Critical role for hyperpolarization-activated cyclic nucleotide-gated channel 2 in the AIF-mediated apoptosis
This study establishes HCN2 channels as physiological relevant ‘calcium gates' that mediate apoptosis-inducing factor-dependent cell death in cancer and primary neuronal cells.
Keywords: apoptosis, apoptosis-inducing factor, calcium signalling, calpain, HCN channel
Abstract
Cellular calcium uptake is a controlled physiological process mediated by multiple ion channels. The exposure of cells to either one of the protein kinase C (PKC) inhibitors, staurosporine (STS) or PKC412, can trigger Ca2+ influx leading to cell death. The precise molecular mechanisms regulating these events remain elusive. In this study, we report that the PKC inhibitors induce a prolonged Ca2+ import through hyperpolarization-activated cyclic nucleotide-gated channel 2 (HCN2) in lung carcinoma cells and in primary culture of cortical neurons, sufficient to trigger apoptosis-inducing factor (AIF)-mediated apoptosis. Downregulation of HCN2 prevented the drug-induced Ca2+ increase and subsequent apoptosis. Importantly, the PKC inhibitors did not cause Ca2+ entry into HEK293 cells, which do not express the HCN channels. However, introduction of HCN2 sensitized them to STS/PKC412-induced apoptosis. Mutagenesis of putative PKC phosphorylation sites within the C-terminal domain of HCN2 revealed that dephosphorylation of Thr549 was critical for the prolonged Ca2+ entry required for AIF-mediated apoptosis. Our findings demonstrate a novel role for the HCN2 channel by providing evidence that it can act as an upstream regulator of cell death triggered by PKC inhibitors.
Introduction
Ca2+-mediated signal transduction regulates multiple cellular processes, including cell motility, DNA transcription, exocytosis and cell death (Berridge et al, 1998). The physiological functions are normally governed by Ca2+ oscillations, whereas perturbation of the intracellular Ca2+ homeostasis may lead to cell death by either apoptosis or necrosis. The cytotoxic Ca2+ response is generally more pronounced and sustained than physiological Ca2+ signalling and can be due to excessive Ca2+ influx by plasma membrane ion channels, or to inappropriate Ca2+ release from intracellular stores, notably, the endoplasmic reticulum (Orrenius et al, 2003).
Non-small-cell lung carcinomas (NSCLC) are resistant to conventional radio- and chemotherapy. Nevertheless, NSCLC cells were shown to be sensitive to staurosporine (STS) and to the more selective protein kinase C (PKC) inhibitor, PKC412 (N-benzoyl-staurosporine), both of which trigger apoptosis in these cells (Joseph et al, 2002; Gallego et al, 2004). Recently, we reported that STS-induced cell death signalling in NSCLC cells involved both a stimulation of Ca2+ influx into the cells and an increase in the intracellular level of reactive oxygen species (ROS); both these events were required for the mitochondrial processing and release of apoptosis-inducing factor (AIF) and subsequent apoptosis (Norberg et al, 2008, 2010a, 2010b).
AIF is a 62-kDa flavoprotein with NADH oxidase activity that is anchored to the inner mitochondrial membrane. The flavine adenine dinucleotide domain is essential for the redox function of AIF, but it does not contribute to its apoptotic function. To promote apoptosis, AIF must first be liberated from its membrane anchor, generating a soluble 57-kDa AIF fragment, that can be released from the mitochondria and translocate to the nucleus, where it promotes chromatin condensation and large-scale DNA fragmentation (Otera et al, 2005; Hangen et al, 2010) AIF-mediated apoptosis is independent of caspase activation and represents an important cell death mechanism in neurons and cells of neuroendocrine origin, for example, NSCLC (Norberg et al, 2010b).
So far, it has been unclear how the PKC inhibitors trigger Ca2+ influx into sensitive cells, and through which plasma membrane ion channel(s) Ca2+ enters the cell. Potential candidates are the family of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. There are four HCN channels (HCN1–4) described, which are permeable to Na+ and K+ (ratio 1:4) and can be inhibited by mM concentrations of Cs+ (Craven and Zagotta, 2006; Biel et al, 2009). Interestingly, two of the four channels, namely, HCN2 and HCN4, were demonstrated to also be permeable to Ca2+, in addition to Na+ and K+ (Yu et al, 2004; Michels et al, 2008). However, the physiological implications of Ca2+ signalling through these channels are not known.
To investigate whether the HCN channels might participate in apoptosis signalling, we used NSCLC cells and primary cultures of rat cortical neurons exposed to PKC412 or STS as experimental model systems. Our data suggest that both kinase inhibitors caused Ca2+ influx through the HCN channel 2 by dephosphorylation of Thr549 within the internal regulatory domain of HCN2. The imported Ca2+ triggered apoptosis mediated by the calpain–AIF axis.
