Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition
Snail1 is subject to O-GlcNAc modification under hyperglycaemic conditions; this stabilizes the transcriptional repressor and hence promotes epithelial–mesenchymal transition.
Keywords: epithelial–mesenchymal transition, O-GlcNAc, OGT, Snail1
Abstract
Protein O-phosphorylation often occurs reciprocally with O-GlcNAc modification and represents a regulatory principle for proteins. O-phosphorylation of serine by glycogen synthase kinase-3β on Snail1, a transcriptional repressor of E-cadherin and a key regulator of the epithelial–mesenchymal transition (EMT) programme, results in its proteasomal degradation. We show that by suppressing O-phosphorylation-mediated degradation, O-GlcNAc at serine112 stabilizes Snail1 and thus increases its repressor function, which in turn attenuates E-cadherin mRNA expression. Hyperglycaemic condition enhances O-GlcNAc modification and initiates EMT by transcriptional suppression of E-cadherin through Snail1. Thus, dynamic reciprocal O-phosphorylation and O-GlcNAc modification of Snail1 constitute a molecular link between cellular glucose metabolism and the control of EMT.
Introduction
Metabolic regulation is important for the proliferation and survival of cancer cells, as indicated by their high rate of aerobic glycolysis (Warburg, 1956; Hsu and Sabatini, 2008; Vander Heiden et al, 2009). Glucose, as a major carbon and energy source, provides ATP and various macromolecules required for cancer cell growth. Through ATP citrate lyase, glucose metabolism of cancer cells is tightly linked to lipid synthesis (Hatzivassiliou et al, 2005) and histone acetylation (Wellen et al, 2009). Therefore, glucose utilization by ATP citrate lyase not only sustains proliferation of cancer cells but also affects gene expression. Indeed, glucose-dependent regulation of histone acetylation has been shown to selectively affect the expression of genes required for aerobic glycolysis (Wellen et al, 2009).
In addition to being a source of ATP and various cellular macromolecules, 2–5% of glucose enters the hexosamine biosynthetic pathway for the synthesis of uridine 5′-diphospho-N-acetylglucosamine (UDP-GlcNAc) (McClain and Crook, 1996), which is transferred by O-GlcNAc transferase (OGT) to serine (Ser) or threonine (Thr) residues of many proteins to yield the O-linked β-N-acetylglucosamine (O-GlcNAc) modification (Wells et al, 2001). This modification is highly dynamic and occurs reciprocal to O-phosphorylation in various nuclear and cytoplasmic proteins (Love and Hanover, 2005; Hart et al, 2007). The reciprocity of O-GlcNAc modification and O-phosphorylation is important in regulating the function of many proteins within signalling cascades and in degrading certain proteins such as β-catenin (Kamemura and Hart, 2003). Serial O-phosphorylation of Ser and Thr is mediated by glycogen synthase kinase-3β (GSK-3β), a pivotal kinase involved in glycogen metabolism and Wnt signalling (Yook et al, 2006). Inhibition of GSK-3β, on the other hand, results in perturbation of O-GlcNAc modification on many transcription factors and structural proteins, indicating diverse roles for the modification in cellular signalling (Wang et al, 2007). As a case in point, oncogenes and tumour suppressor proteins as well as ligand-responsive transcription factors are modified by O-GlcNAc, whose dynamic exchange with O-phosphorylation is involved in the regulation of their activity (Cheng et al, 2000; Kamemura and Hart, 2003; Love and Hanover, 2005; Hart et al, 2007).
Regulation of cell–cell adhesion is essential for embryonic development and cell differentiation as well as for homeostasis of the epithelium. Loss of cell adhesion occurs in many types of cancer and correlates with tumour progression, metastatic potential, and poor prognosis (Peinado et al, 2007). E-cadherin, the prototypic member of the cadherin family, is a major component of adherent junctions and regulates cell adhesion via homophilic interactions between its extracellular domains on opposing plasma membranes and by binding of its cytosolic domain to the cytoskeleton via β-catenin (Gottardi and Gumbiner, 2004). Down-regulation of E-cadherin is a critical step in the epithelial–mesenchymal transition (EMT) and thus carcinoma invasion and progression.
Snail1, a transcriptional repressor of E-cadherin, is a key regulator of EMT (Nieto, 2002; Barrallo-Gimeno and Nieto, 2005). Of note, Snail1 is serially O-phosphorylated by CK1 and GSK-3β prior to β-TrCP-mediated ubiquitination and proteasomal degradation (Zhou et al, 2004; Xu et al, 2010). Conversely, inhibition of GSK-3β by Wnt signalling leads to Snail1 stabilization, resulting in down-regulation of E-cadherin and stimulation of EMT programmes (Yook et al, 2005, 2006). As O-phosphorylation often occurs reciprocally with O-GlcNAc modification, we sought to determine the extent of O-GlcNAc modification of Snail1 and its potential function in regulation of EMT. Here, we show that the O-GlcNAc modification at Ser112 stabilizes Snail1 by suppressing its O-phosphorylation-mediated degradation. Under hyperglycaemic conditions and with enhanced protein O-GlcNAc modification, Snail1 stimulates EMT through transcriptional suppression of E-cadherin. These data provide mechanistic insight into Snail1 function, showing a molecular link between the glucose metabolism of cells and the control of EMT by reciprocal O-phosphorylation and O-GlcNAc modification of Snail1.
