Ubiquitination and deubiquitination of NP protein regulates influenza A virus RNA replication
Monoubiquitination of the NP protein of influenza virus A is critical for efficient viral replication. The deubiquitinating enzyme USP11 removes this modification, inhibiting viral infectivity.
Keywords: influenza A virus, NP, USP11
Abstract
Influenza A virus RNA replication requires an intricate regulatory network involving viral and cellular proteins. In this study, we examined the roles of cellular ubiquitinating/deubiquitinating enzymes (DUBs). We observed that downregulation of a cellular deubiquitinating enzyme USP11 resulted in enhanced virus production, suggesting that USP11 could inhibit influenza virus replication. Conversely, overexpression of USP11 specifically inhibited viral genomic RNA replication, and this inhibition required the deubiquitinase activity. Furthermore, we showed that USP11 interacted with PB2, PA, and NP of viral RNA replication complex, and that NP is a monoubiquitinated protein and can be deubiquitinated by USP11 in vivo. Finally, we identified K184 as the ubiquitination site on NP and this residue is crucial for virus RNA replication. We propose that ubiquitination/deubiquitination of NP can be manipulated for antiviral therapeutic purposes.
Introduction
Influenza virus is an enveloped negative-sense RNA virus that causes major public health problems worldwide. Every year, the global burden of influenza epidemics is believed to be 3–5 million cases of severe illness. Influenza virus, like all other viruses, relies not only on viral proteins but also host cell proteins and their associated mechanisms to complete viral life cycle. Identifying host molecules that participate in each step of virus replication could provide valuable new targets for antiviral therapy (Hao et al, 2008; Karlas et al, 2010; König et al, 2010).
Influenza virus contains eight segments of single-stranded, negative-sense RNA as its genome. Each RNA segment is packaged into ribonucleoprotein (RNP) complexes, which consist of viral nucleoprotein (NP) and the viral RNA polymerase. The RNA genome is transcribed and replicated by the viral RNA-dependent RNA polymerase in the cell nucleus. The viral RNA polymerase consists of three subunits, polymerase basic protein 1 (PB1), polymerase basic protein 2 (PB2), and polymerase acidic protein (PA), which together are responsible for both transcription and replication of viral genome (Wright et al, 2007). However, the precise molecular mechanism of the viral genome replication is not fully understood. It is still unknown how the switching between transcription and replication is regulated, although it has been reported that the NP protein has an important function in this switching (Portela and Digard, 2002).
Posttranslational modification of proteins by ubiquitin (Ub) is a key regulatory event in various cellular activities, including signal transduction, transcription, membrane protein trafficking, nuclear transport, autophagy, and immune responses (Welchman et al, 2005). Ubiquitination is a dynamic and reversible process, and it has become increasingly evident that deubiquitination also has an important function in regulating the ubiquitin-dependent pathway but is less well understood.
Eukaryotic viruses are known to modulate protein ubiquitination to their advantage in various ways. Several viruses have been shown to depend on the proteasome or certain cellular E3 ligases for entry or release (Yu and Lai, 2005; Bieniasz, 2006). Moreover, many viruses regulate protein ubiquitination to overcome host cell defense mechanisms, including major histocompatibility complex (MHC) class-1 antigen presentation, the type-1 interferon response, and apoptosis. To this end, numerous viruses encode proteins that redirect cellular E3 ligases of the ubiquitin–proteasome system (UPS) to proteins with antiviral activity, including the tumor suppressor protein p53, the activators of transcription, and signal transducers (Lindner, 2007). In contrast to ubiquitination, only a few examples of targeting of the protein deubiquitination system by viruses have been reported. They include the specific targeting of the cellular USP7 by the Epstein–Barr nuclear antigen-1 (EBNA1) and the herpes simplex virus type-1 (HSV-1) regulatory protein ICP0 (Everett et al, 1997; Holowaty and Frappier, 2004).
In addition, ubiquitination also regulates functions of viral proteins. For human immunodeficiency virus type-1 (HIV-1) Tat protein, it was observed that the addition of a single ubiquitin molecule to its C-terminus increased its transcriptional function (Bres et al, 2003). However, for HTLV-1 Tax protein, monoubiquitination led to a lower transcriptional activity (Peloponese et al, 2004). In addition, HIV-1 Gag monoubiquitination is important for virion formation (Jäger et al, 2007). Recent studies also found that ubiquitination was required for efficient replication of coxsackievirus B3. The data suggested that proteasome inhibitors reduced coxsackievirus B3 replication through inhibition of viral RNA transcription and protein synthesis (Si et al, 2008). The similar result has also been observed for influenza A virus (Rodriguez et al, 2007), in that the viral protein synthesis was inhibited by treatment with MG132 (Z-Leu-Leu-Leu-al). This result indicated that the ubiquitin–proteasome pathway may be involved in influenza A virus replication in the early phase of viral life cycle.
In this study, we developed and validated an in vitro cell-based assay for high-throughput screening of RNAi libraries, searching for cellular ubiquitinases and deubiquitinases involved in influenza A virus replication. By RNAi screening, we identified a new cellular deubiquitinase USP11, which regulates influenza A virus RNA replication. Furthermore, influenza A virus NP protein is a monoubiquitinated protein, which can be deubiquitinated by USP11 specifically. The ubiquitination and deubiquitination of NP probably regulates influenza A viral genome replication.
Results
Screening of a DUB RNAi library
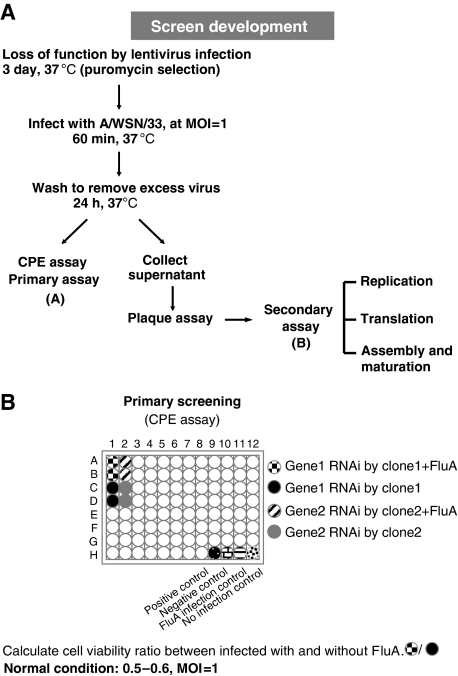
In this study, we first developed and validated a cell-based assay for high-throughput screening of RNAi libraries for cellular factors involved in influenza A virus replication. The assay is based on the determination of cell viability ratio of specific cellular gene knockdown cells infected with or without influenza A virus. During assay development (Figure 1), we defined optimal detection method, plating density, optimal multiplicity of infection (MOI), and positive lentivirus knockdown clone control. A549 cells cultured in a 96-well plate were first infected with specific lentivirus clones at MOI of 2. Each clone was represented by four replicas and selected by puromycin treatment (4 μg/ml). After 3 days, two of the four wells were infected with influenza A virus (A/WSN/33, H1N1) at MOI of 1. At 24 h after infection, cell viability assay was performed and cell viability ratio between cells infected with and without influenza A virus was calculated. Under normal condition, the ratio is 0.5–0.6. The positive control of screening panel used was ISG15, which is a well-characterized cellular inhibitor of influenza A virus infection (Lenschow et al, 2007). As expected, when cellular ISG15 was knocked down, the cell viability ratio was decreased to about 0.28, indicating that more cells were killed by influenza A virus infection in the absence of ISG15. As ISG15 is an ubiquitin-like protein, we hypothesized that other ubiquitin-like proteins may also be involved in influenza A virus life cycle. Therefore, we chose a DUB (deubiquitinating enzyme) RNAi library subset for screening. There are 262 clones in the TRC RNAi DUB library that includes 52 human DUB genes (Supplementary Table I). The criterion for determining hits of screening was set at more than 30% reduction or increase in cell viability ratio. There were five separate clones for each DUB gene; those with three or more clones conforming to the criterion were considered as candidate genes. On the basis of this criterion, a novel cellular deubiquitinase, USP11, was identified as a candidate gene regulating influenza A virus replication or production.