Results
PKC inhibitors trigger hyperpolarization-induced Ca2+ entry into NSCLC cells
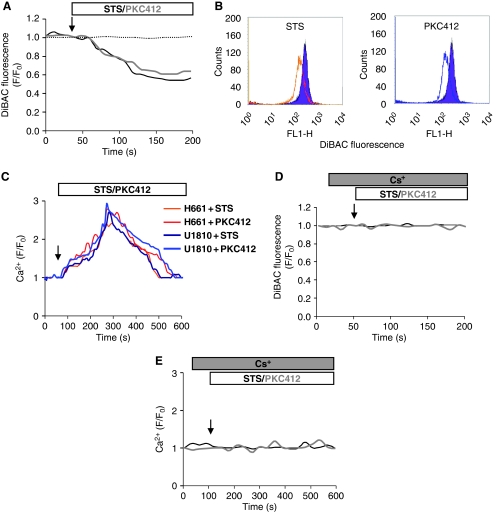
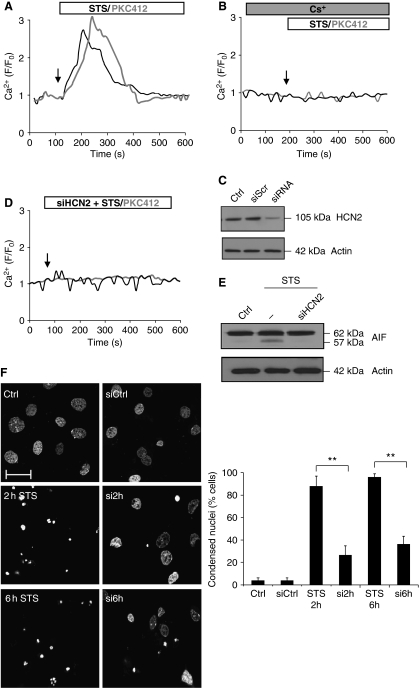
We have previously reported that PKC inhibitors can trigger a Ca2+ influx from the extracellular medium into NSCLC cells (Norberg et al, 2008). To examine which plasma membrane Ca2+ channels might be involved in this response, we first tested the effects of known inhibitors of the voltage-dependent Ca2+ channels (VDCCs), such as nifedipine, Ni2+, Ba2+ and La3+. None of these inhibitors could prevent STS/PKC412-induced Ca2+ influx into the cells (Supplementary Figure S1). The majority of the VDCCs are activated by depolarization, and only few examples of hyperpolarization-activated Ca2+ channels exist (Siegelbaum, 2000). To analyse if STS or PKC412 might affect the polarization of the plasma membrane, cells were loaded with a polarization-sensitive dye, DiBAC, and analysed by time-lapse single-cell recordings (Figure 1A) as well as by flow cytometry (Figure 1B) Hyperpolarization was confirmed by patch-clamp recordings (Supplementary Figure S2). Thus, an early hyperpolarization of the cells was detected upon exposure to any one of the two PKC inhibitors.
Figure 1.
Protein kinase C inhibitors trigger hyperpolarization-mediated Ca2+ entry into NSCLC cells. U1810 cells were loaded with DiBAC, exposed to 0.2–1 μM STS or 1–5 μM PKC412 and analysed for the polarization of the plasma membrane using (A) confocal microscopy or (B) flow cytometry. (C) Representative single-cell Ca2+ recordings of Fluo-4/AM-loaded cells exposed to 0.2–1 μM STS or 1–5 μM PKC412. (D) DiBAC-loaded cells were exposed to 3 mM CsCl before STS or PKC412 treatment. (E) Fluo-4/AM-loaded cells were pre-exposed to 3 mM CsCl before STS or PKC412 treatment. Ratio F/F0 represents fluorescence intensity over baseline.
Next, the effects of STS/PKC412 on the intracellular Ca2+ level in U1810 and H661 NSCLC cell lines were monitored. Figure 1C depicts representative single-cell Ca2+ responses in cells loaded with the Ca2+-sensitive dye Fluo-4/AM and exposed to either one of the kinase inhibitors. Both PKC412 and STS induced a prolonged elevation of the intracellular Ca2+ level in the two cell lines. Yet, other kinase inhibitors, such as Ro-31-8220 and Wortmannin, did not stimulate Ca2+ entry into the cells (Supplementary Figure S3).
As mentioned above, only a few ion channels can be activated by hyperpolarization. One example is the family of HCN channels. Interestingly, two of these channels (HCN2 and HCN4) were demonstrated to also be permeable to Ca2+, in addition to Na+ and K+ (Yu et al, 2004; Michels et al, 2008). However, the cellular implications of Ca2+ signalling by these channels are not known. Therefore, the possible contribution of HCN channels to the Ca2+ entry into cells exposed to PKC inhibitors was investigated. First, the two NSCLC cell lines were exposed to Cs+ before treatment with STS or PKC412, then plasma membrane polarization (Figure 1D) and the cytosolic Ca2+ level (Figure 1E) were monitored. Preincubation with Cs+ inhibited both the hyperpolarization and the Ca2+ entry into STS/PKC412-treated cells, suggesting that under these conditions hyperpolarization was required for the Ca2+ influx, and that this occurred through the HCN channel(s).
PKC inhibitor-induced Ca2+ influx through the HCN2 channel leads to cell death
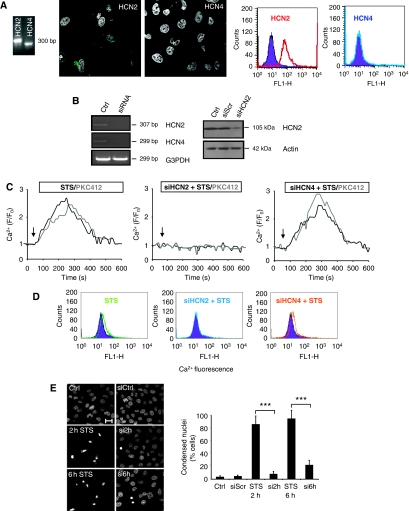
To investigate whether one of the Ca2+-permeable HCN channels was involved in the Ca2+ import triggered by STS/PKC412, the mRNA and protein expression of HCN2/HCN4 was analysed by RT–PCR, immunocytochemistry and FACS, respectively. Both HCN2 and HCN4 mRNAs were expressed in U1810 (Figure 2A) and H661 cells (Supplementary Figure S4). However, the protein expression was detected only for the HCN2 channel, and the immunostainings confirmed its typical plasma membrane localization (Figure 2A).
Figure 2.