Results
The E-cadherin suppressor Snail1 is modified by O-GlcNAc at Ser112
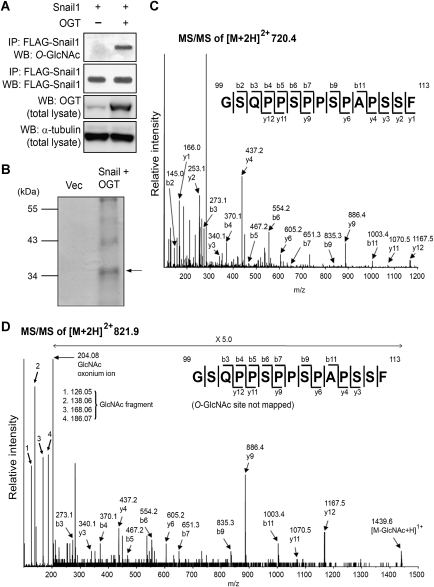
O-GlcNAc modifies various transcription factors and cellular proteins in a process dynamically reciprocal to O-phosphorylation of the same Ser and Thr residues or adjacent residues, and is important in regulating function of the relevant protein (Love and Hanover, 2005; Hart et al, 2007). As the E-cadherin repressor Snail1 is regulated by O-phosphorylation through GSK-3β (Yook et al, 2006) as a key step in its β-TrCP-mediated ubiquitination followed by proteasomal degradation (Zhou et al, 2004; Yook et al, 2006), we sought to determine whether Snail1 is modified with O-GlcNAc. For this purpose, human embryonic kidney (HEK) cells 293 were transfected to express FLAG-tagged Snail1 and immunoprecipitated Snail1 was probed with the O-GlcNAc-specific monoclonal antibody CTD110.6. We were able to unequivocally detect O-GlcNAc on Snail1 in an amount, which could be significantly increased by co-expressed OGT (Figure 1A).
Figure 1.
The E-cadherin repressor Snail1 carries the O-GlcNAc modification. (A) Western blot analysis for O-GlcNAc of immunoprecipitated Snail1 from OGT-expressing or control vector-transfected HEK 293 cells. α-tubulin was included as a loading control. (B) FLAG-tagged Snail1 was immunoprecipitated from OGT-overexpressing HEK 293 cells, separated by SDS–PAGE, and visualized by Coomassie G250 (arrow). This band was in-gel digested and analysed by mass spectrometry. (C) MS/MS spectrum and sequencing result of an unmodified peptide from in-gel digestion at [M+2H]2+ m/z 720.4. The peptide was identified as GSQPPSPPSPAPSSF, corresponding to the 99–113 fragment of Snail1. (D) MS/MS spectrum and sequencing result of an O-GlcNAc-bearing 99–113 peptide at [M+2H]2+ m/z 821.9. Difference in molecular mass between the unmodified peptide (C) and the O-GlcNAc-bearing peptide (D) is ∼203 Da.
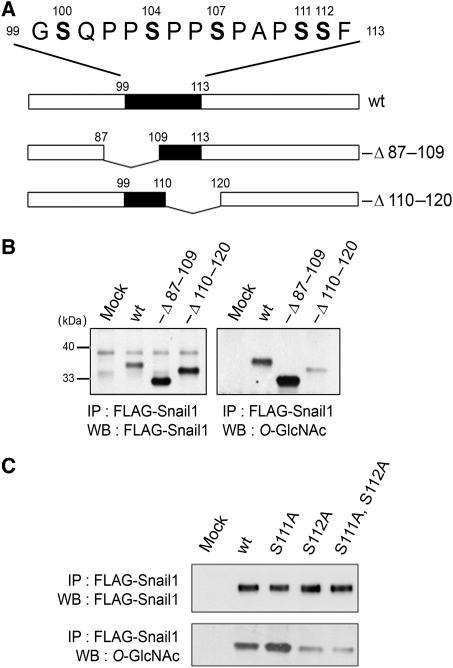
To determine the existence and location of the O-GlcNAc site(s) on Snail1 by mass spectrometry analysis, Snail1 was immunoprecipitated from HEK293 cells co-expressing OGT and subjected to SDS–PAGE (Figure 1B). The Snail1 band was then in-gel digested with both trypsin and chymotrypsin, and analysed by quadrupole time-of-flight tandem mass spectrometry (Q-ToF MS). Both unmodified and O-GlcNAc-modified 99–113 peptides of Snail1 (GSQPPSPPSPAPSSF) were observed at [M+2H]2+ m/z 720.4 and m/z 821.9, respectively (Figure 1C and D). The molecular mass of the O-GlcNAc-bearing peptide was 203.08 Da higher than that of the unmodified peptide and its spectrum showed the GlcNAc oxonium ion and several GlcNAc fragment ions. These indicate that the 99–113 peptide of Snail1 carries O-GlcNAc, although the exact position of the O-GlcNAc site on this peptide could not be defined because of fragmentation of O-GlcNAc from the peptide during Q-ToF MS analysis. To determine the specific O-GlcNAc sites, we created deletions and site-specific point mutants of Snail1 (Figure 2A). When amino acids 110–120 were deleted, much less O-GlcNAc could be detected by immunoblot using the monoclonal antibody CTD110.6 compared with wild type, whereas deleting amino acids 87–109 had no effect on O-GlcNAc detection (Figure 2B), suggesting that the site of O-GlcNAc modification was either Ser111 or Ser112. Indeed, exchange of Ser112 with Ala largely resulted in loss of O-GlcNAc, indicating that this is the major, if not the only Snail1 site carrying the O-GlcNAc modification (Figure 2C).
Figure 2.
Ser112 of Snail1 carries O-GlcNAc. (A) Deletion mutants of Snail1 analysed for the presence of O-GlcNAc. (B) FLAG-tagged wt Snail1, −Δ87–109 Snail1, and −Δ110–120 Snail1 were co-expressed with OGT in HEK293 cells. Wt and deletion mutants of Snail1 were immunoprecipitated with anti-FLAG beads, and immunoblotted for O-GlcNAc. (C) FLAG-tagged wt or point-mutated Snail1 were co-expressed with OGT in HEK293 cells. Wt and point mutants of Snail1 were immunoprecipitated with anti-FLAG beads, and immunoblotted for O-GlcNAc.