Figure 1.
Overview of RNAi screen to identify host factors involved in influenza A virus infection. (A) Schematic diagram of systematic analysis of host genes affecting influenza virus infection in A549 cells (see text for description). (B) The procedure of primary RNAi screening assay.
USP11 inhibits influenza A virus production
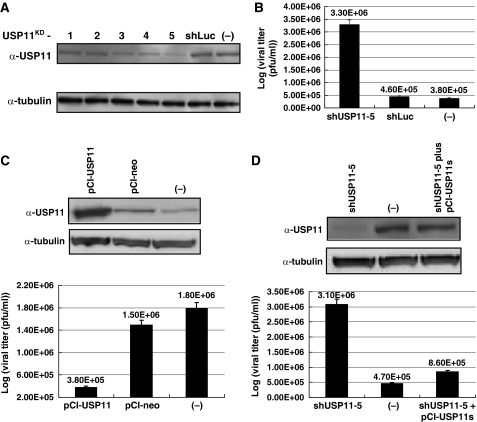
The RNAi primary screening results showed that when cellular USP11 was knocked down, the influenza A virus production was enhanced, as evidenced by lower cell viability, that is, higher CPE. We therefore studied the possible mechanism of inhibition by the USP11 RNAi in detail. The immunoblotting result showed that, among the five shUSP11 clones, clone 5 has the best knockdown efficiency (Figure 2A). Therefore, clone 5 was used for further studies. To verify that USP11 is involved in influenza A virus infection, USP11 knockdown cells were infected with influenza A/WSN/33 virus at MOI of 1. At 24 h after infection, the culture medium was collected and plaque assay was performed to determine virus titers. The result showed that when cellular USP11 was knocked down, the virus titer was increased by about 10-fold (Figure 2B). In addition, when USP11 was overexpressed in 293T cells, the influenza A virus production was reduced to about 20% (Figure 2C). Significantly, when the shUSP11-5-expressing cells were transfected with pCI-USP11s, which can express an USP11 form that is resistant to shUSP11 suppression because of the wobble mutations, the influenza A virus titer dropped to the same level as in the wild-type cells (Figure 2D). This rescue experiment confirmed that the effect of lentivirus shUSP11-5 clone was specifically due to knockdown of USP11. Taken together, we conclude that USP11 can inhibit influenza A virus production.
Figure 2.
The effect of USP11 on influenza A virus infection. (A) Knockdown of USP11 expression as shown by immunoblot analysis using USP11-specific antibody. There were five separate shUSP11 lentivirus clones. shLuc was used as lentivirus infection control. (B) Virus titers (plaque-forming units (PFUs)) after USP11 knockdown. A549 cells were infected with shUSP11-5. At 3 days after infection, cells were infected with influenza A/WSN/33 virus at MOI of 1. At 24 h after infection, supernatant was collected and used to perform plaque assay in MDCK cells. Values are means±s.e.m. of three separate experiments. (C) 293T cells were transfected with pCI-USP11 to overexpress USP11. After 3 days, the cells were infected with influenza A/WSN/33 virus at MOI of 1. At 24 h after infection, supernatant was collected and used to perform plaque assay in MDCK cells. The cellular USP11 expression level was detected by western blot. (D) Rescue experiment for USP11 knockdown cells. pCI-USP11s is a plasmid that can express a USP11 wobble mutant. USP11 was knocked down by lentivirus shUSP11-5 infection. At 24 h after infection, the cells were transfected with pCI-USP11s. At 3 days after transfection, the cells were infected with influenza A/WSN/33 virus at MOI of 1. After 24 h, supernatant was collected to perform plaque assay in MDCK cells. The cell lysates were used to perform immunoblot analysis by using USP11-specific antibody to detect USP11 expression level.
USP11 inhibits influenza A virus genome replication
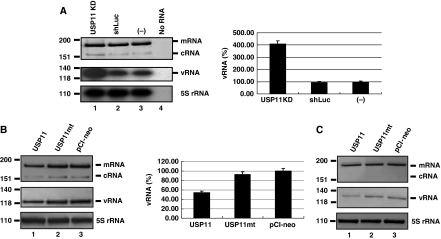
The immunofluorescence staining result showed that USP11 primarily was located in the nucleus of cells as previously reported (Ideguchi et al, 2002; data not shown). Therefore, to determine the role of USP11 in influenza A virus life cycle, we first focused on viral replication and transcription, which take place in the nucleus. USP11 knockdown cells were infected with influenza A virus at MOI of 5 for single-cycle virus infections. At 8 h after infection, total cellular RNA was used for primer extension analysis to detect viral segment 6 (NA) vRNA, cRNA, and mRNA using respective primer sets (Chan et al, 2006; Figure 3A, left panel). The levels of vRNA and cRNA in USP11 knockdown cells were significantly higher than those of the control cells, but the mRNA amount was almost the same between the control and USP11 knockdown cells. We also developed a quantitative RT–PCR (qRT–PCR) procedure to verify the reduction of vRNA. The qRT–PCR protocol was modified from the published data (Tseng et al, 2008) by designing specific tagged primer to detect vRNA (Supplementary Table II). The qRT–PCR result confirmed the primer extension data (Figure 3A, right panel). To elucidate whether the inhibition of influenza A virus replication by USP11 required the deubiquitinating enzyme activity of USP11, a catalytically defective mutant (C275S and C283S) of USP11 was transfected into A549 cells. The cells were infected with virus at MOI of 5 for single-cycle virus infections. The level of vRNA and cRNA in the cells overexpressing the wild-type USP11 were reduced to about 50% of the control cells (Figure 3B), but there was no significant reduction of vRNA or cRNA in cells expressing the catalytically defective USP11 mutant (Figure 3B, lane 2). The result indicated that USP11 inhibited vRNA and cRNA synthesis, but USP11mt did not. Therefore, we concluded that the inhibition of influenza virus RNA replication by USP11 depended on its deubiquitinating enzyme activity. In this particular experiment, there was a slight decrease of viral mRNA in the USP11-overexpressing cells. This reduction could have been a secondary effect of reduction of vRNA by USP11, as mRNA is made using vRNA as the template. To address this possibility, we further dissected the viral transcription activity independent of the virus genome replication by determining the level of viral mRNA synthesis in the presence of cycloheximide (CHX). It has been shown that CHX suppresses viral protein synthesis, and thereby inhibits virus genome RNA replication but not mRNA transcription because the latter does not need new protein synthesis (Vreede et al, 2004). When cells were treated with CHX, the level of mRNA synthesis in the cells overexpressing USP11 was similar to that in control cells (Figure 3C). Taken together, the result indicated that USP11 primarily inhibits influenza A virus RNA replication through its deubiquitinating enzyme activity.
Figure 3.
USP11 inhibits influenza virus RNA replication. (A) Left panel: USP11 knockdown cells were infected with influenza A/WSN/33 virus at MOI of 5. At 8 h after infection, total cellular RNA was analysed by primer extension assay to detect viral NA segment vRNA, cRNA and mRNA. 5S rRNA was used as an internal control. Right panel: The level of viral genome replication in USP11 knockdown cells by using real-time qRT–PCR to detect viral NP segment vRNA. The data were normalized relative to the values detected in A549 cells. (B) The level of virus genome replication in USP11-overexpressing cells. A549 cells were transfected with a plasmid to overexpress wild-type USP11 or catalytically defective mutant USP11 mt. At 48 h after transfection, the cells were infected with influenza A/WSN/33 virus at MOI of 5. At 8 h after infection, total cellular RNA was analysed by primer extension assay to detect viral NA segment RNA species (left panel). The level of viral genome replication in USP11-overexpressing cells was determined by real-time qRT–PCR to detect viral NP segment vRNA. The data were normalized relative to the values detected in the control cells (right panel). (C) The level of viral mRNA synthesis in USP11-overexpressing cells in the presence of cycloheximide (CHX). Cells were infected with influenza A/WSN/33 in the presence of 100 μg/ml cycloheximide at MOI of 5. At 8 h after infection, total cellular RNA was analysed by primer extension assay to detect viral NA segment RNA species.