HCN2 channels mediate the PKC inhibitor-induced Ca2+ influx and subsequent cell death. (A) RT–PCR, immunostainings and FACS analysis of HCN2 and HCN4 expression. Cells were counterstained with DAPI. (B) mRNA level (left panel) and protein level (right panel) after 48 h of 100 nM siRNA silencing of HCN2 and HCN4 (C) Representative single-cell Ca2+ recordings of Fluo-4/AM-loaded cells exposed to 0.2–1 μM STS or 1–5 μM PKC412 in the presence or absence of HCN2 and HCN4. Ratio F/F0 represents fluorescence of baseline. (D) FACS analysis of 10 000 Fluo-4-loaded cells with downregulated HCN2 or HCN4. (E) Cells were exposed to STS for different time periods (indicated in figure), fixed and the nuclei were stained with DAPI. Condensed nuclei were counted and presented in the graph as mean±s.d. (n=4). ***P<0.001. Scale bar, 15 μm. A full-colour version of this figure is available at The EMBO Journal Online.
To examine the possible role of HCN channels in the PKC inhibitor-induced Ca2+ entry, a siRNA approach was chosen. The levels of mRNA (HCN2/HCN4) and protein (HCN2) expression after siRNA downregulation are shown in Figure 2B (left and right panel, respectively). Next, cells were loaded with Fluo-4/AM and exposed to either one of the two PKC inhibitors. Representative Ca2+ traces, shown in Figure 2C, demonstrate that HCN2, but not HCN4, depletion abolished the kinase inhibitor-induced Ca2+ entry. To confirm this, Fluo-4/AM-loaded cells were treated with STS/PKC412 (as in Figure 2C), and 10 000 cells were analysed by flow cytometry (Figure 2D). In order to exclude off-target effect of the siRNA, two distinct non-overlapping siRNAs specific for HCN2 were used. Obtained results confirmed that the HCN2 channel indeed is mediating the influx of Ca2+ (Supplementary Figure S9A).
In addition, a rescue experiment was performed, in which HCN2 was first downregulated by siRNA in U1810 cells, and then mHCN2 was introduced. Ca2+ recordings revealed that the original phenotype observed after treatment with STS was restored by introducing mHCN2 (Supplementary Figure S5). Furthermore, to investigate whether Ca2+ influx by the HCN2 channel was sufficient to trigger apoptosis, the siRNA approach was used before exposure of the cells to STS/PKC412, and the number of cells with condensed nuclei was counted. Although chromatin condensation was observed in many of the control cells treated with STS (Figure 2E, left panel), downregulation of HCN2 significantly delayed this type of cell death manifestation (Figure 2E, right panel). Altogether, our results indicate that the STS/PKC412-induced Ca2+ influx by the HCN2 channel was sufficient to trigger an apoptotic response in NSCLC cells.
Ca2+ entry through HCN2 channels triggers caspase-independent, AIF-mediated cell death
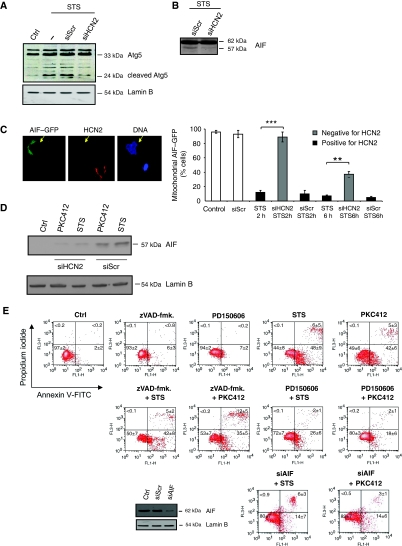
To examine the mechanism of cell death that was triggered by the Ca2+ influx through the HCN2 channel, we first analysed if calpain was activated by monitoring the cleavage of two selective calpain substrates, Atg5 (Figure 3A) (Yousefi et al, 2006) and AIF (Figure 3B), in control cells and cells depleted of HCN2 channels. As shown in Figure 3A, STS-stimulated Atg5 proteolysis was not observed in cells depleted of HCN2. Furthermore, the cleavage of AIF was also suppressed as a result of downregulation of HCN2 (Figure 3B). Accordingly, the mitochondrial liberation of AIF–GFP upon STS treatment was inhibited in cells with downregulated HCN2, and AIF remained in the mitochondria (Figure 3C). Nuclear localization of AIF in HCN2-expressing cells was confirmed using confocal microscopy (Supplementary Figure S9B). Furthermore, nuclear translocation of AIF was suppressed in cells where HCN2 was downregulated (Figure 3D). To confirm that the calpain–AIF signalling pathway was in fact responsible for cell death in this experimental model, four different methods were used. First, FACS analysis of Annexin V/PI-stained cells pre-exposed to either the pan-caspase inhibitor (zVAD-fmk.), the selective calpain inhibitor (PD150606), or siRNA against AIF was performed (Figure 3D and E). Second, condensed nuclei were counted under the same conditions (Supplementary Figure S6). Third, processing/activation of caspases-2, -3, -8 -9 and cleavage of PARP were monitored (Supplementary Figure S7). Finally, caspases-3/-7-like activity was measured (Supplementary Figure S7). In line with previous observations, all these results confirmed that the HCN2-mediated influx of Ca2+ triggered caspase-independent, AIF-mediated apoptosis.
Figure 3.
