Abstract
The purpose of this study was to compare the characteristics of interbody fusion achieved using the hat type cervical intervertebral fusion cage (HCIFC) with those of an autologous tricortical iliac crest graft, Harms cage and the carbon cage in a goat cervical spine model. Thirty-two goats underwent C3-4 discectomy and fusion. They were subdivided into four groups of eight goats each: group 1, autologous tricortical iliac crest bone graft; group 2, Harms cage filled with autologous iliac crest graft; group 3, carbon cage filled with autologous iliac bone; and group 4, HCIFC filled with autologous iliac graft. Radiography was performed pre- and postoperatively and after one, two, four, eight and 12 weeks. At the same time points, disc space height, intervertebral angle, and lordosis angle were measured. After 12 weeks, the goats were killed and fusion sites were harvested. Biomechanical testing was performed in flexion, extension, axial rotation, and lateral bending to determine the stiffness and range of motion. All cervical fusion specimens underwent histomorphological analyses. One week after operation, the disc space height (DSH), intervertebral angle (IVA) and lordosis angle (LA) of HCIFC and carbon cage were statistically greater than those of autologous iliac bone graft and Harms cage. Significantly higher values for DSH, IVA and LA were shown in cage-treated goats than in those that received bone graft over a 12-week period. The stiffness of Harms cage in axial rotation and lateral bending were statistically greater than that of other groups. Radiographic and histomorphological evaluation showed better fusion results in the cage groups than in the autologous bone group. HCIFC can provide a good intervertebral distractability and sufficient biomechanical stability for cervical fusion.
Introduction
Anterior decompression and interbody fusion are widely accepted surgical treatments for patients with cervical spondylosis. Until now, tricortical iliac crest bone graft has been the gold standard, although it is associated with a high rate of donor site morbidity. Additional problems such as pseudarthrosis, graft collapse-induced kyphotic deformity, and graft extrusion have led to a rapid increase in the use of cervical spine interbody fusion cages as an adjunct to arthrodesis [1].
According to Weiner and Fraser [1] cage designs can be subdivided into three groups: screw (horizontal cylinder), box, or cylinder (vertical ring) designs. Cages have been specially designed to provide immediate strong anterior column support. Additionally, cages should retain interbody distraction and should be resistant to subsidence into the adjacent vertebrae to foster a biological environment that guarantees the desired quality of osseous fusion.
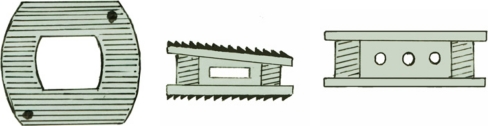
On the basis of a previous study we designed a new kind of cage called the hat type cervical intervertebral fusion cage (HCIFC), shown in Fig. 1, whose interbody immediate stability has been biomechanically evaluated [2, 3]. The purpose of this study was to compare a developing interbody fusion fostered by HCIFC with those of an autologous tricortical iliac crest bone graft, Harms cage and carbon cage in a goat cervical spine interbody fusion model. This study was designed to determine whether there were differences, at a given early stage, in a developing fusion mass between the four interbody fusion techniques with regard to ability to preserve postoperative distraction, biomechanical stability and histological characteristics of intervertebral bone matrix formation.
Fig. 1.
Superior, lateral, and anterior view of hat type cervical intervertebral fusion cage (HCIFC)
Materials and methods
Study design
Thirty-three (two-year-old) adult male goats underwent C3-4 discectomy and fusion. Thirty-two of the goats were randomly assigned to the following groups: group 1, autologous tricortical iliac crest graft (eight goats); group 2, Harms cage filled with autologous cancellous iliac crest bone graft (eight goats); group 3, carbon cage filled with autologous cancellous iliac crest bone graft (eight goats); and group 4, HCIFC filled with autologous cancellous iliac crest bone graft (eight goats).
The goats were evaluated prospectively for 12 weeks, after which they were killed and underwent radiographic, biomechanical, and histological evaluations. All animal-related experimental work was approved by local authorities.