O-GlcNAc modification at Ser112 prevents Snail1 degradation
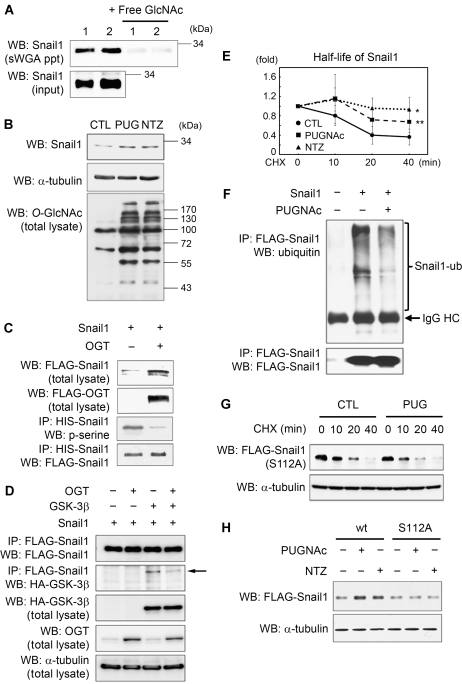
Next, to examine whether endogenous Snail1 is modified by O-GlcNAc, we used succinylated wheat germ agglutinin (sWGA) for affinity purification of A549 total cell lysates (Love and Hanover, 2005; Gambetta et al, 2009) and probed the precipitates with an antibody against Snail1. We successfully detected O-GlcNAc-modified endogenous Snail1 (Figure 3A). The specificity of the lectin-affinity purification was shown by addition of the inhibitory monosaccharide GlcNAc (Figure 3A). We then investigated whether the O-GlcNAc modification on Snail1 is of functional significance for its turnover. As serial O-phosphorylation by GSK-3β (Zhou et al, 2004; Yook et al, 2005) initiates the proteasomal degradation of Snail1 (Yook et al, 2005, 2006), we reasoned that the O-GlcNAc modification of the adjacent Ser112 could affect Snail1 stability. To verify this, we used inhibitors of O-GlcNAcase (OGA), PUGNAc, or NAG-thiazoline (NTZ), which cause a shift in equilibrium from O-phosphorylation to O-GlcNAc modification. Following the treatment of A549 carcinoma cells with either OGA inhibitor, an increased steady state amount of Snail1 as well as enhanced O-GlcNAc modification of various cellular proteins was observed (Figure 3B), although without changes in Snail1 mRNA level (Supplementary Figure 1A).
Figure 3.
Presence of O-GlcNAc stabilizes Snail1 expression by inhibiting O-phosphorylation. (A) A549 cells were grown under hyperglycaemic condition (25 mM) and cell lysates were subjected to sWGA-lectin-affinity purification and the precipitates analysed with western blot for endogenous Snail1. For control, monosaccharide inhibitor GlcNAc (20 mM) was added during sWGA-lectin-affinity purification. Experiments were performed in duplicate (1 and 2). (B) Western blot analysis for Snail1 and O-GlcNAc from A549 cells in the presence/absence of OGA inhibitors PUGNAc or NAG-Thiazoline. α-tubulin was included as a loading control. (C) FLAG/HIS-tagged Snail1 was immunoprecipitated with Ni-Ti agarose from HEK293 cells and the precipitate analysed by western blot with anti-phospho-serine antibody and anti-FLAG antibody. Overexpression of OGT drastically reduces phospho-serine on Snail1. (D) FLAG-Snail1, HA-GSK-3β, and OGT were overexpressed in A549 cells as indicated. FLAG-Snail1 was immunoprecipitated and the precipitate immunoblotted with anti-HA antibody and anti-FLAG antibody, respectively. Arrow indicates HA-GSK-3β. (E) Half-life of Snail1 is increased by OGA inhibitors PUGNAc or NAG-Thiazoline. Cells were treated with cycloheximide for the indicated time and Snail1 level was analysed by western blotting and densitometry (n=3; data are represented as mean±s.d., *P<0.01, **P<0.02 by Student's t-test). (F) Western blot analysis for ubiquitin on Snail1 immunoprecipitated from HEK293 cells in the presence/absence of OGA inhibitor PUGNAc. The cells were treated with MG132 for proteasome inhibition. (G) Half-life of S112A mutant Snail1 in the presence/absence of OGA inhibitor PUGNAc. Cells were treated with cycloheximide for indicated time, and Snail1 level was analysed by western blotting. (H) Western blot analysis of wt and S112A mutant Snail1 in the presence/absence of OGA inhibitors PUGNAc or NAG-Thiazoline. α-tubulin was used as a loading control.
Furthermore, overexpression of OGT in HEK293 cells drastically decreased the level of O-phosphorylation on Ser of Snail1, accompanied by an increase in Snail1 protein expression (Figure 3C). We also observed that OGT overexpression decreased the interaction between Snail1 and GSK-3β (Figure 3D), indicating that O-GlcNAc modification of Snail1 affects GSK-3β-mediated protein stabilization. Indeed, treatment with OGA inhibitors consistently increased Snail1 half-life (Figure 3E), and this was paralleled by suppression of Snail1 ubiquitination (Figure 3F) in a manner similar to that described for p53 (Yang et al, 2006). As expected, no effect was observed on the half-life and expression levels of the S112A Snail1 mutant (Figure 3G and H). Additional experiments in which either OGT (Supplementary Figure 2A) or OGA (Supplementary Figure 2B) were overexpressed corroborated the stabilizing effect of increased O-GlcNAc modification on Snail1.