Ca2+ influx through HCN2 channels triggers caspase-independent AIF-mediated cell death. (A) The calpain-mediated cleavage of Atg5 in the presence or absence of HCN2 was analysed by western blot. The membranes were reprobed for Lamin B to confirm equal protein loading. (B) HCN2 was downregulated by siRNA, and cells were exposed to STS. AIF processing was assessed by western blot. (C) HCN2 was downregulated by an siRNA approach, and 24 h later cells were transfected with AIF–GFP. Cells were subsequently treated with STS for 2 and 6 h and fixed. Cells stained positively/negatively for HCN2 were analysed by immunostaining, and nuclei were counterstained with DAPI. A quantification of the amount of cells harbouring AIF–GFP in mitochondria after STS treatment is presented in the graph as mean±s.d. (n=5). **P<0.01; ***P<0.001 (Student's t-test) (D) Nuclei were isolated from control and HCN2-depleted cells exposed to STS/PKC412. The presence of AIF was analysed by western blotting. Membranes were reprobed for Lamin B to confirm equal loading of samples. (E) FACS analysis of Annexin V/PI-stained cells that were pretreated with either the pan-caspase inhibitor (10 μM zVAD-fmk.), a calpain inhibitor (150 μM PD150606) or siRNA against AIF before STS or PKC412 exposure. Downregulation of AIF was confirmed by western blot analysis. Membranes were reprobed for Lamin B to confirm equal loading of the samples.
Expression of HCN2 sensitizes HEK293 cells to STS/PKC412-evoked Ca2+ influx and apoptosis
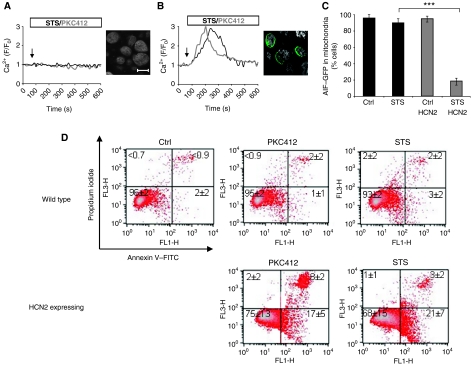
HEK293 cells are deficient of HCN channels and, therefore, are commonly used as a model system to study heterologus expression (Yu et al, 2004). As expected, no HCN2 protein was detected using immunocytochemistry. Moreover, neither STS, nor PKC412, could stimulate Ca2+ influx into these cells (Figure 4A). Hence, we investigated if an STS/PKC412-evoked Ca2+ response and subsequent apoptotic effects could be introduced by expressing the HCN2 channel in HEK293 cells. Indeed, a prolonged PKC inhibitor-induced Ca2+ influx was observed in transfected cells harbouring the ectopic HCN2 channel (Figure 4B). As a control for the expression of HCN2, cells were fixed and immunostained for HCN2 (Figure 4A and B, right panels). Similar to previous observations, AIF was released from the mitochondria upon STS exposure, only in cells expressing HCN2 (Figure 4C). Next, when cells were exposed to the kinase inhibitors and analysed by FACS using Annexin V/PI staining, we observed that HEK293 cells survived STS or PKC412 treatment for at least 24 h (Figure 4D). However, morphological signs of cytotoxicity were detected, suggesting that they might die in a delayed manner. Conversely, the expression of HCN2 could sensitize these cells to STS/PKC412. These data demonstrate that HCN2 was indeed responsible for cellular Ca2+ influx after treatment with PKC inhibitors.
Figure 4.
Expression of mHCN2 sensitizes HEK293 cells to STS/PKC412-induced Ca2+ influx and apoptotic cell death. (A) HEK293 cells were loaded with the Fluo-4/AM dye and exposed to STS or PKC412. Representative single-cell Ca2+ responses are shown in the graph. (B) The HCN2 channel was expressed in HEK293 cells, which were subsequently treated as in (A). (C) Control or HCN2-expressing HEK293 cells were transfected with AIF–GFP and exposed to STS. Cells were fixed and immunostained for HCN2. A quantification of cells with mitochondrial AIF–GFP is presented in the graph. Results are presented as mean±s.d. (n=6) (D) Control or HCN2-expressing HEK293 cells were exposed to STS/PKC412 and subsequently analysed by FACS for Annexin V/PI staining. Ratio F/F0 represents fluorescence of baseline. ***P<0.001 (Student's t-test). Scale bar, 15 μm.
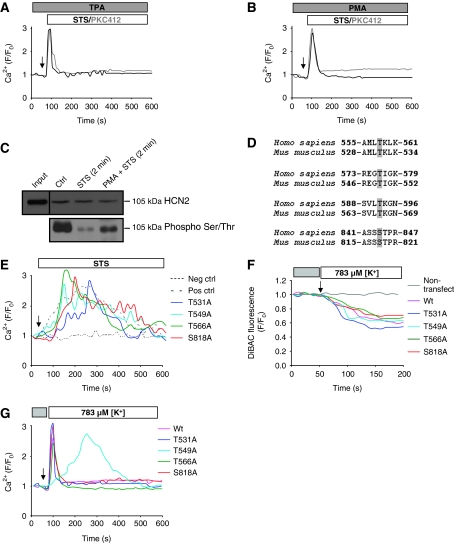
Dephosphorylation of Thr549 is essential for the prolonged Ca2+ entry through the HCN2 channel
To investigate whether the observed kinase inhibitor-induced Ca2+ influx was regulated by a phosphorylation-dependent mechanism, we exposed the cells to PKC activators. Pretreatment of cells with either one of the two different PKC activators (TPA or PMA), before administration of STS or PKC412, prevented the prolonged Ca2+ import through the HCN2 channel (Figure 5A and B and Supplementary Figure S8). Next, the Ser/Thr phosphorylation level of the HCN2 in open/closed states was analysed. The results revealed that the channel is phosphorylated in control cells, and that exposure to STS for only 2 min led to significant dephosphorylation of the channel (Figure 5C). Furthermore, pretreatment of the cells with a PKC activator (PMA) suppressed this decrease. In silico analysis of the HCN2 channel revealed four putative PKC phosphorylation sites within its internal C-terminus domain. Alignment of human and mouse HCN2 sequences shows that these residues are conserved among species and suggests that the regulation of channel activity by phosphorylation might also be conserved (Figure 5D). Site-directed mutagenesis of each of the putative phosphorylation sites was performed, in which Ser/Thr was substituted with Ala. The various mutants were transfected into HEK293 cells, and as expected, recordings of intracellular Ca2+ level showed that the four mutants responded to STS or PKC412 in a similar manner as wild-type cells (non-mutated plasmid) (Figure 5E).