Surgical technique and postoperative care
All goats received 3.2 million units of penicillin intramuscularly before surgery. Surgery was performed after intravenous induction of 100 grams ketamine. For maintenance of anaesthesia, intravenous dosages of 40 mg/kg ketamine were administered. The anterior part of the neck and the left iliac crest were prepared in a sterile fashion, and a right anterolateral approach to the cervical spine was undertaken through a longitudinal skin incision. The longus colli muscle was incised in the midline, and the intervertebral C3-4 disc was exposed. After a Caspar device was used to distract the motion segments, anterior C3-4 discectomy was performed. The endplates were shaved using curette down to bleeding bone, resulting in an excision of 1 mm of each endplate. No attempt was made to excise the posterior longitudinal ligament or expose the spinal canal. For interbody stabilisation, titanium cylinder-design cages (group 2, Harms cage; width 14 mm, depth 14 mm, and cage volume 0.1 cm3), or box-design cages (group 3, carbon cage; width 16 mm, depth 13 mm, and cage volume 0.28 cm3, and group 4, peek HCIFC; width 14 mm, depth 12 mm, and cage volume 0.23 cm3) of appropriate height (mean height 8 mm for three groups) were used (Table 1). Prior to insertion the cavities of the cages were filled with autologous bone or the quantities of the bone graft were determined using water displacement technique (Archimedes principle). The tricortical bone graft was inserted press-fit into the intervertebral space with the cortical surface of the graft anterior (Robinson technique). Finally, the wound was irrigated with saline, and the longus colli muscle was closed using a running suture. The subcutaneous tissue and skin were reapproximated using interrupted sutures, and a soft bandage was applied to the neck.
Table 1.
Height, width, and depth of the cages
| Group | Cage | Type | Company | Material | Height | Width | Depth |
|---|---|---|---|---|---|---|---|
| (mm) | (mm) | (mm) | |||||
| 1 | Iliac bone graft | – | – | Bone | 8 | 14 | 14 |
| 2 | Harms cage | Cylinder | DepuyAcroMed | Titanium | 8 | 14 | 14 |
| 3 | Carbon cage | Box | DepuyAcroMed | Carbon | 8 | 16 | 13 |
| 4 | HCIFC | Box | – | Peek | 8 | 14 | 12 |
After surgery, the goats were observed until fully recovered from anaesthesia. They received two 800,000-unit doses of penicillin intramuscularly per day for five days. Clinical examination was performed daily for the first ten days and weekly thereafter. The goats were allowed activity for the remainder of the experiment. Twelve weeks after surgery, after induction of anaesthesia, the goats were killed by an intravenous injection of potassium chloride. The complete cervical spine, including parts of the occiput and T-1, was excised and cleaned from the surrounding tissue.
Radiographic evaluation
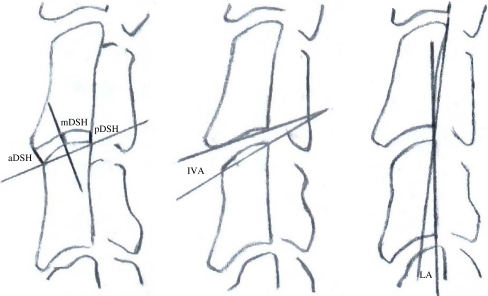
To allow for comparable radiographic evaluation, special fixation devices for the goats cervical spine were developed. Lateral and anteroposterior radiographs were acquired pre- and postoperatively and after one, two, four, eight and 12 weeks. During the same time periods, anterior, middle, and posterior intervertebral disc space height (DSH), intervertebral angle (IVA) and lordosis angle (LA) of the C3-4 motion segment were measured on lateral radiographs (Fig. 2). The mean intervertebral DSH was calculated from anterior, middle and posterior DSH measurements (anterior + middle + posterior DSH/3). After 12 weeks, bone fusion was assessed using the following parameters: (a) no bone fusion, (b) maximum intervertebral gap of more than 5 mm, (c) maximum intervertebral gap of less than 5 mm, and (d) complete bone fusion. The maximum intervertebral gap in the craniocaudal direction was measured directly on lateral X-ray films using a ruler. All radiographic measurements were evaluated by three independent observers [4].
Fig. 2.