USP11 interacts with PB2, PA, and NP
As USP11 is a deubiquitinase, we hypothesized that some components of viral RNP may be a substrate of USP11. We first determined whether USP11 binds to any of the viral RNP components. We performed co-immunoprecipitation assays using cell lysates prepared from 293T cells transiently expressing myc-tagged USP11 and one of the HA-tagged viral RNP proteins. The lysates were immunoprecipitated with anti-myc agarose and blotted with anti-HA antibody. As shown in Figure 4A, PB2, PA, and NP specifically co-precipitated with Myc–USP11 (lanes 3, 9, and 12, respectively). However, no co-precipitation was detected in PB1 (lane 6), NS1 (lane 18), or USP11 only (lane 15). No co-precipitation was detected when anti-myc antibody was absent (lanes 2, 5, 8, 11, 14 and 17) or when either of the two constructs was omitted (Figure 4B–D). These results indicated a specific interaction between USP11 and influenza A viral RNP complex.
Figure 4.
USP11 specifically interacts with PB2, PA and NP. (A) The interaction of USP11 with singly expressed viral proteins. 293T cells were mock transfected (lanes 13–15) or transfected with each plasmid encoding PB2–HA (lanes 1–3), HA–PB1 (lanes 4–6), PA–HA (lanes 7–9), NP–HA (lanes 10–12) or HA–NS1 (lanes 16–18). At 48 h after transfection, the cell lysates were subjected to immunoprecipitation (IP) assays in the absence of antibody (lanes 2, 5, 8, 11, 14 and 17) or presence of anti-myc antibody (lanes 3, 6, 9, 12, 15 and 18). Immunoprecipitated proteins were separated and then visualized by immunoblot with anti-myc and anti-HA antibody. (B) The interaction between USP11 and PB2. PB2–HA and Myc–USP11 were transiently co-expressed (lane 4) or singly expressed (lanes 2 and 3) in 293T cells. Immunoprecipitation (IP) assay was performed with anti-myc antibody. Cell lysates (1/10 IP input) and immunoprecipitated proteins were visualized by immunoblot with anti-USP11 and anti-HA antibody, respectively. The tubulin protein was used as an internal control. (C) The interaction between USP11 and PA. The experimental protocol was as described above. (D) The interaction between USP11 and NP.
NP is a monoubiquitinated protein and can be deubiquitinated by USP11 in vivo
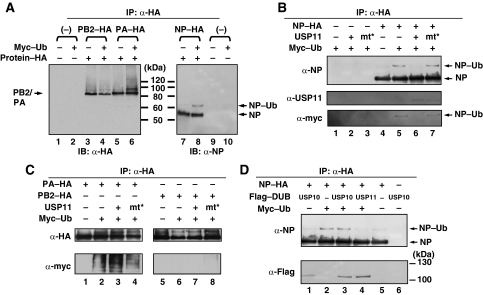
USP11 is a member of ubiquitin-specific proteases (USP) family, which mediates the removal and processing of ubiquitin. The co-immunoprecipitation results showed that USP11 interacts with PB2, PA and NP, respectively. In addition, ubiquitin has been observed in the influenza virus particles (Shaw et al, 2008). We hypothesized that one or more of the RNP complex subunits may be a candidate substrate for USP11 and that ubiquitin is required for influenza A virus RNA replication. To verify this hypothesis, we first attempted to detect ubiquitinated viral protein in the cells. Ubiquitin (Ub) was coexpressed with PB2–HA, PA–HA or NP–HA, respectively, in 293T cells, and the HA-tagged immunoprecipitates were subjected to immunoblot analysis with anti-HA or anti-NP antibody, respectively. The results showed that, for NP, besides the 56-kDa NP, an additional band with a size (65 kDa) consistent with the monoubiquitinated form of NP was detected when it was coexpressed with ubiquitin (Figure 5A, lanes 7 and 8). This result suggested that NP is monoubiquitinated. In addition, when PA was cotransfected with Ub, a high-molecular weight smear was detected by anti-HA (Figure 5A, lane 6) and anti-myc antibodies (Figure 5C lower panel, lane 2), respectively, indicating that PA is a polyubiquitinated protein. By comparison, no larger-molecular weight form of PB2 was detected (Figure 5C, lower panel, lane 6).
Figure 5.
NP is a monoubiquitinated protein and can be deubiquitinated by USP11 in vivo. (A) 293T cells were mock transfected (lanes 1 and 9), or co-transfected with ubiquitin (Ub) and each plasmid encoding PB2–HA (lanes 3 and 4), PA–HA (lanes 5 and 6), or NP–HA (lanes 7 and 8), respectively. At 40 h after transfection, the cell lysates were subjected to immunoprecipitation (IP) assays with anti-HA antibody. Immunoprecipitated proteins were separated and then visualized by immunoblot with anti-HA antibody or anti-NP antibody, respectively. (B) 293T cells were co-transfected with plasmids that can express NP–HA, USP11, USP11–cys mutant and Myc–Ub as indicated. At 40 h after transfection, the cell lysates were prepared and subjected to immunoprecipitation (IP) assays with anti-HA antibody. Immunoprecipitated proteins were separated and then visualized by immunoblot with anti-NP, anti-USP11 and anti-myc antibody, respectively. (C) The protocol was as described in (B). The NP–HA plasmid was replaced by PA–HA or PB2–HA, and visualized by immunoblot with anti-HA and anti-myc antibodies. (D) 293T cells were co-transfected with plasmids that can express NP–HA, Flag–USP10, Flag–USP11 and Myc–Ub as indicated. At 40 h after transfection, the cell lysates were prepared and subjected to immunoprecipitation (IP) assays with anti-HA antibody. Immunoprecipitated proteins were separated and then visualized by immunoblot with anti-NP and anti-Flag antibody respectively.
To verify that NP is a monoubiquitinated protein, we co-transfected plasmids that can express NP–HA, Myc–Ub or USP11, and then subjected HA-tagged immunoprecipitates to immunoblot analysis using the indicated antibodies. The result (Figure 5B) showed that, when both NP and Ub were present (lane 5), there was an additional NP–Ub form, similar to the previous data (Figure 5A). This band could be detected with anti-myc antibody, indicating that it is an ubiquitinated protein (lanes 5 and 7). But, when NP, Ub and USP11 were coexpressed, the NP–Ub signal was diminished (lane 6), whereas USP11 mt did not reduce the NP–Ub signal (lane 7). The result shown in Figures 4C and 5A indicated that PA is a polyubiquitinated protein and can be co-precipitated with USP11. Therefore, we also performed a similar experiment to determine whether PA is a substrate for USP11. As shown in Figure 5C, PA is not a substrate for USP11 (lanes 3 and 4).
Mayer et al (2007) identified several cellular interacting partners of the influenza virus RNP complex by using proteomic-based approaches. USP10 is one of the proteins identified. To determine whether ubiquitinated NP is specifically deubiquitinated by USP11, we co-transfected plasmids that can express NP–HA, Myc–Ub and Flag–USP10 or USP11, and then subjected HA-tagged immunoprecipitates to immunoblot analysis using the indicated antibodies (Figure 5D). The result showed that both USP10 and USP11 specifically co-precipitated with NP–HA (lanes 1, 3 and 4, lower panel), consistent with the published result, and NP could be deubiquitinated by only USP11 but not USP10 (lanes 3 and 4, upper panel). Taken together, we conclude that NP is a monoubiquitinated protein and can be deubiquitinated by USP11 specifically.