Figure 5.
Dephosphorylation of Thr549 is essential for the prolonged Ca2+ influx through HCN2. U1810 cells were pretreated with the PKC activators (A) 1 μM TPA or (B) 300 nM PMA, before exposure to STS or PKC412. (C) The HCN2 channel was immunoprecipitated and analysed by western blot for phosphorylation on Ser/Thr residues. (D) Conserved putative PKC phosphorylation sites in the C terminal of human and mouse HCN2 are marked in grey. (E) The mutant and wild-type forms of the HCN2 channel were expressed in HEK293 cells, which were subsequently loaded with Fluo-4/AM, and the intracellular Ca2+ level was monitored upon exposure to STS. Untransfected HEK293 cells served as negative control and cells transfected with wild-type HCN2 channel was used as a positive control. MitoDsRed was used as a transfection marker. Hyperpolarization was induced by decreasing the K+ level from 4.7 mM (grey box) to 783 μM (white box), and polarization was monitored using DiBAC (F) and the Ca2+ level using Fluo-4/AM (G) in HEK293 cells transfected with wild-type and various mutants of HCN2. Untransfected HEK293 cells served as negative control.
The wild-type HCN2 channel is activated by hyperpolarization and dephosphorylation triggered by PKC inhibitors. Alternatively, the Ala mutants might be opened either by the same mechanism or simply due to the hyperpolarization, as they mimic a dephosphorylated state of the channel. To test this possibility, HEK293 cells transfected with the different mutants were hyperpolarized by decreasing the [K+] level in the incubation medium (Figure 5F). These experiments revealed that the wild-type cells, as well as the Thr531, Thr566 and Ser818 mutants, responded to the hyperpolarization with a transient Ca2+ increase, similar to what was observed using pretreatment with PKC activators, whereas a prolonged Ca2+ elevation was observed with the Thr549 mutant (Figure 5G). Thus, our results suggest that dephosphorylation of the HCN channel on residue Thr549 is pivotal for the prolonged Ca2+ influx mediated by the HCN2 channel upon treatment of NCSLC cells with PKC inhibitors.
PKC inhibition leads to the influx of Ca2+ through the HCN2 channel also in cortical neurons
Current in vivo data suggest that the AIF-mediated pathway might be particularly important for neuronal cell death (Zhu et al, 2007a, 2007b; Hangen et al, 2010). Hence, we decided to investigate whether a mechanism similar to that observed in NSCLC cells might operate in primary cultures of rat cortical neurons. Similar to NSCLC cells, STS or PKC412 exposure resulted in a sustained increase in the intracellular Ca2+ level (Figure 6A). The Ca2+ influx was blocked by preincubation with Cs+ (Figure 6B). Next, the HCN2 protein was downregulated using the siRNA approach (Figure 6C); this treatment prevented PKC inhibitor-induced Ca2+ entry (Figure 6D) and the subsequent processing of AIF (Figure 6E). Furthermore, by assessing the number of cortical neurons with condensed nuclei upon treatment with STS, we found that siRNA depletion of the HCN2 channel did suppress cell death also in this model system (Figure 6F). In summary, our results reveal that PKC inhibitor-induced Ca2+ import through the HCN2 channel is sufficient to trigger apoptosis also in cortical neurons and suggest that this might be a general mechanism of AIF activation in cell types expressing this channel.
Figure 6.
PKC inhibition leads to the entry of Ca2+ through the HCN2 channel in primary culture of cortical neurons. The cells were loaded with Fluo-4/AM and exposed to (A) STS or PKC412 and (B) CsCl before STS or PKC412. (C) Western blot analysis of the HCN2 level after siRNA-induced downregulation. HCN2 was downregulated by siRNA and cells were either (D) loaded with Fluo-4/AM and exposed to STS or PKC412 or (E) exposed to STS followed by western blot analysis of AIF cleavage. (F) Cells were exposed to STS for different time periods (indicated in figure), fixed and the nuclei were stained with DAPI. Condensed nuclei were counted and presented in the graph as mean±s.d. (n=4). **P<0.01. Scale bar, 15 μm.
Discussion
Intracellular Ca2+ overload can lead to apoptotic as well as necrotic cell death, and can be due to excessive Ca2+ entry from the extracellular milieu, impaired Ca2+ extrusion or inappropriate Ca2+ release from intracellular stores. Excess Ca2+ can cause cytotoxicity and cell death by the activation of a variety of degradative enzymes, including Ca2+-dependent proteases, or by impairment of mitochondrial function (Orrenius et al, 2003).
NSCLC cells are resistant to conventional anticancer treatments, but can be killed by the PKC inhibitors, STS and PKC412. The responsible cell death pathway involves the influx of extracellular Ca2+, leading to calpain-mediated mitochondrial processing and nuclear translocation of AIF, which causes chromatin condensation, DNA fragmentation and apoptotic cell death (Joseph et al, 2002; Norberg et al, 2008, 2010a, 2010b). However, the precise regulation of this mechanism and the identity of the plasma membrane channels responsible for the Ca2+ entry remain elusive.
