Abstract
Infection after total knee arthroplasty (TKA) is a devastating complication, and two-stage reimplantation has evolved as an effective treatment option. This study was undertaken to compare the clinical results and radiological changes associated with static or mobile cement spacer placement for the treatment of infected TKA. Between July 2000 and February 2007, 36 consecutive patients were treated by two-stage reimplantation using antibiotic-impregnated cement spacers (AICS) for infected TKAs. Static spacers were used in 20 knees and mobile spacers in 16 knees. Clinical outcomes included success rates of TKR revisions, ranges of motion (ROM), and Hospital for Special Surgery knee scores (HSS), pain and function scores of the Knee Society (KS), joint exposure methods, and bone loss. In this study, mobile spacers provided better ranges of motion and functional knee scores without concomitant increases in infection rate and bone loss in the initial and mid-term periods.
Introduction
The increasing numbers of total knee arthroplasties (TKAs) performed have resulted in an increase in the overall number of infections associated with TKA [3]. Though the infection rate following TKAs has decreased primarily due to the use of prophylactic antibiotics, the current infection rate is reported to be 1–2% for primary TKAs and 4–8% for revision surgery [1, 9, 10]. And infection following TKA remains a formidable challenge to both surgeons and patients [4].
The management modalities used to treat infected TKR can be categorised as single-stage reimplantation (removal of an infected TKA with irrigation and debridement and reimplantation of a new TKA during same operation) and two-stage reimplantation. The first step in the two-stage reimplantation procedure involves prosthesis removal with irrigation and debridement and cement spacer insertion. Thus, new prostheses are implanted after infections have been eradicated. Two-stage reimplantation has success rates from 90% to 96% and remains the gold standard for the treatment of infected TKAs [2, 19]. Antibiotic-impregnated cement spacers (AICS) are available as static and mobile types. Static AICS have been used to deliver high doses of antibiotics locally and to minimise contractures of collateral ligaments, thus facilitating second-stage reimplantation [18]. Due to immobilisation between surgical stages, static AICS result in joint stiffness and exposure difficulty at time of reimplantation; accordingly, mobile AICS were developed to solve these problems [13, 15].
The purpose of this study was to compare clinical results and radiological changes associated with the use of static and mobile AICS for the treatment of infected TKA.
Materials and methods
Patient demographics and evaluation methods
From July 2000 to May 2005, 20 patients were treated by using static AICS and from June 2003 to February 2007, 16 patients were treated by using mobile AICS for infected TKAs. All patients were followed-up for at least two years after revision TKA. In the static group, there were two men and 18 women, with a mean overall age of 66.5 years (range, 49–77 years). The mean follow-up after two-stage reimplantation was 36 months (range, 24–62 months). In the mobile group, there were two men and 14 women with a mean age of 60.2 years (range, 47–72 years), and the mean follow-up after two-stage reimplantation was 29 months (range, 25–45 months). Finally, ten patients were referred for uncontrolled infected knee arthroplasty from other hospitals. The mean interval from primary TKA to a diagnosis of infected TKA was 11.3 months (range, 2.5–48 months). The infecting organisms in the two study groups are shown in Table 1. Successful two-stage reimplantation was defined as no evidence of infection at least one year after revision TKA. Clinically we evaluated success rates, average range of motion (ROM), Hospital for Special Surgery (HSS) scores [14], as well as Knee Society (KS) pain and function scores [7] prior to revision TKA and at last follow-up visits after revision TKA. The knee ROM and clinical results were checked using goniometer and questionnaire by one orthopaedic surgeon (S. J. Park). An average ROM measurement was conducted three times and we used the average of the three values.
Table 1.
Infecting organisms (results of culture)
| Organisms | Spacer type | |
|---|---|---|
| Static | Mobile | |
| MRSA | 6 | 2 |
| MSSA | 4 | 4 |
| Staphylococcus epidermis | 0 | 1 |
| Aspergillus species | 1 | 0 |
| Candida species | 2 | 4 |
| E. coli | 2 | 0 |
| Others | 4 | 0 |
| No growth | 1 | 5 |
| Total | 20 | 16 |
MRSA Methicillin resistant staphylococcus aureus, MSSA Methicillin susceptible staphylococcus aureus
Ambulation methods during AICS placement and exposure methods at revision TKAs were compared. We assessed bone loss by comparing radiographs obtained immediately after AICS placement with those obtained before reimplnatation (Figs. 1 and 2). The amount of bone loss in the patients who received static and mobile spacers was evaluated by overlapping two radiographs obtained immediately postoperatively and before reimplantation, and spacer migration as well as radiolucency at the bone–cement interface were evaluated.