K184 is the ubiquitination site on NP and crucial for virus RNA replication
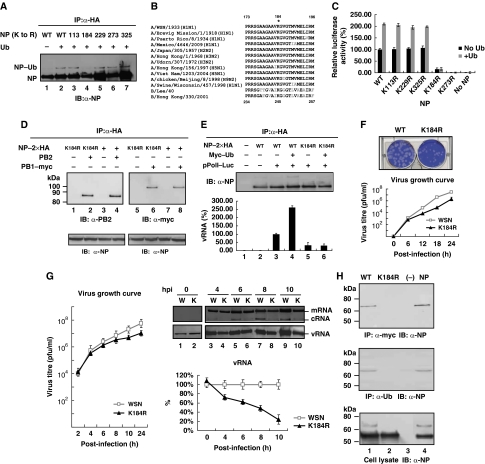
The NP possesses regions that are highly conserved among various influenza A virus strains. To identify the ubiquitination site on NP, we performed site-directed mutagenesis to replace all the crucial conserved lysine residues with arginine based on a published report (Li et al, 2009b) and expressed the HA-tagged forms of mutant and wild-type NP together with Ub into 293T cells. The results showed that the wild type and most of the mutant NP (including K113R, K229R, K273R and K325R), but not K184R mutant, were modified by ubiquitination (Figure 6A). These results indicated that K184 is important for NP ubiquitination and may be the ubiquitination site. The sequence alignment showed that K184 is highly conserved among different subtypes of influenza A virus and influenza B virus (Figure 6B).
Figure 6.
K184 is the ubiquitination site on NP and crucial for virus RNA replication. (A) 293T cells were co-transfected with constructs expressing HA-tagged wild-type NP or its substitution mutants and Ub as indicated. At 40 h after transfection, the cell lysates were prepared and subjected to immunoprecipitation (IP) assays with anti-HA antibody. Immunoprecipitated proteins were separated and then visualized by immunoblot with anti-NP antibody. (B) K184 is highly conserved on NP among different subtypes of influenza A virus (from nt173 to 196) and influenza B virus (from nt234 to 257). (C) Ubiquitination on K184 of NP is important for influenza A virus genome RNA replication using the mini-replicon reporter system. USP11 knockdown cells were transfected with pPolI–Luc and plasmids for the expression of the viral PB2, PB1, PA and NP (wild-type or mutant, black bars). Half of the cells were co-transfected with a plasmid that can express Ub (grey bars). Renilla luciferase was used as an internal control. As a negative control, 293T cells were transfected with the same plasmids, in the absence of the NP expression plasmid. Luciferase assay was performed at 48 h after transfection. The data were normalized relative to the values detected in the cells transfected with wild-type NP. Values are means±s.e.m. of three separate experiments. (D) 293T cells were co-transfected with constructs expressing HA-tagged wild-type NP or K184R mutant and plasmid encoding PB2 (lanes 1–4), or PB1–myc (lanes 5–8), respectively. At 40 h after transfection, the cell lysates were subjected to immunoprecipitation (IP) assays with anti-HA antibody. Immunoprecipitated proteins were separated and then visualized by immunoblot with anti-PB2 antibody or anti-myc antibody, respectively. (E) RNA-binding protein immunoprecipitation (RIP) assay. USP11 knockdown cells were co-transfected with plasmids that can express NP–2xHA, Myc–Ub and pPolI–Luc that can produce vRNA-like molecules, and then subjected to immunoprecipitation using anti-HA antibody to isolate NP–vRNA complex. Immunoprecipitated proteins were separated and then visualized by immunoblot with anti-NP antibody (upper panel); qRT–PCR analysis was carried out to check vRNA in the RNP complex (lower panel). The data were normalized relative to the values detected in cells co-transfected with wild-type NP and pPolI–Luc cells (lane 3). (F) The plaque morphology of NP-K184R and wild-type virus (upper panel), and the growth curve of NP-K184R virus (bottom panel). MDCK cells were infected with NP-K184R and wild-type virus at MOI of 0.01. The supernatant was collected at 6, 12, 18 and 24 h after infection, and then used for plaque assay in MDCK cells. Values are means±s.e.m. of three separate experiments. (G) A549 cells were infected with influenza A/WSN/33 virus or NP-K184R mutant virus at MOI of 5. At the indicated time points after infection, total cellular RNA was analysed by primer extension assay to detect viral NA segment vRNA, cRNA and mRNA (upper panel). qRT–PCR was performed to detect quantitative data of vRNA (bottom panel). Each time point of wild-type virus vRNA amount was designated as 100%. Values are means±s.e.m. of three separate experiments. (H) USP11-knockdown cells were transfected with a plasmid that can express Myc–Ub. At 48 h after transfection, the cells were infected with wild-type or NP-K184R mutant virus at MOI of 5. At 8 h after infection, cell lysates were prepared and subjected to immunoprecipitation (IP) assays with anti-myc or anti-Ub antibody. Cells were co-transfected with a plasmid that can express NP as a positive control (lane 4). Immunoprecipitated proteins were separated and then visualized by immunoblot with anti-NP antibody. Cell lysates without prior immunoprecipitation were also probed with anti-NP (lower panel).
To assess the effects of K184R on viral RNA replication and transcription, we tested the replication function of K184R by using a mini-replicon system (Li et al, 2009b), containing luciferase as the reporter, which assays viral RNA replication activity. The results showed that when K184 was substituted with arginine, the replication and transcription activities were decreased to 30% (Supplementary Figure 1), indicating that K184 is important for viral RNA replication, consistent with a recent study (Wasilenko et al, 2009), but inconsistent with another report (Li et al, 2009b). We repeated the same mini-replicon experiment in USP11 knockdown cells (Figure 6C), and co-transfected a plasmid that can express Ub as a control (grey bars). The result showed that the luciferase activity of the K184 mutant was as low as 15% of the wild-type construct in USP11 knockdown cells. The difference of 30 versus 15% between the two approaches suggests that USP11 may have additional targets in the cells. In addition, it is noted that the luciferase activity of K184R mutant was not affected by overexpression of ubiquitin, whereas the luciferase activity was increased in WT and most of the other NP mutants (including K113R, K229R and K325R). These data taken together support the hypothesis that the replication defect in K184R was most likely due to the failure of ubiquitination. Among these NP mutants, the K273R mutant was totally devoid of luciferase activity, indicating that K273 is crucial for viral genome replication and/or transcription for reasons other than ubiquitination, consistent with the previous studies that demonstrated that this mutation blocked the interaction of NP with two of the polymerase subunits (PB1 and PB2; Biswas et al, 1998; Li et al, 2009b).
A recent study showed that the direct interaction between NP and the viral polymerase results in a modification of the polymerase in favour of unprimed initiation of RNA replication (Newcomb et al, 2009). In addition, Biswas et al (1998) showed that NP interacted with PB1 and PB2, but not PA. To determine whether the defective replication of NP-K184R was caused by the impaired interaction between NP-K184R and the viral polymerase, we co-transfected plasmids that can express NP–2 × HA, PB2 or PB1–myc, and then subjected HA-tagged immunoprecipitates to immunoblot analysis using the indicated antibodies. The result (Figure 6D) showed that there were no significant differences between the interactions of NP-K184R and wild-type NP with PB2 or PB1 (lanes 2 and 4; lanes 6 and 8). Therefore, the defective replication of NP-K184R is not caused by its defective interaction with virus polymerase.
We next examined whether this mutation affected the binding of NP to vRNA by the RNA binding protein immunoprecipitation (RIP) assay. USP11 knockdown cells were co-transfected with plasmids that can express NP–2 × HA, Myc–Ub and pPolI–Luc that can produce vRNA-like molecules, and then subjected HA-tagged immunoprecipitates to isolate NP–vRNA complex. The immunoblot result showed that the NP protein expression levels were equivalent in each reaction (Figure 6E, upper panel). The vRNA isolated from the RNP complex was quantified by qRT–PCR (Figure 6E, lower panel). The result showed that, when both wild-type NP and Ub were present (lane 4), the amount of vRNA precipitated was about 2.5-fold more than that in the absence of Ub, indicating that ubiquitination can improve RNA-binding affinity of NP. In addition, when K184 was substituted with arginine, the amount of vRNA precipitation was decreased to 20%, indicating that this amino acid is crucial for RNA binding (lane 5). Furthermore, with NP-K184R mutant, there was no significant difference between the vRNAs precipitated in the absence and presence of Ub (lanes 5 and 6), indicating that K184 is the ubiquitination site of NP. Taken together (lane 3 versus lane 5; lane 4 versus lane 6), the qRT–PCR result showed that impaired RNA binding of K184R is caused by defective ubiquitination, leading to defective replication of NP-K184R (Figure 6C).