Recently, it was reported that two of the HCN channels previously identified in the plasma membrane of multiple cell types are also permeable to Ca2+, in addition to K+/Na+ (Yu et al, 2004; Michels et al, 2008). This finding suggested a possible role for these channels in mediating cellular Ca2+ influx. Unlike most other plasma membrane Ca2+ channels, HCN channels are activated by hyperpolarization. By monitoring polarization of the plasma membrane, we found that treatment of NSCLC cells with STS or PKC412 resulted in hyperpolarization of the plasma membrane and Ca2+ influx into the cells (see Figure 1A, 2B and C). It was reported previously that the Ca2+ current flow through the HCN2 channel triggered by hyperpolarization pulses is rather slow in comparison with that seen in other Ca2+ channels (Yu et al, 2004). In line with these reports, we observed a time-dependent, sustained Ca2+ influx through the HCN2 channel that was followed by a normalization of the Ca2+ level (see Figure 1A). The imported Ca2+ caused an elevation of the intracellular Ca2+ level that persisted for several minutes, which was sufficient to trigger apoptosis through activation of the calpain–AIF axis (see Figure 2E, G and H and Supplementary Figure S6). All these apoptotic events occurred in a time window where caspase activation was not yet detected (Supplementary Figure S7).
Opening of HCN channels is regulated by cAMP binding and phosphorylation-dependent mechanisms. For instance, tyrosine kinases of the Src kinase family have been shown to phosphorylate HCN1, HCN2 and HCN4, resulting in a more rapid activation of the channel (Zong et al, 2005; Biel et al, 2009). Moreover, inhibition of Ser/Thr p38 MAP kinase has been reported to cause hyperpolarization and activation of the HCN channel (Poolos et al, 2006). Our results suggest that there is a constitutive phosphorylation by PKC of Thr549 within the conserved C-terminal regulatory domain of the channel, and that the PKC inhibitors, PKC412 or STS, trigger both hyperpolarization and dephosphorylation of Thr549. Hence, hyperpolarization alone would only trigger a transient Ca2+ influx through the HCN channel. Similarly, when PKC activators are present to prevent dephosphorylation events, STS/PKC412 could only stimulate a transient Ca2+ response (see Figure 5A and B). Such a Ca2+ transient has been reported earlier to be insufficient to activate an apoptotic response (Norberg et al, 2008). However, when Thr549 was mutated and could no longer be phosphorylated, hyperpolarization per se resulted in a prolonged Ca2+ elevation in the absence of any drug exposure of cells. Hence, it appears that the dephosphorylation of Thr549 is pivotal for the involvement of HCN2 in cell death signalling.
There are multiple isoforms of PKC, including PKC-α and PKC-epsilon, which have been reported to promote proliferation and survival of NSCLC cells. These PKC isoforms were also found to be overexpressed in NSCLC. Therefore, protein kinases C are attractive drug targets, and several PKC inhibitors have been designed that are currently being investigated in clinical trials (Reyland, 2009). Our results support this approach, as exposure of NSCLC cells to either one of the PKC inhibitors, STS or PKC412, led to intracellular Ca2+ elevation and subsequent AIF-mediated cell death. In particular, PKC412 is clinically relevant as it is currently used in combination with Cisplatin and Gemcitabine for the treatment of patients with NSCLC, and it has been reported to interact strongly with PKC-α, -β and -γ (Reyland, 2009). Moreover, PKC412 was shown to have greater antitumour activity in nude mice bearing human H460 NSCLC tumour xenografts as compared with other conventional cytotoxic agents, including doxorubicin, cyclophosphamide, cisplatin and gemcitabine (Monnerat et al, 2004). Our findings might therefore have particular implications for treatment of NSCLC.
Materials and methods
Cell culture and transient transfection
U1810 and H661 NSCLC cells were grown in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 U/ml streptomycin and 2% (w/v) glutamine. HEK293 cells were grown in DMEM medium containing 10% (v/v) heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin. Cells were grown in a humidified 5% CO2 atmosphere at 37°C at a cell density allowing exponential growth. For the plasmid transfections, cells were seeded on coverslips and on the following day transfected with either pEGFP–AIF or pcDNA3.1–mHCN2 using the Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer's instruction. pMitoDsRed was used as a transfection marker.
Culture of cerebral cortical neurons
Primary cultures of cerebral cortical neurons were prepared from Sprague–Dawley rat pups, 1–3 days old. The cerebral cortices were dissected in Hank's balanced salt solution (HBSS). HBSS was removed and the tissues were treated with 0.25% trypsin at 37°C for 15 min. This was followed by a 5 min treatment with 0.1% DNase I at 37°C. Cells were then diluted to 7 × 105 cells/ml in DMEM medium containing 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mmol/l L-glutamine, and were seeded on plates precoated with 50 μg/ml poly-L-lysine. After 3 h, DMEM was replaced with neurobasal medium (GIBCO) containing B27 Supplement (GIBCO), 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mmol/l L-glutamine. Half of the cell culture medium was replaced after three DIV with neurobasal medium containing the supplements specified above and 10−7 mol/l aracytine.
Immunocytochemistry
Cells were seeded on coverslips, fixed for 20 min in 4% formaldehyde at 4°C and then washed with PBS. Incubations with primary antibodies diluted (1:400) in PBS containing 0.3% Triton X-100 and 0.5% bovine serum albumin (BSA) were performed at 4°C overnight. The slides were washed the following day with PBS before incubation with secondary antibodies (1:200) at room temperature for 1 h. Nuclei were counterstained with DAPI (10 μg/ml) by 5 min incubation at room temperature. The following primary antibodies were used: rabbit anti-HCN2 (Alomone), goat anti-HCN2 and goat anti-HCN4 (Santa Cruz Biotechnology). Secondary FITC-conjugated antibodies (Molecular probes) were directed to goat (Alexa 488). Stained slides were mounted using Vectashield H-1000 (Vector Laboratories Inc.) and examined under a Zeiss LSM 510 META confocal laser scanner microscope (Zeiss).