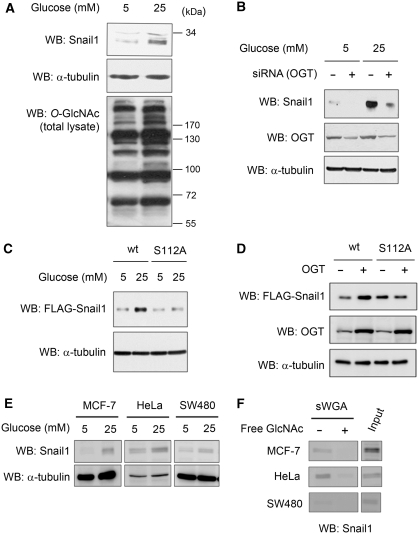
Snail1 levels are influenced by cellular glucose concentration
As the extent of protein O-GlcNAc modification is directly related to the glucose influx through the hexosamine biosynthetic pathway (Kang et al, 2009), we next studied the effect of glucose concentration in the culture medium on Snail1 levels. An increase in endogenous Snail1 protein was clearly observed under hyperglycaemic culture conditions (25 mM glucose) as compared with normoglycaemic conditions (5 mM glucose) (Figure 4A). This was apparently not due to increased de novo synthesis of Snail1 as the hyperglycaemic condition had no effect on its mRNA level (Supplementary Figure 1B). Rather, this effect was related to the O-GlcNAc modification as siRNA-mediated knock-down of OGT strongly reduced the effect of hyperglycaemic culture conditions on Snail1 expression (Figure 4B). Furthermore, hyperglycaemia in combination with OGT overexpression increased the amount of Snail1, but not that of mutant S112A Snail1 (Figure 4C and D). Together, these results suggest that enhanced glucose influx directly affects O-GlcNAc modification on Snail1, which in turn results in increased Snail1 protein level. Next, we investigated the generality of the glucose concentration on the O-GlcNAc modification of Snail1. By analysing MCF-7, HeLa, and SW480 cells, we found that their amount of Snail1 increased under hyperglycaemic culture conditions (25 mM glucose) as compared with normoglycaemic conditions (5 mM glucose) (Figure 4E). To demonstrate the O-GlcNAc modification on Snail1 in these cell lines, sWGA-affinity purification of total cell lysates was performed and Snail1 demonstrated by immunoblot analysis of the precipitate (Figure 4F). Specificity was shown by addition of the inhibitory monosaccharide GlcNAc during sWGA-affinity purification (Figure 4F). These results show that O-GlcNAc modification on Snail1 commonly occurs in various cancer cell lines.
Figure 4.
Hyperglycaemic condition increases Snail1 in an OGT-dependent manner. (A) Western blot analysis for endogenous Snail1 and O-GlcNAc from A549 cells under normoglycaemic (5 mM) and hyperglycaemic (25 mM) conditions. α-tubulin is shown as the loading control. (B) siRNA-mediated knock-down of OGT and western blot analysis for endogenous Snail1 or OGT from A549 cells under normo- and hyperglycaemic conditions. α-tubulin was included as a loading control. (C) Western blot analysis of wt or S112A mutant Snail1 from HEK293 cells under normo- and hyperglycaemic conditions. α-tubulin is shown as the loading control. (D) Western blot analysis of wt or S112A mutant Snail1 in HEK293 cells overexpressing OGT. α-tubulin is shown as the loading control. (E) Western blot analysis for endogenous Snail1 from MCF-7, HeLa, and SW480 cells under normoglycaemic (5 mM) and hyperglycaemic (25 mM) conditions. α-tubulin is shown as the loading control. (F) MCF-7, HeLa, and SW480 cells were grown under hyperglycaemic condition (25 mM) and their cell lysates were subjected to sWGA-lectin-affinity purification and the precipitates analysed with western blot for endogenous Snail1. The inhibitory monosaccharide GlcNAc (20 mM) was added during sWGA-lectin-affinity purification.
O-GlcNAc modification on Snail1 down-regulates E-cadherin and promotes EMT
Snail1 has been proposed to have important functions in embryonic development and cancer progression through its function as an E-cadherin repressor and its role in EMT (Nieto, 2002; Peinado et al, 2007). To determine the functional significance of O-GlcNAc-mediated stabilization of Snail1 on E-cadherin expression, we performed the luciferase gene reporter assay for the E-cadherin proximal promoter and investigated endogenous E-cadherin mRNA levels. As it is known that Snail1, upon binding to the three E-boxes of the human E-cadherin promoter, blocks transcription of E-cadherin (Batlle et al, 2000), we used a triple E-box mutant (3xMut) as the negative control of the gene reporter assay.
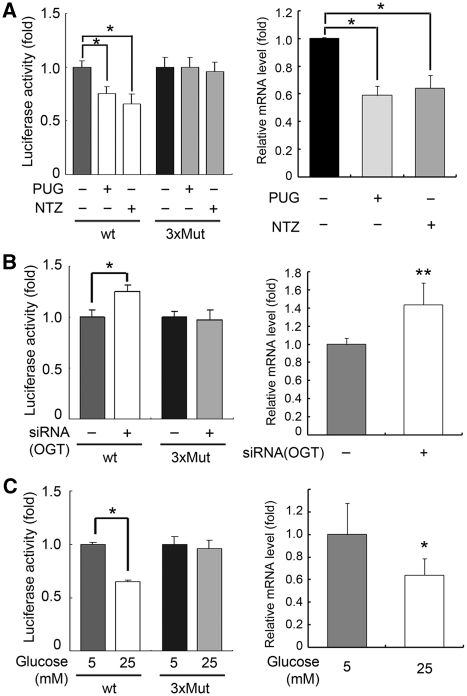
The A549 cells were treated with OGA inhibitors to maximize the amount of O-GlcNAc-modified protein. Both OGA inhibitors used in our experiments resulted in suppression of E-cadherin promoter activity and E-cadherin mRNA transcription, while no effect was observed when the triple E-box mutant was analysed (Figure 5A). Conversely, following siRNA-mediated knock-down of endogenous OGT (see Figure 4B) to prevent the O-GlcNAc modification, E-cadherin promoter activity and E-cadherin mRNA transcription increased compared with the triple E-box mutant (Figure 5B). In agreement with our observation that hyperglycaemic conditions increase O-GlcNAc modification on Snail1 (see Figure 4A–C), hyperglycaemic conditions resulted in suppression of E-cadherin promoter activity and E-cadherin mRNA transcription (Figure 5C). Together, these data strongly suggest that glucose is involved in the control of E-cadherin transcription through increased O-GlcNAc modification on Snail1.
Figure 5.