Radiographic measurements. Left: anterior disc space height (aDSH), posterior disc space height (pDSH), and middle disc space height (mDSH). Center: intervertebral angle (IVA). Right: lordosis angle (LA)
Biomechanical analysis
After the goats were killed, the specimens were biomechanically tested in a nonconstrained testing apparatus using a nondestructive flexibility method previously described [5]. Pure bending moments were applied to the C3-4 motion segments by using a system of cables and pulleys to induce flexion, extension, left and right lateral bending, and left and right axial rotation. Tension was applied to the cables with a uniaxial testing machine. Three-dimensional displacement of each motion segment was measured.
The vertebrae were mounted in pots using polymethylmethacrylate. The lower pot was rigidly attached to the base of the testing apparatus. This test setup resulted in a compressive preload of 25 N because of the weight of the upper fixation pot, which represents the mean weight of the head of the goats. Moments were applied in a quasistatic manner in increments of 1 N·m to a maximum of 6 N·m. Specimens were preconditioned with three cycles of 6-N·m load with a velocity of 1.2 mm/s of the transverse bar. The fourth cycle was measured. The mean apparent stiffness values in the EZ were calculated from the corresponding load–displacement curves.
Histomorphological analysis
All C3-4 motion segments were harvested at 12 weeks for histological examination of the bone. The motion segments were fixed for seven days in 10% buffered formaldehyde followed by dehydration in ascending concentrations of ethanol and embedded without being decalcified in methylmethacrylate.
For histomorphological analysis longitudinal sections in the sagittal plane were cut at 6 µm by using a microtome and a 40° stainless-steel knife. Masson-Goldner staining was used for histomorphological analysis, which included evaluation of bone fusion according to the A through D parameters previously defined. The maximum intervertebral gap in the craniocaudal direction was measured directly on midsagittal sections.
Statistical analysis
Comparison of data was performed using one-way analysis of variance for independent samples followed by Tukey post-hoc analysis for multiple comparison procedures. Statistically significant differences were defined at a 95% confidence level. The values are given as mean ± standard deviation. The SPSS software supported statistical evaluation.
Results
Failure parameters and complications
One goat died of an anaesthesia-related complication on day 0. This animal was excluded from the study and replaced by another animal.
In group 1, one goat developed a haematoma at the donor site of the iliac crest graft. In group 3, one goat developed wound healing problems at the donor site. Both complications resolved without further difficulty on conservative treatment.
Volume of the implants
The volume of the implants was evaluated prior to insertion by using the water-displacement technique. The mean volume of the autologous tricortical iliac crest bone graft was 1.43 ± 0.05 cm3. The mean volumes of the autologous iliac crest graft-filled Harms, carbon and HCIFC cages were 1.52 ± 0.06 cm3, 1.5 ± 0.07 cm3 and 1.49 ± 0.07 cm3, respectively.
Radiographic results
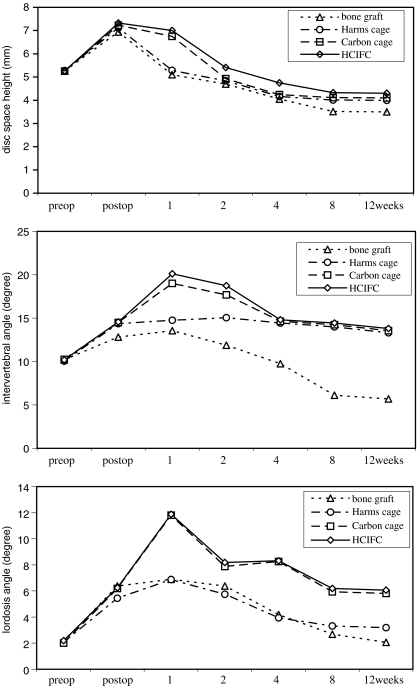
With regard to preoperative baseline values of all radiographic parameters, there were no intergroup differences. At one week, mean DSH values for the HCIFC and carbon cages were significantly higher than Harms cage and tricortical iliac crest bone graft (P < 0.001). At two and four weeks the DSH of HCIFC was significantly larger than that of other groups (P < 0.05). At eight and 12 weeks all cage groups showed significantly higher values for mean DSH than group 1 (P < 0.05). However, no differences were found in DSH among the three cage types during the other experimental period (Fig. 3, upper graph). At two, four, eight and 12 weeks all three cage-treated groups (groups 2, 3 and 4) showed significantly higher values for IVA (P < 0.05; Fig. 3, centre graph) compared with the autologous tricortical iliac crest graft-treated group (group 1). During the experimental period, no significant differences in IVA were found among the cage groups, except for at the one- and two-week time points, at which the box-design HCIFC and carbon cage showed a significantly higher IVA than the cylinder-design Harms cage (P < 0.05). At one, four, eight and 12 weeks the box-design cages (groups 3 and 4) showed significantly higher values for LA (P < 0.05; Fig. 3, lower graph) compared with the autologous iliac crest graft group (group 1) and the cylinder-design cage group (group 2).