Immunoblotting
Equal amount of the protein samples were mixed with loading buffer, boiled for 5 min, and subjected to 15% SDS–PAGE at 40 mA followed by transfer to nitrocellulose membranes for 90 min at 120 V. Membranes were blocked for 30 min with 5% non-fat milk in TBS at room temperature and subsequently probed with rabbit anti-cleaved Atg5 (Abgent), goat anti-AIF (Santa Cruz Biotechnology), rabbit anti-actin (Sigma) or rabbit anti-HCN2 (Alomone). ECL™ (Amersham Biosciences) was used for revealing the blots. The primary antibodies were diluted in TBS containing 1% BSA, 0.05% Tween-20 and 0.1% NaN3. Horseradish peroxidase-conjugated secondary antibodies (Pierce) were diluted in 2.5% blocking buffer.
Live cell imaging
To perform Ca2+ or polarization measurements the cells were incubated (30 min at 37°C in 5% CO2) in KREBS medium containing 5 μM Fluo-4/AM (Molecular Probes) together with 0.1% Pluronic F-127 (Molecular Probes) or 150 nM DiBac. The Hepes medium contained 130 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 1 mM MgSO4, 1.2 mM KH2PO4, 20 mM Hepes (pH 7.4) and 5 mM dextrose. Hyperpolarization that was induced by decreasing the extracellular K+-level was performed as follows. Cells were incubated in 500 μl KREBS medium and subsequently the K+ concentration was decreased to 17% (783 μM) by the addition of K+-free KREBS. Images were acquired at 0.2 Hz. During the time-lapse experiments all drugs were bath-applied and examined under a Zeiss LSM 510 META scanning laser confocal microscope (Zeiss). The following drugs were used: 0.2–1 μM STS and 1–5 mM PKC412, 1 μM TPA and 300 nM PMA.
Flow cytometry
Cells were loaded with DiBac or Fluo-4/AM as described above and treated with 1 μM STS or 3 mM PKC412 immediately before FL-1 FACS analysis of 10 000 cells. Cell death was assessed using the Annexin V-FLUOS staining kit (Roche), according to the manufacturer's instruction.
For analysis of HCN2 and HCN4 staining, cells were washed in PBS and fixed with 0.25% paraformaldehyde for 10 min at room temperature. Next, cells were stained with HCN2 and HCN4 antibodies as described in Materials and methods section, and Immunocytochemistry.
RNA isolation and RT–PCR
Total RNA was isolated from U1810 or H661 cells using the RNeasy kit (Qiagen). The quality of the RNA was controlled by gel electrophoresis of 18S and 28S ribosomal RNA. For the first-strand cDNA synthesis, the RevertAid™ M-MuL V RT enzyme (Fermentas) was used in combination with gene-specific primers according to the manufacturer's instruction. The PCR was performed using the Platinum®Pfx DNA polymerase system (Invitrogen) and the following specific primers (Invitrogen): HCN2 (forward primer, CGGCCCAGTGGCTGAGAGGA; reverse primer, CACTGCTGCCTCGGCTCGTC), HCN4 (forward primer, GGCCACTTCCACAAGGCGCT; reverse primer, CACAAGGGACGGCGGCTCAG), G3PDH (forward primer, CCTGGCCAAGGTCATCCATG; reverse primer CTGACACGTTGGCAGTGGG). The conditions for HCN2 and G3PDH PCR reactions were as follows: 94°C for 1 min (hot start) followed by 95°C for 1 min, 55°C for 30 s and 72°C for 1 min. For the PCR of HCN4 57°C was used as annealing temperature. Samples were removed every fifth cycle to ensure exponential growth of the PCR product, which was then analysed on a 1% agarose gel.
Measurement of caspases-3/-7-like activity
The measurement of caspase substrate (Peptide Institute, Osaka, Japan) cleavage was performed as follows: 2 × 106 cells were washed once with PBS, resuspended in 25 μl of PBS and placed on a microtiter plate. Cells were subsequently mixed with DEVD-AMC (50 μM), dissolved in standard reaction buffer (100 mM Hepes, pH 7.25, 10% sucrose, 10 mM DTT, 0.1% CHAPS). Cleavage of the fluorogenic peptide substrate was monitored by AMC liberation in a Fluoroscan II plate reader (Thermo Electron Co., Waltham, MA, USA) using 355 nm excitation and 460 nm emission wavelengths.
siRNA approach
Silencing of HCN2, HCN4 and AIF expression was achieved by transfection of anti-HCN2 (ONTARGETplus SMARTpool L-006201-00-0005, Dharmacon), anti-HCN4 (ONTARGETplus SMARTpool L-006203-00-0005, Dharmacon) and anti-AIF (PDC8) (ONTARGETplus SMARTpool L-011912-00-0005, Dharmacon) using INTERFERIN siRNA transfection reagent (Polyplus transfection). The level of the mRNAs was monitored by RT–PCR and the level of protein by western blot.