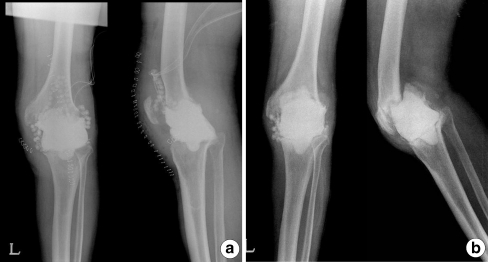
Fig. 1.
A 73-year-old man with left infected total knee arthroplasty (TKA). a Immediately after static spacer insertion. b Just before reimplantation there was static spacer migration with bone loss of femur and tibia
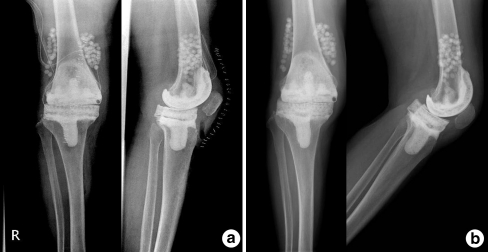
Fig. 2.
A 66-year-old woman with right infected total knee arthroplasty (TKA). a Immediately after mobile spacer insertion. b Just before reimplantation there was no appreciable bone loss of femur or tibia
Operative technique and the protocol used to eradicate infection
During the first stage surgery, all patients underwent extensive debridement at the time of implant removal. A complete synovectomy was performed, and all non-viable soft tissues and bone were resected. Medullary canals were thoroughly debrided. Antibiotics used in the cement depended on the sensitivity profile of the infecting organism. Antibiotic-loaded cement power (1 g erythromycin per 40 g bone cement power) was mixed with 2 g vancomycin and 4.5 g tazocin in most cases, and amphotericin B was added if fungus was isolated.
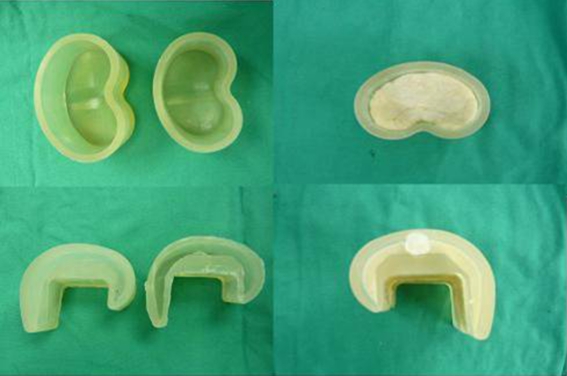
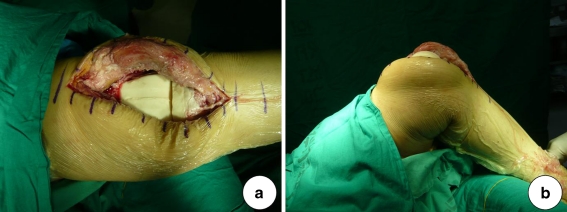
For static spacers, antibiotic-loaded cement was mixed and allowed to become doughy before being used to fill the extension gap. The limb was held aligned at 15° of knee flexion and distracted to maintain the extension gap. For mobile spacers, we prepared specially-made silicone molds (Hangil, Seoul, Korea) and antibiotic-loaded cement was poured into the molds (Fig. 3). After polymerisation of the cement, the spacers were removed from the molds. Excess cement was removed, and edges were smoothed with a burr. The femoral spacer was inserted followed by the tibial spacer. The limb was placed in full extension, in proper alignment and tension. Range of motion, stability, and patellar tracking were assessed (Fig. 4). Lateral retinacular release was performed if necessary. Antibiotics were administered intravenously according to the sensitivities of infecting organisms for three weeks, and then an additional three-week course of oral antibiotics was given. Before second stage revision TKAs, the clinical absence of infection and normal laboratory findings (ESR, CRP) were required after withdrawal of antibiotics for at least two weeks. After surgery, weight-bearing as tolerated was allowed in both groups and knee range of motion was allowed after applying a knee brace in the mobile group.
Fig. 3.
Silicone mobile type cement spacer mold
Fig. 4.