Fig. 3.
Radiographic analysis. Upper: mean disc space height (DSH) of the different groups throughout the observation period. Centre: mean intervertebral angle (IVA) of the different groups throughout the observation period. Lower: mean lordosis angle (LA) of the different groups throughout the observation period
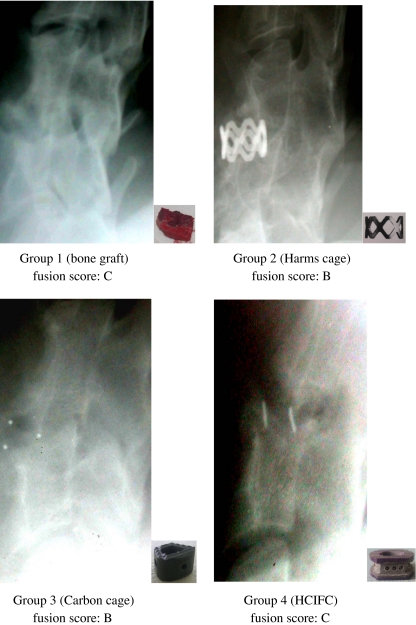
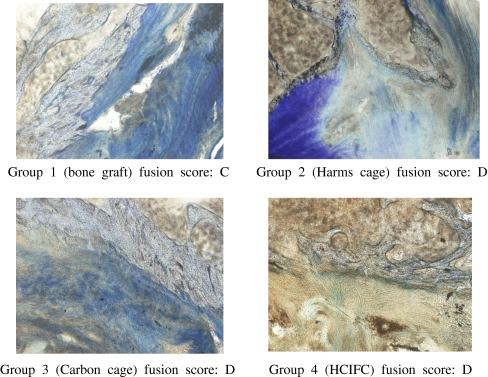
After 12 weeks, bone fusion was evaluated radiographically (Fig. 4). In the cage groups (groups 2, 3, and 4), a slightly more advanced interbody fusion was found compared with that in group 1 (Table 2).
Fig. 4.
Radiographic analysis. After 12 weeks, the extent of osseous fusion was evaluated on radiographs. The small pictures within the radiographs show the different implants
Table 2.
Radiographic and histomorphological fusion results after 12 weeks
| Fusion parameter (score) | Radiographic | Histomorphological | ||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 1 | Group 2 | Group 3 | Group 4 | |
| A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 5 | 4 | 4 | 3 | 5 | 4 | 4 | 3 |
| C | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 |
| D | 0 | 1 | 1 | 2 | 0 | 2 | 1 | 2 |
Biomechanical results
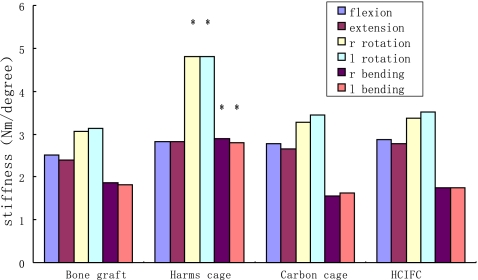
Biomechanical results for stiffness and range of motion (ROM) are depicted in Fig. 5 and Table 3.
Fig. 5.
Bar graph demonstrating results of biomechanical stiffness of different groups. * = P<0.05 compared with the bone graft, Carbone cage and HCIFC Carbone
Table 3.