Site-directed mutagenesis
The pcDNA3.1–mHCN2 plasmid was kindly provided by Prof Hang-Gang Yu, West Virginia University School of Medicine, Morgantown. Quick-change II XL Site-Directed Mutagenesis kit (Stratagene) was used according to the manufacturer's instructions to generate the mutations of putative PKC phosphorylation sites within the regulatory C-terminal domain of HCN2: T531A, T549A, T566A, S818A. The following primers were used: T531A (forward primer, TCACAGCCATGCTGGCAAAGCTCAAATTTG; reverse primer, CAAATTTGAGCTTTGCCAGCATGGCTGTGA), T549A (forward primer, CCGAGAGGGGGCCATCGGGAAGA; reverse primer, TCTTCCCGATGGCCCCCTCTCGG), T566A (forward primer, TGAGCGTGCTCGCCAAGGGCAACAA; reverse primer, TTGTTGCCCTTGGCGAGCACGCTCA), S818A (forward primer, GGCCAGCAGCGCCACGCCGC; reverse primer, GCGGCGTGGCGCTGCTGGCC). Desired substitutions were confirmed by sequencing.
Statistical analysis
Data from at least three independent experiments are presented as mean±s.d. Student's unpaired t-test was used for the comparisons between different groups. A value of P<0.05 was considered as statistically significant.
Supplementary Material
Acknowledgments
We are grateful to Dr Vladimir Gogvadze for valuable discussions. We thank Professor Hang-Gang Yu for his generous gift of pCDNA3.1–mHCN2. This work was supported by grants from the Swedish Research Council, the Swedish and the Stockholm Cancer Societies, the Swedish Childhood Cancer Foundation, Swedish Foundation for Strategic Research (CEDB), the Knut and Alice Wallenberg Foundation (CLICK), the EC FP-6 (Chemores) as well as the FP7 (Apo-Sys) programs.
Author contributions: EN, PU, SO and BZ designed research; EN, MK, OK performed research; SS performed electrophysiological recordings; EN, GS, PU, SO and BZ analysed data; and EN, PU, SO and BZ wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Berridge MJ, Bootman MD, Lipp P (1998) Calcium—a life and death signal. Nature 395: 645–648 [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X (2009) Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847–885 [DOI] [PubMed] [Google Scholar]
- Craven KB, Zagotta WN (2006) CNG and HCN channels: two peas, one pod. Annu Rev Physiol 68: 375–401 [DOI] [PubMed] [Google Scholar]
- Gallego MA, Joseph B, Hemstrom TH, Tamiji S, Mortier L, Kroemer G, Formstecher P, Zhivotovsky B, Marchetti P (2004) Apoptosis-inducing factor determines the chemoresistance of non-small-cell lung carcinomas. Oncogene 23: 6282–6291 [DOI] [PubMed] [Google Scholar]
- Hangen E, Blomgren K, Benit P, Kroemer G, Modjtahedi N (2010) Life with or without AIF. Trends Biochem Sci 35: 278–287 [DOI] [PubMed] [Google Scholar]
- Joseph B, Marchetti P, Formstecher P, Kroemer G, Lewensohn R, Zhivotovsky B (2002) Mitochondrial dysfunction is an essential step for killing of non-small cell lung carcinomas resistant to conventional treatment. Oncogene 21: 65–77 [DOI] [PubMed] [Google Scholar]
- Michels G, Brandt MC, Zagidullin N, Khan IF, Larbig R, van Aaken S, Wippermann J, Hoppe UC (2008) Direct evidence for calcium conductance of hyperpolarization-activated cyclic nucleotide-gated channels and human native If at physiological calcium concentrations. Cardiovasc Res 78: 466–475 [DOI] [PubMed] [Google Scholar]
- Monnerat C, Henriksson R, Le Chevalier T, Novello S, Berthaud P, Faivre S, Raymond E (2004) Phase I study of PKC412 (N-benzoyl-staurosporine), a novel oral protein kinase C inhibitor, combined with gemcitabine and cisplatin in patients with non-small-cell lung cancer. Ann Oncol 15: 316–323 [DOI] [PubMed] [Google Scholar]
- Norberg E, Gogvadze V, Ott M, Horn M, Uhlen P, Orrenius S, Zhivotovsky B (2008) An increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ 15: 1857–1864 [DOI] [PubMed] [Google Scholar]
- Norberg E, Gogvadze V, Vakifahmetoglu H, Orrenius S, Zhivotovsky B (2010a) Oxidative modification sensitizes mitochondrial apoptosis-inducing factor to calpain-mediated processing. Free Radic Biol Med 48: 791–797 [DOI] [PubMed] [Google Scholar]
- Norberg E, Orrenius S, Zhivotovsky B (2010b) Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF). Biochem Biophys Res Commun 396: 95–100 [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565 [DOI] [PubMed] [Google Scholar]
- Otera H, Ohsakaya S, Nagaura Z, Ishihara N, Mihara K (2005) Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J 24: 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Bullis JB, Roth MK (2006) Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase. J Neurosci 26: 7995–8003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland ME (2009) Protein kinase C isoforms: multi-functional regulators of cell life and death. Front Biosci 14: 2386–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum SA (2000) Presynaptic facilitation by hyperpolarization-activated pacemaker channels. Nat Neurosci 3: 101–102 [DOI] [PubMed] [Google Scholar]
- Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8: 1124–1132 [DOI] [PubMed] [Google Scholar]
- Yu X, Duan KL, Shang CF, Yu HG, Zhou Z (2004) Calcium influx through hyperpolarization-activated cation channels (I(h) channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci USA 101: 1051–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, Neagu MR, Nilsson M, Eriksson PS, Hagberg H, Luban J, Kroemer G, Blomgren K (2007a) Cyclophilin A participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med 204: 1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson PS, Hagberg H, Culmsee C, Plesnila N, Kroemer G, Blomgren K (2007b) Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ 14: 775–784 [DOI] [PubMed] [Google Scholar]
- Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, Li R, Mistrik P, Gerstner A, Much B, Baumann L, Michalakis S, Zeng R, Chen Z, Biel M (2005) A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem 280: 34224–34232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.