental knee arthroplasty. A survival analysis of an independent series. J Bone Joint Surg Br. 2001;83(2):191–194. doi: 10.1302/0301-620X.83B2.10966. [DOI] [PubMed] [Google Scholar]
- 32.Verdonk R, Cottenie D, Almqvist KF, et al. The Oxford unicompartmental knee prosthesis: a 2–14 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2005;13(3):163–166. doi: 10.1007/s00167-004-0522-8. [DOI] [PubMed] [Google Scholar]
- 33.Vorlat P, Putzeys G, Cottenie D, et al. The Oxford unicompartmental knee prosthesis: an independent 10-year survival analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):40–45. doi: 10.1007/s00167-005-0621-1. [DOI] [PubMed] [Google Scholar]