Range of motion (ROM) of the four groups after 12 weeks
| Test mode (degrees) | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| Flexion | 4.0 ± 0.6 | 3.5 ± 0.5 | 3.7 ± 0.6 | 3.5 ± 0.6 |
| Extension | 4.1 ± 0.6 | 3.7 ± 0.7 | 3.9 ± 0.5 | 3.7 ± 0.6 |
| Right rotation | 3.6 ± 0.6 | 2.5 ± 0.7 * | 3.4 ± 0.6 | 3.4 ± 0.7 |
| Left rotation | 3.6 ± 0.6 | 2.4 ± 0.7 * | 3.5 ± 0.6 | 3.5 ± 0.7 |
| Right bending | 5.0 ± 0.7 | 4.7 ± 0.9 | 5.6 ± 0.8 | 5.2 ± 0.8 |
| Left bending | 5.1 ± 0.8 | 4.8 ± 1.0 | 5.8 ± 0.7 | 5.3 ± 0.9 |
* P < 0.05 compared with groups 1, 3, and 4
The highest stiffness values (P < 0.001) and the lowest ROM values (P < 0.05) in rotation and bending were consistently found in Harms cage in group 2.
Histomorphological results
Histomorphological analysis supported the findings of radiographic and biomechanical examinations.
The spines fixed with autologous iliac crest graft (group 1) showed extensive callus formation with cartilage and connective tissue cells, most of which were seen ventrally. The tricortical bone graft, however, collapsed and was mainly resorbed. Those spines fixed with the three types of cage (groups 2, 3, and 4) showed islands of bone between the endplates with cartilage and fibrous tissue components. These findings were accompanied by capillary ingrowth and resorptive lacunae. The tissue surrounding the cages appeared similar in three groups (Fig. 6).
Fig. 6.
Histomorphological analysis
Bone fusion was evaluated histomorphologically (Table 2). In the three cage groups a more advanced interbody fusion mass was found compared with that in the tricortical iliac crest graft group. In the cylinder-design cages, however, a slightly more advanced bone matrix formation was shown than in the box-design cage.
Discussion
On the basis of a previous study we designed a new kind of cage called the hat type cervical intervertebral fusion cage (HCIFC) as shown in Fig. 1. HCIFC is box-shaped with superior and inferior oval surfaces and wedged with anterior height greater than posterior. Superior and inferior hat edges are extended, increasing the surface area and decreasing the thickness of the cage [2, 3]. Biomechanical in vitro testing showed that the HCIFC can provide adequate primary stability for cervical interbody fusion [2, 3].
The object of this study was to compare characteristics of interbody fusion among an autologous tricortical iliac crest bone graft, a cylinder-design Harms cage, a box-design carbon cage and HCIFC. This study was designed to determine whether there were differences among the four interbody fusion materials with regard to the ability to preserve postoperative distraction, biomechanical stability, and histological characteristics of intervertebral bone matrix formation.
In this study the goat spine was used as a model for the human spine. Anatomical, biomechanical, and bone mineral density evaluation of the goat cervical spine has shown good comparability with the human spine [6, 7]. Anatomical evaluation has demonstrated an average disc space height of 6 mm in the goat cervical spine, which is approximately 1 mm higher than in the human cervical spine [8]. Additionally, similar upper end plate parameters of C4 and C5 in both species were observed. Therefore, human implants fit the goat cervical spine.