We also produced a recombinant virus incorporating the NP-K184R substitution by reverse genetic techniques (Hoffmann et al, 2000) and examined its biological properties. The plaque size of the NP-K184R mutant virus was smaller than that of the wild-type (Figure 6F). The virus growth curve data (Figure 6F) also indicated that NP-K184R mutant virus was defective in virus production from early time on, consistent with the defect in RNA replication (Figure 6C).
To further dissect the role of NP ubiquitination in viral RNA synthesis, cells were infected with NP-K184R mutant at MOI of 5, and total cellular RNA was used for primer extension analysis at indicated time points (Figure 6G, right upper panel). At 0 h after infection, only vRNA, which represents viral genomic RNA derived from the infecting virus particles, was detected (Figure 6G, lanes 1 and 2). The amounts of cRNA and vRNA increased from 0 to 8 h after infection as RNA replications proceed (lanes 1, 3, 5 and 7 for wild-type RNA, and lanes 2, 4, 6 and 8 for the mutant RNA, respectively). Significantly, the vRNA and cRNA amounts of K184R mutant were less than those of the wild type at each time point (except 0 h), indicating that K184R mutant virus is defective in virus RNA replication, most likely due to lack of ubiquitination. In contrast, the mRNA amount was very similar between K184R mutant and wild-type throughout (lanes 3–8), suggesting that NP ubiquitination is not involved in viral mRNA transcription. We also performed qRT–PCR to detect vRNA (Figure 6G, right lower panel); the result showed that at 10 h after infection, the vRNA amount was decreased to about 25%, but at this time point, the mRNA amount also decreased, probably as a secondary effect of reduction of vRNA. Therefore, the result showed that K184 on NP is crucial for viral RNA replication.
Finally, we performed an additional experiment to directly demonstrate that NP protein was ubiquitinated during virus infection. USP11 knockdown cells were transfected with a plasmid that can express Myc–Ub. At 48 h after transfection, the cells were infected with wild-type or NP-K184R mutant virus at MOI of 5; at 8 h after infection, cell lysates were subjected to immunoprecipitation with anti-myc or anti-Ub antibody, and the immunoprecipitates were subjected to immunoblot analysis by using anti-NP antibody. The result (Figure 6H) showed that the NP–Ub signal can be detected in wild-type (lane 1), but not in NP-K184R mutant virus (lane 2), indicating that NP protein was ubiquitinated at K184 during virus infection. Taken together, we conclude that K184 is ubiquitination site on NP protein and is crucial for viral RNA replication through increasing RNA-binding affinity.
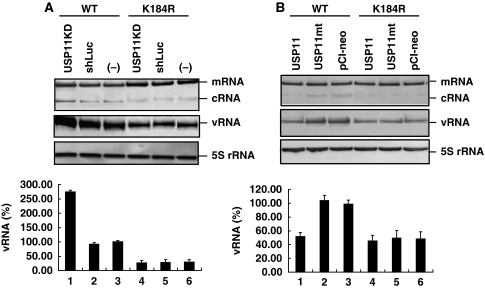
USP11 inhibits viral RNA replication through deubiquitinating NP
We further dissected the mechanism of inhibition of influenza A virus RNA replication by USP11. USP11 knockdown or control cells were infected with wild-type or NP-K184R mutant virus at MOI of 5, and total cellular RNA was used for primer extension and qRT–PCR analysis at 8 h after infection. When cellular USP11 was knocked down, the amounts of vRNA and cRNA of the wild-type virus increased by about 2.5-fold (Figure 7A, lane 1 versus lanes 2 and 3), indicating that USP11 inhibits viral RNA replication as previously mentioned; with the mutant virus defective in NP ubiquitination (K184R), however, there was no significant difference in viral RNA synthesis between USP11 knockdown and control cells (Figure 7A, lanes 4–6). Furthermore, the amounts of viral RNA of K184R mutant were lower than the wild type (about 25% of the wild-type cells infected with the wild-type virus; see lane 3). In addition, the difference in the amount of vRNA between wild-type and K184R mutant viruses was about 10-fold in USP11 knockdown cells (lane 1 versus lane 4). We also performed a converse experiment, namely, USP11-overexpressing or control cells were infected with wild-type or K184R mutant virus (Figure 7B). The amount of vRNA and cRNA of wild-type virus in the cells overexpressing the wild-type USP11 were reduced to about 50% of that in the control cells (Figure 7B, lane 1 versus lane 3), or in the cells expressing the catalytically defective USP11 mutant (lane 2). In contrast, overexpression of USP11 did not affect the amount of vRNA and cRNA of K184R mutant virus (lanes 4–6). Interestingly, the amount of wild-type vRNA in the USP11 overexpressing cells was almost the same as that of K184R mutant (lane 1 versus lane 4). The results were consistent with mini-replicon data (Supplementary Figures 2 and 3). These results combined suggest that USP11 inhibits viral RNA replication through deubiquitinating NP. As a consequence, once NP ubiquitination was defective, such as in K184R mutant virus, the effect of cellular USP11 inhibition was diminished. This result further supports the conclusion that USP11 inhibits viral RNA replication through deubiquitinating NP.
Figure 7.
USP11 inhibits viral RNA replication through deubiquitinating on K184 of NP. (A) USP11 knockdown or control cells were infected with wild-type or NP-K184R mutant virus at MOI of 5. At 8 h after infection, total cellular RNA was analysed by primer extension assay (upper panel). qRT–PCR was performed to detect the amount of vRNA (bottom panel). The data were normalized relative to the values detected in wild-type cells infected with wild-type virus (lane 3). Values are means±s.e.m. of three separate experiments. (B) The same experiment as mentioned in (A) performed in USP11 overexpressing or control cells. The data were normalized relative to the values detected in cells transfected with vector and infected with wild-type virus (lane 3). Values are means±s.e.m. of three separate experiments.
Discussion
Ubiquitination is a posttranslational modification, in which ubiquitin chains or single ubiquitin molecules are linked to target proteins, giving rise to poly- or monoubiquitination (Weissman, 2001). Polyubiquitin chains of four subunits or longer are required for efficient recognition and degradation of ubiquitinated proteins by the proteasome. Other functions of ubiquitin that do not involve the proteasome have also been demonstrated. Some proteins are modified by a single ubiquitin or short ubiquitin chains. Instead of sending proteins to their death through the proteasome, monoubiquitination is a regulator of the location and activity of diverse cellular proteins. Histone is a well-characterized monoubiquitinated protein. Previous studies have shown that ubiquitination may alter conformation or oligomeric state of histone, and thus affect protein–protein or protein–nucleic acid interactions (Hicke, 2001). The proliferating cell nuclear antigen (PCNA) is another example of monoubiquitinated modification of regulation. PCNA is loaded onto DNA strands as a trimeric ring in which it functions as a clamp and processivity factor for DNA polymerase. It is essential for DNA replication, DNA repair and silencing. After exposure of human cells to DNA-damaging agents that block the progress of the replication fork, monoubiquitinated PCNA mediates the switch from replicative DNA polymerases to polymerases specializing in translesion synthesis (Kannouche et al, 2004).
Previous literatures have shown that UPS has an important function in early steps of influenza virus infection. The treatment of host cells with MG132 or another proteasome inhibitor lactacystin affected the early stages of virus replication. The confocal laser scanning microscopic images showed that influenza virus selectively uses the ubiquitin/vacuolar protein-sorting pathway for entry into host cells (Khor et al, 2003). Recently, Shaw et al (2008) used two complementary mass spectrometry approaches to perform a comprehensive proteomic analysis of purified influenza virus particles. Ubiquitin is among the 36 host-encoded proteins in influenza virus particles. In this study, we have identified the deubiquitinating enzyme USP11 as a new cellular factor involved in influenza A virus RNA replication. It can interact with PB2, PA and NP proteins, which are subunits of the viral RNP complex. Furthermore, it could cleave monoubiquitin from NP protein specifically. We have further tested other deubiquitinase, including USP10 and USP32, neither of which can regulate influenza virus RNA replication (CS Tseng, unpublished observations).