O-GlcNAc modification on Snail1 regulates E-cadherin expression. (A) Left panel: gene reporter assay for wt or 3xMut E-cadherin promoter in the presence (+) or absence (−) of OGA inhibitors PUGNAc or NAG-Thiazoline under normoglycaemic conditions (n=3, data are presented as mean±s.d., *P<0.01 by Student's t-test). Right panel: quantitative real-time (RT)–PCR for E-cadherin mRNA in the presence (+) or absence (−) of OGA inhibitors PUGNAc or NAG-Thiazoline under normoglycaemic conditions (n=4, data are presented as mean±s.d., *P<0.01 by Student's t-test). GAPDH was used for normalization. (B) Left panel: gene reporter assay for wt or 3xMut E-cadherin promoter following siRNA knock-down of OGT under hyperglycaemic conditions (n=3, data are presented as mean±s.d., *P<0.01 by Student's t-test). Right panel: quantitative RT–PCR for E-cadherin mRNA after siRNA knock-down of OGT hyperglycaemic conditions (n=3, data are presented as mean±s.d., **P<0.02 by Student's t-test). GAPDH was used for normalization. (C) Left panel: gene reporter assay for wt or 3xMut E-cadherin promoter under normoglycaemic (5 mM) or hyperglycaemic (25 mM) conditions (n=3, data are presented as mean±s.d., *P<0.01 by Student's t-test). Right panel: quantitative RT–PCR for E-cadherin mRNA under normo- or hyperglycaemic conditions (n=4, data are presented as mean±s.d., *P<0.01 by Student's t-test). GAPDH was used for normalization.
OGT is ubiquitous in cells and tissues (Hanover et al, 1999; Hart et al, 2007). The fact that Snail1 expression is important for the invasion and metastasis formation in human cancers raises the possibility that OGT activation and subsequent Snail1 stabilization may have a function in the recurrence and metastatic progression of cancer. Therefore, we examined OGT and Snail1 expression in human pancreatic cancer tissues. In all examined cases, OGT and Snail1 protein were consistently co-expressed in the invasive cancer cells (Supplementary Figure 3), suggesting that O-GlcNAc-mediated stabilization of Snail1 is relevant in the condition of human cancer.
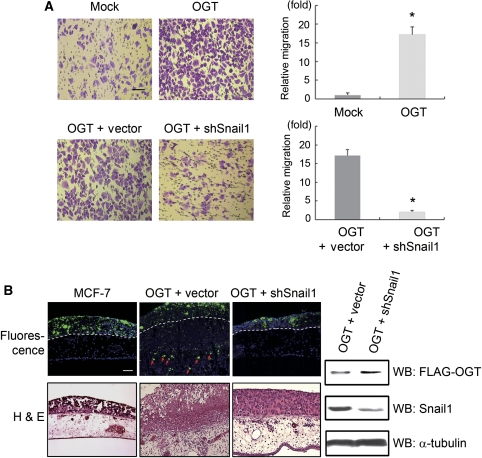
Given that increased O-GlcNAc modification of Snail1 results in its accumulation, which in turn suppresses E-cadherin proximal promoter activity, we sought to determine the effect of O-GlcNAc modification on Snail1 in terms of cancer cell migration and invasive progression using non-invasive MCF-7 cells. As shown in Figure 6, OGT-overexpressing MCF-7 cells exhibited robust cellular migration and invasion as compared with non-transfected MCF-7 cells. Importantly, these migratory and invasive properties were largely rescued by shRNA-mediated knock-down of Snail1, and the invasive properties because of OGT-mediated Snail1 expression were confirmed by immunohistochemical detection of OGT and Snail1 in the chorioallantoic membrane (CAM) samples (Supplementary Figure 4). Inversely, siRNA-mediated OGT knock-down inhibited cell migration compared with control. Moreover, knock-down of OGT abolished cell migration by overexpression of wild-type Snail1, while cell migration by overexpression of S112A mutant Snail1 was not abolished (Supplementary Figure 5). These data suggest that Snail1 is one of the important regulators responsible for OGT-mediated cellular migration and the invasive programme (Figure 6).
Figure 6.
Snail1 O-GlcNAc modification induces cell migration and in vivo invasion. (A) The migratory activity of MCF-7 cells transfected with flag-tagged OGT-expression vector relative to cells transfected with a control vector (Mock) alone or in combination with shRNA vector control (vector) or shRNA for Snail1 (shSnail) in tandem was determined after 2 days in culture. OGT induces cell migration, which was largely attenuated by knock-down of Snail1 (scale bar, 100 μm, n=5, data are presented as mean±s.d., *P<0.01 by Student's t-test). (B) Overexpression of OGT in non-invasive MCF-7 cells turns them invasive in chick chorioallantoic membrane (CAM) assay. Combined expression of OGT and shSnail1 in non-invasive MCF-7 prevents the OGT effect by suppression of Snail1. MCF-7 cells labelled with fluorescent nanobeads were cultured atop the CAM of live chick embryos for 3 days. Tissues were then removed and unstained frozen sections examined by fluorescence microscopy to directly visualize the invasive activity of the green-coloured cancer cells (upper panels, scale bar, 100 μm), or H&E-stained cross-sections prepared for light microscopy (lower panels). The upper face of the chorioallantoic membrane is indicated by the dashed white lines in the fluorescent images. Arrows denote invasive cancer cells. Immunoblot analysis detects transfected OGT (anti-flag) and knock-downed endogenous Snail1 in MCF-7 cells to verify the shRNA for Snail1 (right panels).
Discussion
Snail1 has a regulatory function in the EMT programme, a key feature of tumour development and progression, and is important for organ development in the embryo. This study revealed the presence of O-GlcNAc, an important regulatory posttranslational protein modification, on Ser112 of Snail1. Glucose level regulated O-GlcNAc on Snail1, resulting in an increase of O-GlcNAc modification with hyperglycaemia. Importantly, the O-GlcNAc modification stabilized Snail1, whose increased amounts caused down-regulation of E-cadherin through transcriptional repression and resulted in EMT.