Distractive properties
Cages should retain interbody distraction and should be resistant to subsidence into the adjacent vertebrae to guarantee the desired quality of fusion [1]. The authors of several clinical studies have demonstrated that tricortical iliac crest graft-assisted interbody fusion induced a decrease in DSH during the postoperative period [9]. Sandhu et al. [10] demonstrated in a sheep model that screw-design cages preserved interbody distraction more effectively than an autologous iliac crest bone graft. Analysis of the data in this study demonstrated that, at the time of surgery, three cages and the tricortical iliac crest graft were able to distract intervertebral spaces beyond their baseline measurements to nearly the same values. In all conditions, however, significant subsidence developed beyond DSH values during the 12-week observation period. Three cages were able to decrease the loss of DSH significantly compared with the tricortical iliac crest graft. Although the box-design cage showed significantly less subsidence after one week than the cylinder-design cage, three cages demonstrated similar DSH values at final measurements. The DSH of HCIFC was significantly higher than that of other cages after two and four weeks, and higher than that of the carbon cage after eight and 12 weeks, although there was no significant difference, which showed good distractive properties of HCIFC. The loss of DSH of the cages resulted from subsidence into the subchondral bone of the adjacent vertebral bodies; the loss of the intervertebral space in the tricortical iliac crest graft group resulted mainly from gradual graft collapse. Two factors that influence the subsidence tendency of an intervertebral implant are the shape of the implant, especially its contact area at the implant–endplate interface, together with preparation of the endplates. A small surface area and destructive preparation of the endplates increase the subsidence risk [11]. The hat edges of the HCIFC surface which increase the contact area can decrease the tendency to subsidence.
Biomechanical properties
In this in vivo experiment the Harms cage showed significantly more biomechanical stiffness in bending and rotation and a lower ROM in rotation than the tricortical iliac crest bone graft. Our in vitro study [2, 3] found that the stiffness of Harms cage in flexion and rotation was significantly less than that of tricortical iliac crest bone graft, which is in contrast to the report of in vivo experiments. The biomechanical stiffness in bending for Harms cage was higher than for tricortical bone graft in accordance with our in vivo study. Although the stiffness of HCIFC and carbon cage was higher than that of tricortical bone graft after 12 weeks, we found no significant difference between them. The in vitro study [2, 3] showed a significantly lower biomechanical stiffness in flexion and higher stiffness in rotation associated with HCIFC and carbon cages than with the tricortical bone graft. These in vitro results are also in crucial contrast to in vivo results obtained by our own working group. The significantly higher stiffness in bending and rotation was demonstrated for the Harms cage used in this study than for HCIFC and carbon cages after 12 weeks. In our previous in vitro study [2, 3], we found significantly higher stiffness in flexion, extension and bending and lower stiffness in rotation for the Harms cage than for HCIFC and carbon cages. The initial biomechanical in vivo stability of the cage combined with the graft composite is nearly exclusively a result of the biomechanical properties of the cage. Only secondarily, the interbody fusion mass arising from the incorporated bone graft contributes to biomechanical stability [12, 13]. With the biomechanical in vitro results of three cages [2, 3], the significantly greater biomechanical stability in Harms cage compared with HCIFC and the carbon cage in vivo was an effect of an accelerated interbody fusion in the former. The differences between biomechanical in vitro and in vivo results may be a result of the “biological qualities” of different cage designs in vivo, suggesting that currently available biomechanical in vitro tests are not highly predictive of the in vivo performance of any interbody fusion cage.
Histological characteristics
Histological analysis demonstrated that interbody fusion in the three cages was better than in the bone graft group. Additionally, significantly more bone matrix formation was found in the Harms cage than in HCIFC and carbon cages. In this experimental setting, stiff collars were not used for the animals postoperatively, which made the tricortical iliac crest grafts subject to an unstable fusion environment in group 1. This explains the extensive amount of callus formation with cartilage and connective tissue mostly seen anteriorly. The limited biomechanical properties of the tricortical iliac crest bone graft resulted in a compression and sometimes fragmentation of the graft, which was followed by extensive osteoclastic activity and finally graft resorption. If an internal stabilisation plate were provided, the group with tricortical bone would have performed much better. In contrast, because the bone grafts packed inside the cages have a biomechanically protected and, consequently, biologically improved environment for fusion, a more stable interbody fusion mass develops, especially in the cylinder-design Harms cage group. Biomechanical loads in vivo consist of shear and compression. Whereas shear loads promote bone matrix formation, compressive loads do not [14]. In contrast to the bone graft, the cage mainly functions as a protector against compressive loads. Therefore, this cage-related factor contributed to the more stable interbody fusion mass in the cage groups compared with the bone graft group. The ideal biological environment for bone fusion is achieved by optimum grafting techniques and the maximum filling of the intervertebral space with graft material. As cage volume increases, however, graft volume decreases. Therefore, biologically, the ideal cage would be the one with the smallest cage volume that will provide adequate mechanical stability, because this cage will allow for the maximum filling of the intervertebral space with graft material [1]. Although three cages in this study were of similar volumes when filled with bone grafts (Harms cage, 1.52 cm3; HCIFC, 1.49 cm3; carbon cage, 1.50 cm3), the volume of the isolated cages was substantially different (Harms cage, 0.1 cm3; HCIFC, 0.23 cm3; carbon cage, 0.28 cm3). Therefore, a higher bone graft volume was incorporated in the cylinder-design Harms cage, which potentially contributed to the more stable intervertebral fusion mass in this group.