USP11 was originally isolated and characterized with the name ubiquitin C-terminal hydrolase on the X chromosome (UHX1; Swanson et al, 1996). It contains both Cys and His boxes, which are responsible for its deubiquitinating activity. It is a ubiquitious protein distributed across a wide range of human tissues and localized in the nucleus. It has been demonstrated that USP11 interacts with RanBPM, a Ran-binding protein, which is required for correct microtubule nucleation (Ideguchi et al, 2002). The published result also showed that USP11 participates in DNA-damage repair functions within the BRCA2 pathway independently of BRCA2 deubiquitination (Schoenfeld et al, 2004). USP11 also regulates the IκB kinase-α (IKKα)–p53 signalling pathway in response to tumor necrosis factor-α (TNFα; Yamaguchi et al, 2007). Recent studies further showed that USP11 can stabilize HPV16-E7 and further regulate its biological activity (Lin et al, 2008). Interestingly, two groups also observed that USP11 is required for West Nile virus and hepatitis C virus infection, but the mechanism remains unknown (Krishnan et al, 2008; Li et al, 2009a).
The influenza A virus nucleoprotein (NP) is a 56-kDa basic RNA-binding protein, which is phosphorylated in vivo (Wright et al, 2007). It binds single-stranded RNA with no sequence specificity through the interactions between its basic residues and the phosphodiester backbone with stacks between its aromatic residues and nucleotide bases (Ye et al, 2006). The N-terminal 180-amino-acid portion of NP bears an RNA-binding domain, which can be subdivided into two smaller regions (residues 1–77 and 79–180), and retain RNA-binding activity (Albo et al, 1995). Owing to its binding to ssRNA, NP is able to self-associate to form large oligomers. In addition, NP can interact with a variety of other macromolecules of both viral and cellular origin. It has been shown that NP interacts directly with PB2 and PB1 but not with PA (Biswas et al, 1998), and that M1 interacts with RNP complex specifically through an interaction between M1 and NP (Ye et al, 1999). It is believed that the interactions between NP and M1 are required for virion assembly. Furthermore, NP can interact with several cellular proteins, including filamentous (F) actin (Digard et al, 1999), nuclear import receptors of the importin-α class (O'Neill and Palese, 1995), the nuclear export receptor CRM1 (Elton et al, 2001) and a DEAD-box helicase BAT1/UAP56 (Momose et al, 2001).
The RNP complex is the basic functional unit for replication and transcription of influenza viral genome. Previous data have shown that the template-size RNA can not be synthesized if there is no NP in the RNP complex (Honda et al, 1988), and NP is essential for the synthesis of vRNA and cRNA (Shapiro and Krug, 1988). Several hypotheses have been proposed for the role of NP in the switch between mRNA and cRNA synthesis (Portela and Digard, 2002). The encapsidation hypothesis proposes that NP does not have a regulatory function but is merely an essential co-factor to coat the nascent cRNA and vRNA segments (Shapiro and Krug, 1988). The template modification hypothesis considers that NP regulates transcription initiation and termination by interaction with template RNA to change its structure (Hsu et al, 1987; Klumpp et al, 1997). The polymerase modification hypothesis proposed that the ability of NP to bind directly to PB1 and PB2 alters the transcription activity of polymerase through direct protein–protein interaction (Biswas et al, 1998). Moreover, a recent study showed that a direct interaction between NP and the viral polymerase results in a modification of the polymerase in favour of unprimed initiation (Newcomb et al, 2009).
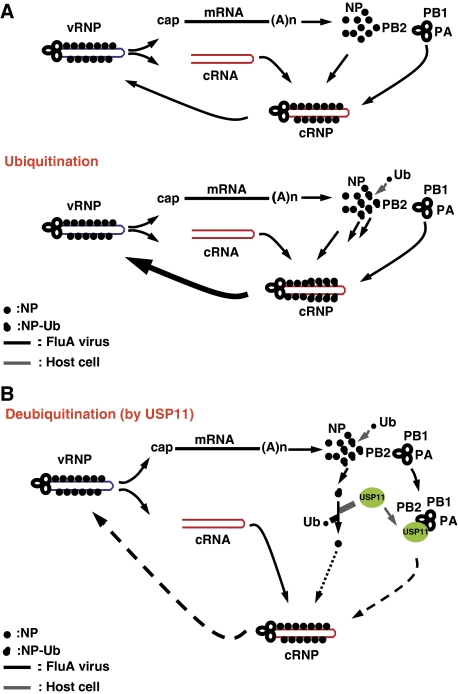
Our results have shown that USP11 inhibited influenza A virus RNA replication and that NP is a monoubiquitinated protein that can be deubiquitinated by USP11 specifically. We have also identified K184 as the ubiquitination site of NP. When K184 was mutated, the viral genome replication was inhibited, consistent with the recently published data (Wasilenko et al, 2009). However, the extent of interaction between NP-K184R and virus polymerase is similar to that between the wild-type NP and the polymerase. The result is consistent with a previous study that demonstrated that the fragment of NP interacting with the viral polymerase did not include K184 (Biswas et al, 1998). In addition, protein structural studies with influenza A virus NP revealed that the potential RNA-binding pockets consist of several positively charged amino acids, including K184 (Ye et al, 2006). The positive charge of K184 may facilitate binding of the negatively charged RNA. However, our data showed that even when K184 was substituted with positively charged arginine, the vRNA-binding affinity and virus RNA replication were still defective, indicating that the defect in RNA replication was caused by reduction of vRNA-binding affinity through impaired NP ubiquitination. It should be noted that protein modification, such as ubiquitination, is a dynamic process, constantly being ubiquitinated and deubiquitinated. Therefore, even though our biochemical studies showed that only a small fraction of NP is ubiquitinated, a larger fraction of NP molecules could, in fact, be ubiquitinated. Vreede et al (2004) proposed ‘stabilization' model that nascent cRNA is degraded by host cell nucleases unless it is stabilized by newly synthesized viral RNA polymerase and NP. On the basis of this model, both mRNA and cRNA are synthesized early in infection, but cRNA is degraded. However, in later infection, once viral polymerase and NP are synthesized, cRNA is stabilized as cRNP complex that can be replicated to vRNA. In addition, a recent study (Perez et al, 2010) showed that influenza A virus-derived small viral RNAs (svRNAs) can trigger the viral switch from transcription to replication through interactions with the viral polymerase machinery. Our result showed that, there were no significant differences between the interactions of NP-K184R and wild-type NP with PB2 or PB1, which are subunits of viral polymerase. In addition, our study also showed that ubiquitination of NP on K184 can alter the interactions of NP with RNA. We hypothesize that monoubiquitination of NP can alter the interactions of NP with RNA, thus stabilizing cRNA, and thereby increase RNA replication efficiency (Figure 8A), but not transcription. It is possible that multiple factors regulate different steps of viral RNA replication and transcription. Conversely, cellular deubiquitinase USP11 can interact with PB2, PA and NP, and thereby affect the function of RNP complex during RNA replication. Significantly, USP11 can cleave single ubiquitin from NP, and thereby inhibit the RNA replication efficiency (Figure 8B). This mechanism offers a new way to fine-tune viral RNA replication through NP modification.
Figure 8.
A proposed model for the regulation of influenza A virus RNA replication by ubiquitination/deubiquitination of NP. (A) Upper panel: the stabilization model proposed by Vreede et al (2004). Lower panel: ubiquitination of viral NP protein may increase its binding affinity to cRNA, and thereby stabilized cRNP and increase RNA replication. (B) Deubiquitination of NP by cellular USP11 may lower its binding affinity to cRNA. In addition, USP11 also can interact with viral PB2 and PA protein, thereby inhibiting viral RNA replication.
Materials and methods
Virus strains and cell culture
The A/WSN/33 (WSN) strain of influenza virus was used throughout. Detailed protocols are available in the Supplementary data.