Human and other mammalian Snail1 proteins have a highly conserved β-catenin-like consensus motif. This region of Snail1 is O-phosphorylated by GSK-3β, followed by β-TrCP-dependent ubiquitination and rapid proteasomal degradation, indicating that O-phosphorylation by GSK-3β is a determining step in Snail1 metabolism (Dominguez et al, 2003; Yook et al, 2005, 2006). It has been shown that Wnt signalling and β-catenin activity regulate the Axin2-mediated nuclear export function of GSK-3β (Yook et al, 2006). Through Wnt signalling, GSK-3β serially phosphorylates several Ser/Thr sites on Snail1. The O-phosphorylation of Ser96, Ser104, and Ser107 is prerequisite to generation of a recognition site for the ubiquitin-ligase β-TrCP (Yook et al, 2005). In this study, we found that O-GlcNAc modification of Ser112 stabilized Snail1 by inhibiting its O-phosphorylation. This in turn resulted in down-regulation of E-cadherin and initiated the EMT. Hence, the O-GlcNAc modification on Snail1 has the same functional importance as for the tumour suppressor p53 (Yang et al, 2006). O-phosphorylation of p53 at Thr155, which causes its ubiquitination and degradation, is inhibited by O-GlcNAc modification of the adjacent Ser149 (Yang et al, 2006). Although no crystal structure of Snail1 is currently available, it similarly appears that O-GlcNAc modification of Ser112 affects casein kinase1's priming phosphorylation of Ser104 and Ser 107, inhibiting GSK-3β-mediated degradation of Snail1 (Xu et al, 2010).
Transgenic mice overexpressing Snail1 about 20% above normal frequently develop carcinomas and leukaemia, which shows the importance of regulated Snail1 expression for normal organ homeostasis in vivo (Perez-Mancera et al, 2005). On the other hand, persistent hyperglycaemic condition in diabetic rats has been shown to result in enhanced OGT expression in various tissues and a general increase in protein O-GlcNAc modification (Akimoto et al, 2003). Our present observation of a dynamic interplay between O-phosphorylation and O-GlcNAc modification of Snail1 and the influence of hyperglycaemia clearly bears a relation to both conditions and provides insight into the correlation between metabolic disease and EMT. It also suggests hyperglycaemic conditions as observed in diabetics could be one of the factors eventually resulting in dysregulation of Snail1 expression and subsequent initiation of the EMT programme. As OGT is a glucose sensor, its catalytic activity being controlled by substrate UDP-GlcNAc, whose concentrations are determined by glucose influx (Love and Hanover, 2005; Hart et al, 2007), our results link the glucose sensing function of OGT, stabilization of Snail1 through O-GlcNAc modification by OGT, and long-term consequences of diabetes. Epidemiological studies have provided compelling evidence that metabolic disorders such as obesity and diabetes mellitus are associated with a higher risk of developing cancer compared with normoglycaemic individuals, irrespective of other confounding factors (Calle and Kaaks, 2004; Coughlin et al, 2004; Jee et al, 2005; Larsson et al, 2005, 2007; LeRoith et al, 2008). Although chronic hyperinsulinaemia and serum levels of insulin-like growth factor receptor (IGF-R) have been related to increased cancer risk and mortality in diabetics (Fernandez et al, 2001; Calle and Kaaks, 2004), our data indicate that glucose levels might be another important contributing condition. As we have shown, hyperglycaemic condition is an important regulator for Snail1 as the resulting increased O-GlcNAc modification stabilizes it prior to transcriptional suppression of E-cadherin and induction of EMT. Interestingly, activation of IGF-R mediated by NFκB also induces EMT through transcriptional up-regulation of Snail1 (Kim et al, 2007). Indeed, we have recently shown that NFκB is activated by O-GlcNAc modification at Thr352, resulting in decreased binding to IκB (Yang et al, 2008). Together, these results also suggest that chronic hyperglycaemic condition such as in diabetes mellitus contributes to the development and progression of cancer and that Snail1 might be an important part of this cascade.
What other possible effects could result from hyperglycaemia-induced stabilization of Snail1? A main function of Snail1 is to regulate cell movement during embryonic development (Barrallo-Gimeno and Nieto, 2005). In this context, it has long been recognized that the risk of congenital malformation and prenatal mortality is greatly increased by diabetes mellitus during pregnancy (Nielsen et al, 2005; Kapoor et al, 2007). The level of O-GlcNAc protein modification normally fluctuates during early embryogenesis (Dehennaut et al, 2009), and its dysregulation may lead to developmental abnormalities, as OGT knock-out in mice is embryonic lethal (Shafi et al, 2000). This suggests that a maternal hyperglycaemic condition could be responsible for congenital malformations because of increased O-GlcNAc modification of important regulatory proteins such as the Snail1, NFκB, and p53. Other complications of chronic hyperglycaemia are organ fibrosis and extra deposition of extracellular matrix components. Aberrant activation of Snail1 has been reported to disrupt renal tissue homeostasis and to result in fibrosis (Boutet et al, 2006). Moreover, we have also shown that OGA inhibition regulates transcription of EMT genes such as claudin-1, occludin, vimentin, and fibronectin (Supplementary Figure 6). It would thus be interesting to analyse the Snail1 O-GlcNAc state under chronic hyperglycaemia in various tissues affected by fibrosis.
In summary, hyperglycaemia-regulated O-GlcNAc modification of Snail1 leads to loss of its E-cadherin suppressor function. As this may have a key function in diverse pathologies, further functional studies are required to determine the effects of O-GlcNAc on Snail1, particularly in the development and progression of cancer and other diseases.