Conclusion
Compared with the tricortical bone graft, three cage designs, especially HCIFC, were associated with significantly better distractive properties. A significantly greater biomechanical stiffness in rotation and bending and an accelerated interbody fusion was associated with the cylinder-design cages than the box-design cage and the tricortical bone graft. HCIFC can provide a good intervertebral distractability and enough biomechanical stability for cervical fusion.
Footnotes
Patent 200410015636.X.
References
- 1.Weiner BK, Fraser RD. Spine update lumbar interbody fusion cages. Spine. 1998;23:634–640. doi: 10.1097/00007632-199803010-00020. [DOI] [PubMed] [Google Scholar]
- 2.Gu YT, Jia LS, Chen TY. Biomechanical study of a hat type cervical intervertebral fusion cage. Int Orthop. 2007;31:101–105. doi: 10.1007/s00264-006-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu YT, Jia LS, Chen XS, et al. Biomechanical study of hat type cervical intervertebral fusion cage. Int Orthop. 2005;31:101–105. doi: 10.1007/s00264-006-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandziora F, Schollmeier G, Scholz M, et al. Influence of cage design on interbody fusion in a sheep cervical spine model. J Neurosurg (Spine 3) 2002;96:321–332. doi: 10.3171/spi.2002.96.3.0321. [DOI] [PubMed] [Google Scholar]
- 5.Kandziora F, Pflugmacher R, Scholz M, et al. Comparison between sheep and human cervical spines: an anatomic, radiographic, bone mineral density, and biomechanical study. Spine. 2001;26:1028–1037. doi: 10.1097/00007632-200105010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Goh JC, Wong HK, Thambyah A, et al. Influence of PLIF cage size on lumbar spine stability. Spine. 2000;25:35–39. doi: 10.1097/00007632-200001010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kim KW, Ha KY, Moon MS, et al. Volumetric change of the graft bone after intertransverse fusion. Spine. 1999;24:428–433. doi: 10.1097/00007632-199903010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Kozak JA, Doherty BJ, et al. Interspace distraction and graft subsidence after anterior lumbar fusion with femoral strut allograft. Spine. 1993;18:2393–2400. doi: 10.1097/00007632-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu HS, Turner S, Kabo M, et al. Distractive properties of threaded interbody fusion device: an in vivo model. Spine. 1996;21:1201–1210. doi: 10.1097/00007632-199605150-00013. [DOI] [PubMed] [Google Scholar]
- 11.Wilke HJ, Kettler A, Goetz C, et al. Subsidence resulting from simulated postoperative neck movements: an in vitro investigation with a new cervical fusion cage. Spine. 2000;25(21):2762–2770. doi: 10.1097/00007632-200011010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Mittlmeier T, Pflugmacher R, Schfer J, et al. Cervical interbody fusion cages: biomechanical comparison of screw, box and cylinder designs. Europ Spine J. 2000;9:297–298. [Google Scholar]
- 13.Kanayama M, Cunningham BW, Haggerty CJ, et al. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J Neurosurg (Spine 2) 2000;93:259–265. doi: 10.3171/spi.2000.93.2.0259. [DOI] [PubMed] [Google Scholar]
- 14.Patwardhan AG, Havey RM, Ghanayem AJ, et al. Load-carrying capacity of the human cervical spine in compression is increased under a follower load. Spine. 2000;25:1548–1554. doi: 10.1097/00007632-200006150-00015. [DOI] [PubMed] [Google Scholar]