An intraoperative photograph obtained after mobile spacer insertion shows proper alignment and range of motion. a Knee full extension. b Knee 90° flexion
At the second stage procedure, preoperative planning included extensive exposure, intraoperative cultures, cell counts, and intraoperative frozen sections. The surgical approach for second-stage reimplantation followed the principles of revision TKAs. Use of the previous first stage incision was most common, and more extensive exposure was frequently necessary due to intervals between stages and associated scar tissue formation. We based reconstruction plans on the underlying bone and soft tissue defects. After second stage operations, we routinely reviewed patients clinically and checked ESR and CRP and for any signs of recurrent infection.
Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, Illinois, USA). The chi-square test was used to compare joint opening methods in the two groups. The Fisher’s exact test was used to compare reinfection rates. The Mann-Whitney U test was used to compare ranges of motion, HSS scores, as well as KS pain and function scores in the two groups. P values of <0.05 were considered to be statistically significant.
Results
In the static and mobile groups, reinfections within two years after TKA revision developed in three (15%, three of 20) and one (6.3%, one of 16) cases, respectively. These reinfection rates were not significantly different (P = 0.698).
Prior to second stage surgery, patients with a static AICS achieved an average 9° (range, 0–20°) of mean range of motion (ROM) compared with an average of 80° (range, 50–140°) in the mobile group (p = 0.01) (Table 2). At final follow-up after reimplantation, patients with a static AICS achieved an average ROM of 92° (range, 65–140°) and patients with a mobile AICS achieved 108° (range, 85–140°), which were significantly different (p = 0.04). Just prior to reimplantation, the average HSS scores in the static and mobile groups were 48.2 (range, 31–52 points) and 57.2 points (range, 46–71 points), respectively (p = 0.02). At final follow-up, average HSS scores in the static and mobile groups were 80.0 points (range, 74–97 points) and 87.0 points (range, 76–95 points), respectively (p = 0.04), and this difference was also statistically significant. At final follow-up, average KS pain and function scores in the static and mobile groups were 46.0 (range, 45–50 points) and 50.0 points (range, 10–100 points) and 42.0 (range, 20–50 points) and 76.0 points (range, 50–100 points), respectively. Average KS function scores were significantly different (p = 0.03), while KS pain scores were not (p = 1.00).
Table 2.
Clinical results just prior to reimplantation and at last follow-up
| Time | Clinical results | Spacer type | P-value | |
|---|---|---|---|---|
| Static | Mobile | |||
| Just before reimplantation | Mean ROM | 9° (0–20°) | 80° (50–140°) | 0.01 |
| Extension | 5° (0–25°) | 5° (0–20°) | 0.01 | |
| Flexion | 14° (5–30°) | 85° (50–140°) | 0.01 | |
| HSS score | 48.2 (31–52) | 57.2 (46–71) | 0.02 | |
| Last follow-up | Mean ROM | 92° (65–140°) | 108° (85–140°) | 0.04 |
| Extension | 7° (0–15°) | 2° (0–5°) | 0.01 | |
| Flexion | 99° (75–140°) | 110° (85–140°) | 0.01 | |
| HSS score | 80 (74–97) | 87 (76–95) | 0.04 | |
| KS pain score | 46 (45–50) | 42 (20–50) | 1.00 | |
| KS function score | 50 (10–100) | 76 (50–100) | 0.03 | |
ROM range of motion, HSS Hospital for Special Surgery knee score, KS Knee Society
Prior to second stage surgery, four patients could walk with a knee brace in both groups (P = 0.75) (Table 3).
Table 3.
Ambulation during the cement spacer state
| Ambulation status | Spacer type | |
|---|---|---|
| Static | Mobile | |
| Bed ridden | 2 | 0 |
| Wheel chair | 10 | 4 |
| Crutch | 4 | 8 |
| Walking with brace | 4 | 4 |
No inter-group difference was observed in terms of the need for extensive exposure techniques. Seven V-Y quadriceps plasties and eight tibial tuberosity osteotomies were performed in patients that received static AICS, and there were six V-Y quadriceps plasties and five tibial tuberosity osteotomies in patients who received a mobile AICS (Table 4) (p = 0.65).
Table 4.