Materials and methods
Reagents and DNA transfection
A549, HEK 293, MCF-7, HeLa, and SW480 cells were cultured in DMEM (Gibco, Grand Island, NY) supplemented with 10% foetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. A human Snail1 expression vector with a C-terminal FLAG and HIS epitope tag were described previously (Yook et al, 2005, 2006). Mutants of Snail1 cDNA with FLAG and HIS epitope, including the −Δ87–109, −Δ110–120, S111A, S112A, S111A, and S112A mutants, were produced by PCR-based methods using a wild-type Snail1 cDNA as template, followed by subcloning into the pCR3.1 expression vector. A human OGT cDNA was subcloned into the pcDNA3.1-HIS-V5 mammalian expression vector (Invitrogen) and the p3xFLAG-CMV-7.1 expression vector (Sigma, St Louis, MO). siRNA of OGT and control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). DNA plasmid and siRNA were transiently transfected into HEK 293 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
PUGNAc was purchased from Toronto Research Chemicals Inc. (ON, Canada) and NTZ was kindly provided by Dr Kwan Soo Kim (Yonsei University, Seoul, Korea). Cells were treated with 100 μM PUGNAc or 50 μM NTZ for 24 h before cell harvest or for 6 h before transfection. Cycloheximide and MG132 were purchased from Sigma. Wild-type or mutant Snail1-FLAG proteins were detected using anti-FLAG antibody (F-7425 for polyclonal and F-3156 for monoclonal, Sigma). CTD110.6, an antibody against O-GlcNAc was purchased from Covance (Princeton, NJ). Antibodies against OGT (Sigma) and against phosphor-Ser (clone 4A4, Upstate) were commercially available. Endogenous Snail1 was detected with an anti-Snail1 monoclonal antibody (Cell Signaling Technology). Antibodies against α-tubulin and ubiquitin were purchased from Santa Cruz Biotechnology. Immunoblot analysis and immunoprecipitation were performed as described previously (Yook et al, 2005; Yang et al, 2006).
sWGA-affinity purification
A549 cells were lysed with NET lysis buffer (150 mM NaCl, 50 mM Tris, pH 7.4, 1 mM EDTA, 0.5% Nonidet P-40) and cell lysates were incubated with agarose-conjugated sWGA (Vector Laboratories, Burlingame, CA) for 3 h at 4°C. For control of specificity, 20 mM GlcNAc was added. Precipitates were washed four times with lysis buffer and proteins were eluted by boiling in SDS sample buffer.
Quantitative real-time PCR
Detection of mRNA transcripts was performed using a 7300 Real-Time PCR System (Applied Biosystems, Forster City, CA) and SYBR Green (Takara, Japan). First-strand cDNA was synthesized using SuperscriptIII (Invitrogen) according to the manufacturer's instructions. Primers that amplify human Snail1, E-cadherin, or GAPDH were designed with Primer3 software (Whitehead Institute for Biomedical Research). PCR were as follows: Snail1 forward, 5′-TGGATACAGCTGCTTTGAGC; Snail1 reverse, 5′-GGAGCTTCCCAGTGAGTCTG; E-cadherin forward, 5′- ATGGGGTCTTGCTATGTTGC; E-cadherin reverse, 5′- AAGGCAGAAGGATTGCTTGA; GAPDH forward, 5′- CGACCACTTTGTCAAGCTCA; GAPDH reverse, 5′- CCCTGTTGCTGTAGCCAAAT. Quantitative real-time PCR (RT–PCR) was performed in triplicate, and the mRNA levels of Snail1 and E-cadherin were normalized to GAPDH.
Transwell cell migration assay
For migration assays, MCF-7 cells were transfected with flag-tagged OGT expression vector (0.5 μg) in combination with 1.0 μg of shRNA vector control or shRNA for Snail1 with lipofectamine. After 24 h, cells were trypsinized and 1 × 105 cells seeded into Transwell inserts (8.0 μm pore, BD Biosciences). After an 18 h culture period, the upper side of the membrane was rubbed with cotton swap and the numbers of cells migrating to the basal side insert were counted following 0.25% crystal violet staining. Cell counts were determined in five random fields from three replicate wells.
Chick CAM invasion assay
The CAM assay for in vivo invasion was performed as described previously (Yook et al, 2005, 2006). In brief, control-transfected MCF-7 cells, OGT with shRNA vector-transfected cells, or OGT with shRNA for Snail1-transfected cells labelled with fluoresbrite carboxylate nanospheres (Polysciences) were cultured atop the CAM of 11-day-old chick embryos for 3 days. Invasion was monitored in haematoxylin–eosin-stained cross-sections of fixed CAM and by immunofluorescence microscopy.
Tissue samples and immunohistochemistry
Samples of paraffin-embedded primary pancreatic adenocarcinomas were obtained from the archive of the Department of Pathology, Gangnam Severance Hospital, Seoul, Korea. Following antigen retrieval by microwave heating in citrate buffer (DakoCytomation, Glostrup, Denmark), sections from CAM assay specimens or pancreactic adenocarcinoma samples were incubated with antibodies against OGT, Snail1, and keratin, and bound antibody was visualized with the avidin–biotin-complex peroxidase technique (Dako). Sections were stained with methyl green. In controls, primary antibodies were omitted.
Mapping of O-GlcNAc site using mass spectrometry
To map O-GlcNAc sites, ESI-MS/MS was performed as previously described (Yang et al, 2006). Overexpressed wild-type Snail1-FLAG proteins were purified using FLAG-M2 agarose (Sigma) and subjected to SDS–PAGE. In-gel digestion with trypsin (Promega, Madison, WI) (25 ng/μl) and chymotrypsin (Roche) (25 ng/μl) was simultaneously performed for 16 h at 37°C. The peptide analysis was carried out using a QSTAR Pulsar quadrupole TOF mass spectrometer (Applied Biosystems) equipped with nanoelectrospray ion sources (Protana, Odense, Denmark). Database search for sequenced peptides was performed in the National Center for Biotechnology Information (Bethesda, MD) non-redundant database using the MASCOT software package (Matrix Sciences, London, UK).
Reporter gene assays
Reporter constructs for Ecad(-108)-Luc (wt) or Ecad(-108)/EboxA.MUT/EboxB.MUT/EboxC.MUT-Luc (3x Mut) were described previously (Yook et al, 2005, 2006). A549 cells were transfected with 1.0 μg of reporter gene Ecad(-108)-Luc or Ecad(-108)/EboxA.MUT/EboxB.MUT/EboxC.MUT-Luc. Reporter gene activities were measured with a dual luciferase assay system (Promega) 48 h after transfection and normalized by measuring co-transfected control pRL-SV40 renilla luciferase activity. Reporter gene activities are presented as light units relative to that obtained from mock-transfected cells. The results are expressed as averages of ratios of reporter activities.