RNAi knockdown
All RNAi reagents were purchased from the National RNAi Core Facility located at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, supported by the National Research Program for Genomic Medicine Grants of NSC (NSC 97-3112-B-001-016). The target of the most effective lentivirus-based RNAi for USP11 is shUSP11-5: 5′-CCGTGACTACAACAACTCCTA-3′. The control RNAi is shLuc. Cells were seeded at appropriate density on 96-well (100 μl per well) or 6-well (2 ml per well) tissue culture plates and incubated overnight. Cells were maintained in DMEM containing 8 μg/ml polybrene. The RNAi lentivirus was added to cells at an MOI of 5 and incubated overnight. Then the medium was replaced with fresh medium containing 4 μg/ml puromycin and incubated further for 3 days. For primary RNAi screening, cell viability assay was performed by using CellTiter Glo® Luminescent Cell Viability Kit (Promega) according to the manufacturer's protocol.
Plasmid construction
Details are provided in the Supplementary data.
Primer extension assay
Primer extension assay was performed by using the primer extension system-AMV reverse transcriptase kit (Promega). A total of 5 μg of total RNA was mixed with 0.5 pmole each of two DNA primers, labelled at 5′-end with [γ-32P]ATP and T4 polynucleotide kinase (Promega). The mixture was heated at 50°C for 2 h, and followed by cooling at room temperature for 10 min. Primer extensions were performed after addition of 1 U of avian myeloblastosis virus reverse transcriptase (Promega) in the reaction buffer provided with the enzyme for 2 h at 42°C. Two NA-specific primers were used in the same reverse transcription reaction: 5′-TGGACTAGTGGGAGCATCAT-3′ to detect vRNA and 5′-TCCAGTATGGTTTTGATTTCCG-3′ to detect cRNA and mRNA (Vreede et al, 2004). The sequence 5′-TCCCAGGCGGTCTCCCATCC-3′ was used as a primer to detect 5S rRNA (Chan et al, 2006). Transcription products were analysed on 6% polyacrylamide gels containing 7 M urea in TBE buffer and detected by autoradiography.
Quantitative RT–PCR
Total RNAs were extracted by using RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol, and subjected to reverse transcription with specific primers to vRNA (Supplementary Table II). For the detection of vRNA, 200 ng of total RNA was reverse transcribed in a 20-μl final reaction mixture volume containing 1 × rTth reverse transcriptase buffer, 0.5 μM RT primer, 40 U RNaseOUT recombinant RNase inhibitor (Invitrogen), 1 mM MnCl2, 0.2 mM (each) dATP, dGTP, dCTP and dTTP (Invitrogen), and 5 U rTth (Applied Biosystems). The RT reaction was performed at 70°C for 15 min and terminated by adding 2 μl of 10 × chelating buffer. The single-stranded cDNAs were subjected to real-time quantitative PCR by using the QuantiTect SYBR Green PCR kit (Qiagen) with specific primer sets. Briefly, 5 μl of 10-fold serially diluted cDNA was mixed with 45 μl of the master mixture. The reaction consisted of an initiating step of 15 min at 95°C, followed by 40 cycles of amplification, with each cycle consisting of 15 s at 94°C, 30 s at 50°C, and 25 s at 72°C. The reaction, data acquisition and analyses were performed using the iCycler iQ5 v2.0 system (Bio-Rad).
Immunoprecipitation
The 293T cells were transfected in 60-mm dishes by use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's directions. Approximately 48 h after transfection, cells were washed with TBS and lysed in 250 μl of M-PER® reagent (Pierce). After centrifugation at 16 000 g at 4°C for 20 min, 200 μl of whole cell extract was incubated with 10 μl of the indicated antibody agarose slurry (Pierce) and rotated for overnight at 4°C. The column was washed three times with TBS-T buffer and eluted by 25 μl 2 × non-reducing sample buffer (Pierce). Samples were analysed by western blotting using the indicated antibody.
Luciferase assay
For determining polymerase activities of replication complexes containing NP mutant, a mini-replicon using firefly luciferase reporter system was used (Li et al, 2009b). The 293T cells were cotransfected with plasmids for expression of the viral proteins PB2, PB1, PA, NP (wild type or substitution mutant) and pPolI-Luc. Renilla luciferase-expressing plasmid was used as an internal control to normalize transfection efficiency. At 48 h after transfection, cells were collected and the luciferase activity was measured by using Dual-Glo Luciferase assay system (Promega) according to the manufacturer's protocol.
Plasmid-driven reverse genetics
K184R recombinant virus used in this study was generated by reverse genetics. The eight fragments of A/WSN/33 viral genome were amplified by RT–PCR, and viral cDNAs were inserted into the vector of pcDNA3.1 containing pol1 and CMV promoters similar to pHW2000. Recombinant viruses were generated by eight plasmids co-transfection into MDCK/293T cells (Hoffmann et al, 2000). Supernatants were collected, titrated and froze at −80°C ready for use.
RNA-binding protein immunoprecipitation (RIP) assay
RIP assay was performed by using the Magna RIP™ kit (Millipore). USP11 knockdown cells were transfected in 10-cm dishes by use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's directions. Approximately 48 h after transfection, cells were washed twice with ice-cold PBS and scraped off. After centrifugation at 1500 r.p.m. at 4°C for 5 min and discard the supernatant. Cells were lysed in 250 μl of complete RIP lysis buffer. The 100 μl whole cell extract was incubated with 900 μl RIP immunoprecipitation buffer containing the HA-tagged magnetic beads and rotated for overnight at 4°C. Samples were washed three times with RIP wash buffer and 20 μl samples were analysed by immunoblotting using the indicated antibody to check immunoprecipitation efficiency. The remnant samples were incubated with Proteinase K buffer at 55°C for 30 min with shaking to digest the protein. RNAs were extracted by using standard phenol/chloroform protocol and subjected to qRT–PCR with specific primers (Supplementary Table II).
Supplementary Material
Acknowledgments
We thank Dr Shin-Ru Shih and Dr Yi-Ling Lin for helpful discussions and suggestions.
Footnotes
The authors declare that they have no conflict of interest.