Exposure at revision TKR
| Joint opening method | Spacer type | |
|---|---|---|
| Static | Mobile | |
| Medial parapatellar | 5 | 5 |
| V-Y quadriceps plasty | 7 | 6 |
| Tibia tubercle osteotomy | 8 | 5 |
Tibial and femoral bone loss occurred in patients who received static spacers (Fig. 1). Fifteen patients (75%) with a static spacer had either tibial or femoral bone loss. Tibial bone loss was present in ten patients (50%), and femoral bone loss in 13 (65%). Eight patients had tibial and femoral bone loss. No bone loss was found in the mobile spacer group (Fig. 2).
Discussion
Two-stage reimplantation has proven to be the most successful means of treating infected TKA [2, 4, 5, 11–13, 16, 17, 19]. Patients who are more frail with more medical problems may be better candidates for static spacers simply because the technique is quicker at the time of removal and reimplantation. However, immobilisation between stages using the static spacer causes joint stiffness, poor ROM, exposure difficulties at revision surgery, and patient dissatisfaction [12]. To overcome these disadvantages, a protocol for two-stage reimplantation using a mobile spacer has been developed.
The purpose of our study was to determine whether mobile AICS improves knee function, patient convenience during the cement spacer state, eases joint exposure at revision, and prevents bone loss without a concomitant increase in infection rate. This study analysed 36 consecutive patients with infected TKA. The successful two-stage reimplantation in this study was defined as no evidence of infection at least two years after revision TKA. Fehring et al. [8] examined reinfection rates for static versus mobile spacers, and the mean follow-up durations for static and mobile were 36 months (range, 24–72 months) and 27 months (range, 24–36 months), respectively. They reported reinfection rates for static and mobile spacers of 12% and 7%, respectively. Emerson et al. [6] reported reinfection rates for patients with static spacers and mobile spacers of 7.6% and 9% at 36 months, respectively. In our study, mean follow-up duration after TKR revision was 36 months (range, 24–62 months) with the static spacer and 29 months (range, 25–45 months) with the mobile spacer. This mean follow-up duration is similar to that in the study by Fehring et al. Reinfection occurred in three patients (15%) who received a static AICS and in one patient (6.3%) who received a mobile AICS, which was not significantly different. These results show that allowing some ROM between stages did not increase the reinfection rate. But Emerson et al. [6] reported reinfection was seen at 1.4, 2.1 and 4.5 years after revision surgery with the static spacer and 2.3 and 3.6 years with the mobile spacer. Reinfection may occur at any stage in the follow-up; thus, a further follow-up to review the reinfection rate over the long-term in both groups, is needed especially in the mobile group.
Using a similar mobile spacer technique, Emerson et al. [6] reported that patients with mobile spacers had significantly better average ROM at follow-up than patients with static spacers (107° vs. 93.7°). But Fehring et al. [8] reported that mobile spacers offered no functional advantage over static spacers because there was no statistical significance of mean ROM (105° in mobile spacers, 98° in static spacers). In our study, patients treated with a mobile AICS had significantly better ROMs and HSS scores than patients treated with static spacers during the cement spacer state and at final revision TKA follow-ups (Table 2).
Hsu et al. [13] reported that mobile AICS allowed satisfactory range of motion during the life of the cement spacer. This decreased the amount of soft tissue contracture, and thus facilitated surgical exposure and soft tissue balancing during revision [6, 13]. However, in our study, extensive exposures, such as V-Y quadriceps plasty and tibial tubercle osteotomy, were necessary in both groups. Accordingly, we were unable to find a statistically significant difference between static and mobile AICS in terms of joint exposure at revision (Table 4). We thought that the average ROM of 80° (range, 50–140°) for mobile AICS achieved was not sufficient for tibial anterior subluxation and adequate exposure using the medial parapatellar approach at revision surgery. All seven cases of static spacer which were approached by V-Y quadriceps plasty had an extension lag. Mean extension lag was 12.5 (range, 10–15). Two cases out of six with the mobile spacer which were approached by V-Y quadriceps plasty had a five degree extension lag.