Supplementary Material
Acknowledgments
We thank SJ Weiss and XY Li for reading of the paper, and also thank Eph Tunkle for preparation of the paper. This work was supported by grants from the National Research Foundation of Korea by the Ministry of Education, Science, and Technology Grant (2010-0018923 and 2010-0027736) and by the World Class University programme through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10086-0). Additionally, SYP, SJ and JGK are fellowship awardees of the Brain Korea 21 programme. This work was made possible through the use of research facilities in the Yonsei Center for Biotechnology. This study was also supported by a grant from the National R&D Program for Cancer Control, Ministry of Health, Welfare & Family Affairs (0720270, A080916), Korea Research Institute of Chemical Technology (SI-0905), and a National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2009-0067248, 2009-0079653, 2009-0094029, KRF-2008-331-E00328).
Footnotes
The authors declare that they have no conflict of interest.
References
- Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H (2003) Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest Ophthalmol Vis Sci 44: 3802–3809 [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132: 3151–3161 [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2: 84–89 [DOI] [PubMed] [Google Scholar]
- Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA (2006) Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25: 5603–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4: 579–591 [DOI] [PubMed] [Google Scholar]
- Cheng X, Cole RN, Zaia J, Hart GW (2000) Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry 39: 11609–11620 [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 159: 1160–1167 [DOI] [PubMed] [Google Scholar]
- Dehennaut V, Lefebvre T, Leroy Y, Vilain JP, Michalski JC, Bodart JF (2009) Survey of O-GlcNAc level variations in Xenopus laevis from oogenesis to early development. Glycoconj J 26: 301–311 [DOI] [PubMed] [Google Scholar]
- Dominguez D, Montserrat-Sentis B, Virgos-Soler A, Guaita S, Grueso J, Porta M, Puig I, Baulida J, Franci C, Garcia de Herreros A (2003) Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol Cell Biol 23: 5078–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Le Roith D (2001) Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev 15: 1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetta MC, Oktaba K, Muller J (2009) Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science 325: 93–96 [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM (2004) Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 167: 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA, Lai Z, Lee G, Lubas WA, Sato SM (1999) Elevated O-linked N-acetylglucosamine metabolism in pancreatic beta-cells. Arch Biochem Biophys 362: 38–45 [DOI] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446: 1017–1022 [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB (2005) ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8: 311–321 [DOI] [PubMed] [Google Scholar]
- Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134: 703–707 [DOI] [PubMed] [Google Scholar]
- Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM (2005) Fasting serum glucose level and cancer risk in Korean men and women. JAMA 293: 194–202 [DOI] [PubMed] [Google Scholar]
- Kamemura K, Hart GW (2003) Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription. Prog Nucleic Acid Res Mol Biol 73: 107–136 [DOI] [PubMed] [Google Scholar]
- Kang JG, Park SY, Ji S, Jang I, Park S, Kim HS, Kim SM, Yook JI, Park YI, Roth J, Cho JW (2009) O-GlcNAc protein modification in cancer cells increases in response to glucose deprivation through glycogen degradation. J Biol Chem 284: 34777–34784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor N, Sankaran S, Hyer S, Shehata H (2007) Diabetes in pregnancy: a review of current evidence. Curr Opin Obstet Gynecol 19: 586–590 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV (2007) Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol 27: 3165–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 121: 856–862 [DOI] [PubMed] [Google Scholar]
- Larsson SC, Orsini N, Wolk A (2005) Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 97: 1679–1687 [DOI] [PubMed] [Google Scholar]
- LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S (2008) Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes 116 (Suppl 1): S4–S6 [DOI] [PubMed] [Google Scholar]
- Love DC, Hanover JA (2005) The hexosamine signaling pathway: deciphering the ‘O-GlcNAc code'. Sci STKE 2005: re13. [DOI] [PubMed] [Google Scholar]
- McClain DA, Crook ED (1996) Hexosamines and insulin resistance. Diabetes 45: 1003–1009 [DOI] [PubMed] [Google Scholar]
- Nielsen GL, Norgard B, Puho E, Rothman KJ, Sorensen HT, Czeizel AE (2005) Risk of specific congenital abnormalities in offspring of women with diabetes. Diabet Med 22: 693–696 [DOI] [PubMed] [Google Scholar]
- Nieto MA (2002) The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3: 155–166 [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428 [DOI] [PubMed] [Google Scholar]
- Perez-Mancera PA, Perez-Caro M, Gonzalez-Herrero I, Flores T, Orfao A, de Herreros AG, Gutierrez-Adan A, Pintado B, Sagrera A, Sanchez-Martin M, Sanchez-Garcia I (2005) Cancer development induced by graded expression of Snail in mice. Hum Mol Genet 14: 3449–3461 [DOI] [PubMed] [Google Scholar]
- Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD (2000) The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 97: 5735–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Pandey A, Hart GW (2007) Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol Cell Proteomics 6: 1365–1379 [DOI] [PubMed] [Google Scholar]
- Warburg O (1956) On respiratory impairment in cancer cells. Science 124: 269–270 [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB (2009) ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324: 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291: 2376–2378 [DOI] [PubMed] [Google Scholar]
- Xu Y, Lee SH, Kim HS, Kim NH, Piao S, Park SH, Jung YS, Yook JI, Park BJ, Ha NC (2010) Role of CK1 in GSK3beta-mediated phosphorylation and degradation of snail. Oncogene 29: 3124–3133 [DOI] [PubMed] [Google Scholar]
- Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW (2006) Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol 8: 1074–1083 [DOI] [PubMed] [Google Scholar]
- Yang WH, Park SY, Nam HW, Kim do H, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW (2008) NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA 105: 17345–17350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ (2005) Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem 280: 11740–11748 [DOI] [PubMed] [Google Scholar]
- Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, Cha SY, Ryu JK, Choi YJ, Kim J, Fearon ER, Weiss SJ (2006) A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol 8: 1398–1406 [DOI] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC (2004) Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6: 931–940 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.