References
- Albo C, Valencia A, Portela A (1995) Identification of an RNA binding region within the N-terminal third of the influenza A virus nucleoprotein. J Virol 69: 3799–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniasz PD (2006) Late budding domains and host proteins in enveloped virus release. Virology 344: 55–63 [DOI] [PubMed] [Google Scholar]
- Biswas SK, Boutz PL, Nayak DP (1998) Influenza virus nucleoprotein interacts with influenza virus polymerase proteins. J Virol 72: 5493–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bres V, Kiernan RE, Linares LK, Chable-Bessia C, Plechakova O, Treand C, Emiliani S, Peloponese J-M, Jeang K-T, Coux O, Scheffner M, Benkirane M (2003) A non-proteolytic role for ubiquitin in Tat-mediated transactivation of the HIV-1 promoter. Nat Cell Biol 5: 754–761 [DOI] [PubMed] [Google Scholar]
- Chan AY, Vreede FT, Smith M, Engelhardt OG, Fodor E (2006) Influenza virus inhibits RNA polymerase II elongation. Virology 351: 210–217 [DOI] [PubMed] [Google Scholar]
- Digard P, Elton D, Bishop K, Medcalf E, Weeds A, Pope B (1999) Modulation of nuclear localization of the influenza virus nucleoprotein through interaction with actin filaments. J Virol 73: 2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton D, Simpson-Holley M, Archer K, Medcalf L, Hallam R, McCauley J, Digard P (2001) Interaction of the influenza virus nucleoprotein with the cellular CRM1-mediated nuclear export pathway. J Virol 75: 408–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett RD, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J (1997) A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J 16: 1519–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Sakurai A, Watanabe T, Sorensen E, Nidom CA, Newton MA, Ahlquist P, Kawaoka Y (2008) Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature 454: 890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L (2001) Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol 2: 195–201 [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG (2000) A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci USA 97: 6108–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowaty MN, Frappier L (2004) HAUSP/USP7 as an Epstein–Barr virus target. Biochem Soc Trans 32: 731–732 [DOI] [PubMed] [Google Scholar]
- Honda A, Ueda K, Nagata K, Ishihama A (1988) RNA polymerase of influenza virus: role of NP in RNA chain elongation. J Biochem 104: 1021–1026 [DOI] [PubMed] [Google Scholar]
- Hsu MT, Parvin JD, Gupta S, Krystal M, Palese P (1987) Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle. Proc Natl Acad Sci USA 84: 8140–8144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideguchi H, Ueda A, Tanaka M, Yang J, Tsuji T, Ohno S, Hagiwara E, Aoki A, Ishigatsubo Y (2002) Structural and functional characterization of the USP11 deubiquitinating enzyme, which interacts with the RanGTP-associated protein RanBPM. Biochem J 367: 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger S, Gottwein E, Krausslich H-G (2007) Ubiquitination of human immunodeficiency virus type 1 Gag is highly dependent on Gag membrane association. J Virol 81: 9193–9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannouche PL, Wing J, Lehmann AR (2004) Interaction of human DNA polymerase η with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell 14: 491–500 [DOI] [PubMed] [Google Scholar]
- Karlas A, Machuy N, Shin Y, Pleissner K-P, Artarini A, Heuer D, Becker D, Khalil H, Ogilvie LA, Hess S, Maurer AP, Muller E, Wolff T, Rudel T, Meyer TF (2010) Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 463: 818–822 [DOI] [PubMed] [Google Scholar]
- Khor R, McElroy LJ, Whittaker GR (2003) The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic 4: 857–868 [DOI] [PubMed] [Google Scholar]
- Klumpp K, Ruigrok RWH, Baudin F (1997) Roles of the influenza virus polymerase and nucleoprotein in forming a functioal RNP structure. EMBO J 16: 1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza MB, Liang Y, Gao Q, Andrews SE, Bandyopadhyay S, De Jesus P, Tu BP, Pache L, Shih C, Orth A, Bonamy G, Miraglia L et al. (2010) Human host factors required for influenza virus replication. Nature 463: 813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, Brass AL, Adametz R, Tsui M, Qian F, Montgomery RR, Lev S, Mason PW, Koski RA, Elledge SJ, Xavier RJ, Agaisse H, Fikrig E (2008) RNA interference screen for human genes associated with West Nile virus infection. Nature 455: 242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ, Lai C, Frias-Staheli N, Giannakopoulos NV, Lutz A, Wolff T, Osiak A, Levine B, Schmidt RE, García-Sastre A, Leib DA, Pekosz A, Knobeloch K-P, Horak I, Virgin HW (2007) IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci USA 104: 1371–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Brass AL, Ng A, Hu Z, Xavier RJ, Liang TJ, Elledge SJ (2009a) A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci USA 106: 16410–16415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Watanabe T, Hatta M, Watanabe S, Nanbo A, Ozawa M, Kakugawa S, Shimojima M, Yamada S, Neumann G, Kawaoka Y (2009b) Mutational analysis of conserved amino acids in the influenza A virus nucleoprotein. J Virol 83: 4153–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Chang H-S, Yu WCY (2008) USP11 stabilizes HPV-16E7 and further modulates the E7 biological activity. J Biol Chem 283: 15681–15688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner HA (2007) Deubiquitination in virus infection. Virology 362: 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer D, Molawi K, Martínez-Sobrido L, Ghanem A, Thomas S, Baginsky S, Grossmann J, García-Sastre A, Schwemmle M (2007) Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J Proteome Res 6: 672–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose F, Basler CF, O'Neill RE, Iwamatsu A, Palese P, Nagata K (2001) Cellular splicing factor RAF-2p48/NPI-5/BAT1/UAP56 interacts with the influenza virus nucleoprotein and enhances viral RNA synthesis. J Virol 75: 1899–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb LL, Kuo R-L, Ye Q, Jiang Y, Tao YJ, Krug RM (2009) Interaction of the influenza A virus nucleocapsid protein with the viral RNA polymerase potentiates unprimed viral RNA replication. J Virol 83: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill RE, Palese P (1995) NPI-1, the human homolog of SRP-1, interacts with influenza virus nucleoprotein. Virology 206: 116–125 [DOI] [PubMed] [Google Scholar]
- Peloponese J-M Jr, Iha H, Yedavalli VRK, Miyazato A, Li Y, Haller K, Benkirane M, Jeang K-T (2004) Ubiquitination of human T-cell leukemia virus type 1 tax modulates its activity. J Virol 78: 11686–11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JT, Varble A, Sachidanandam R, Zlatev I, Manoharan M, García-Sastre A, tenOever BR (2010) Influenza A virus-generated small RNAs regulate the switch from transcription to replication. Proc Natl Acad Sci USA 107: 11525–11530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A, Digard P (2002) The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J Gen Virol 83: 723–734 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Perez-Gonzalez A, Nieto A (2007) Influenza virus infection causes specific degradation of the largest subunit of cellular RNA polymerase II. J Virol 81: 5315–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld AR, Apgar S, Dolios G, Wang R, Aaronson SA (2004) BRCA2 is ubiquitinated in vivo and interacts with USP11, a deubiquitinating enzyme that exhibits prosurvival function in the cellular response to DNA damage. Mol Cell Biol 24: 7444–7455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GI, Krug RM (1988) Influenza virus RNA replication in vitro: synthesis of viral template RNAs and virion RNAs in the absence of an added primer. J Virol 62: 2285–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ML, Stone KL, Colangelo CM, Gulcicek EE, Palese P (2008) Cellular proteins in influenza virus particles. PLoS Pathog 4: e1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Gao G, Wong J, Wang Y, Zhang J, Luo H (2008) Ubiquitination is required for effective replication of Coxsackievirus B3. PLoS ONE 3: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson D, Freund C, Ploder L, McInnes R, Valle D (1996) A ubiquitin C-terminal hydrolase gene on the proximal short arm of the X chromosome: implications for X-linked retinal disorders. Hum Mol Genet 5: 533–538 [DOI] [PubMed] [Google Scholar]
- Tseng C-H, Jeng K-S, Lai MMC (2008) Transcription of subgenomic mRNA of hepatitis delta virus requires a modified hepatitis delta antigen that is distinct from antigenomic RNA synthesis. J Virol 82: 9409–9416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreede FT, Jung TE, Brownlee GG (2004) Model suggesting that replication of influenza virus is regulated by stabilization of replicative intermediates. J Virol 78: 9568–9572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilenko JL, Sarmento L, Pantin-Jackwood MJ (2009) A single substitution in amino acid 184 of the NP protein alters the replication and pathogenicity of H5N1 avian influenza viruses in chickens. Arch Virol 154: 969–979 [DOI] [PubMed] [Google Scholar]
- Weissman AM (2001) Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol 2: 169–178 [DOI] [PubMed] [Google Scholar]
- Welchman RL, Gordon C, Mayer RJ (2005) Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol 6: 599–609 [DOI] [PubMed] [Google Scholar]
- Wright PF, Neumann G, Kawaoka Y (2007) Orthomyxoviruses. In Fields Virology, Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (eds), 5th edn, pp 1691–1740. Lippincott Williams & Wilkins: Philadelphia, PA [Google Scholar]
- Yamaguchi T, Kimura J, Miki Y, Yoshida K (2007) The deubiquitinating enzyme USP11 controls an IκB Kinase α (IKKα)-p53 signaling pathway in response to tumor necrosis factor α (TNFα). J Biol Chem 282: 33943–33948 [DOI] [PubMed] [Google Scholar]
- Ye Q, Krug RM, Tao YJ (2006) The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 444: 1078–1082 [DOI] [PubMed] [Google Scholar]
- Ye Z, Liu T, Offringa DP, McInnis J, Levandowski RA (1999) Association of influenza virus matrix protein with ribonucleoproteins. J Virol 73: 7467–7473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G-Y, Lai MMC (2005) The ubiquitin–proteasome system facilitates the transfer of murine coronavirus from endosome to cytoplasm during virus entry. J Virol 79: 644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.