Fehring et al. [8] assessed bone loss by comparing radiographs obtained intraoperatively after resection with those obtained immediately before reimplantation. He reported that 15 (60%) cases were associated with either tibial or femoral bone loss. In our study, we assessed bone loss by comparing radiographs obtained immediately after cement spacer insertion with those obtained before reimplnatation (Figs. 2 and 3). No appreciable bone loss was observed in the patients who received a mobile spacer, but 15 of 20 (75%) patients with static spacers developed unexpected bone loss between stages. This bone loss might occur for the following reasons. First, bone loss was correlated with the length of the cement spacer insertion period. In our study, patients treated with a mobile AICS had a shorter cement spacer period than patients treated with static spacers (3.3 months vs. 4.2 months). Second, tibial bone loss was associated with spacers which were smaller than the tibial plateau and rested on cancellous bone rather than on the cortical rim. In our study, six cases rested on cancellous bone rather than the tibial plateau’s cortical rim. Third, motion of mobile spacers occurred mainly at the cement–cement interface, and micro-motion of the cement–bone junction of the mobile spacers occurred less than that of the static spacer. Micro-motion of the cement–bone junction of the static spacer may precipitate further bone loss.
Our comparative study consisted of two consecutively treated groups, so that the follow-up period for the two groups was different. This could be a limitation of the study. In conclusion, our study shows that two-stage reimplantation with antibiotic-loaded cement mobile spacers provides a more effective means of treating infected TKAs than static spacers. Mobile spacers were found to provide better ranges of motion not only during the cement spacer state but also after reimplantation. Hence, mobile spacers were found to result in good functional outcomes without concomitant increases in reinfection rates and bone loss in the initial and mid-term periods. Thus, we should have a further follow-up to review the reinfection rate for the long-term in both groups.
Conflict of interest statement
No support in the form of grants, equipment or other items has occurred for this project. The authors have received nothing of value.
This manuscript does not contain information about medical devices.
References
- 1.Anderson JA, Sculco PK, Heitkemper S, Mayman DJ, Bostrom MP, Sculco TP. An articulating spacer to treat and mobilize patients with infected total knee arthroplasty. J Arthroplast. 2009;24(4):631–635. doi: 10.1016/j.arth.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Antoci V, Phillips MJ, Antoci V, Jr, Krackow KA. The treatment of recurrent chronic infected knee arthroplasty with a 2-stage procedure. J Arthroplast. 2009;24(1):159.e13–17. doi: 10.1016/j.arth.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Borden LS, Gearen PF. Infected total knee arthroplasty: a protocol for management. J Arthroplast. 1987;2(1):27–36. doi: 10.1016/S0883-5403(87)80028-1. [DOI] [PubMed] [Google Scholar]
- 4.Burnett RSJ, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two-stage exchange for infection in TKA. Clin Orthop Relat Res. 2007;464:164–178. doi: 10.1097/BLO.0b013e318157eb1e. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan JJ, Katz RP, Johnston RC. One-stage revision surgery of the infected hip. A minimum 10-year follow up study. Clin Orthop Relat Res. 1999;369:139–143. doi: 10.1097/00003086-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Emerson RH, Jr, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–138. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 8.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gristina AG, Kolkin J. Current concepts review: total joint replacement and sepsis. J Bone Jt Surg Am. 1983;65:128–134. [PubMed] [Google Scholar]
- 10.Haddad FS, Masri BA, Campbell D, et al. The PROSTALAC functional spacer in two stage revision for infected knee replacements. Prosthesis of antibiotic-loaded acrylic cement. J Bone Jt Surg Br. 2000;82:807–812. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 11.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125–131. doi: 10.1097/01.blo.0000149241.77924.01. [DOI] [PubMed] [Google Scholar]
- 13.Hsu YC, Cheng HC, Ng TP, Chiu KY. Antibiotic-loaded cement articulating spacer for 2-stage reimplantation in infected total knee arthroplasty: a simple and economic method. J Arthroplast. 2007;22(7):1060–1066. doi: 10.1016/j.arth.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee replacement prostheses. J Bone Jt Surg Am. 1976;78:754–765. [PubMed] [Google Scholar]
- 15.Kent M, Rachha R, Sood M. A technique for the fabrication of a reinforced moulded articulating cement spacer in two-stage revision total hip arthroplasty. Int Orthop. 2009 doi: 10.1007/s00264-009-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplast. 1998;13(3):331–338. doi: 10.1016/S0883-5403(98)90179-6. [DOI] [PubMed] [Google Scholar]
- 17.Meek RM, Masri BA, Dunlop D, et al. Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of the PROSTALAC articulating spacer. J Bone Jt Surg Am. 2003;85:1888–1892. doi: 10.2106/00004623-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Pitto RP, Castelli CC, Ferrari R, Munro J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int Orthop. 2005;29:305–308. doi: 10.1007/s00264-005-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Jt Surg Am. 1990;2:272–278. [PubMed] [Google Scholar]