Abstract
The DNA polymerase-primase from Drosophila lacks 3'----5' exonuclease activity. However, a potent exonuclease can be detected after separating the 182-kDa polymerase subunit from the other three subunits of the enzyme (73, 60, and 50 kDa) by glycerol gradient sedimentation in the presence of 50% ethylene glycol. The exonuclease activity cosediments with the polymerase subunit, suggesting that the two activities reside in the same polypeptide. The 3'----5' exonuclease excises mismatched bases at the 3' termini of primed synthetic and natural DNA templates. Excision of a mispaired base at the 3' terminus occurs at a 10-fold greater rate than excision of the correctly paired base. When replication fidelity is measured by the bacteriophage phi X174 am3 reversion assay, the isolated polymerase subunit is at least 100-fold more accurate than either the intact polymerase-primase or a complex of the 182- and 73-kDa subunits. These results suggest that the 3'----5' exonuclease functions as a proofreading enzyme during Drosophila DNA replication in vitro and very likely in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbotts J., Loeb L. A. On the fidelity of DNA replication: use of synthetic oligonucleotide-initiated reactions. Biochim Biophys Acta. 1985 Jan 29;824(1):58–65. doi: 10.1016/0167-4781(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Banks G. R., Holloman W. K., Kairis M. V., Spanos A., Yarranton G. T. A DNA polymerase from Ustilago maydis. 1. Purification and properties of the polymerase activity. Eur J Biochem. 1976 Feb 2;62(1):131–142. doi: 10.1111/j.1432-1033.1976.tb10106.x. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Black V. L., So A. G. A new mammalian DNA polymerase with 3' to 5' exonuclease activity: DNA polymerase delta. Biochemistry. 1976 Jun 29;15(13):2817–2823. doi: 10.1021/bi00658a018. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. The effect of template secondary structure on vaccinia DNA polymerase. J Biol Chem. 1979 Aug 25;254(16):7820–7826. [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. A comparison of associated enzyme activities in various deoxyribonucleic acid polymerases. J Biol Chem. 1973 May 25;248(10):3398–3404. [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Enzymatic synthesis of oligodeoxynucleotides. Biochemistry. 1971 Feb 2;10(3):536–542. doi: 10.1021/bi00779a029. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- Crute J. J., Wahl A. F., Bambara R. A. Purification and characterization of two new high molecular weight forms of DNA polymerase delta. Biochemistry. 1986 Jan 14;25(1):26–36. doi: 10.1021/bi00349a005. [DOI] [PubMed] [Google Scholar]
- Drake J. W. Comparative rates of spontaneous mutation. Nature. 1969 Mar 22;221(5186):1132–1132. doi: 10.1038/2211132a0. [DOI] [PubMed] [Google Scholar]
- Fansler B. S., Loeb L. A. Sea urchin nuclear DNA polymerase. Methods Enzymol. 1974;29:53–70. doi: 10.1016/0076-6879(74)29009-8. [DOI] [PubMed] [Google Scholar]
- Field J., Gronostajski R. M., Hurwitz J. Properties of the adenovirus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9487–9495. [PubMed] [Google Scholar]
- Kaguni L. S., DiFrancesco R. A., Lehman I. R. The DNA polymerase-primase from drosophila melanogaster embryos. Rate and fidelity of polymerization on single-stranded DNA templates. J Biol Chem. 1984 Jul 25;259(14):9314–9319. [PubMed] [Google Scholar]
- Kaguni L. S., Rossignol J. M., Conaway R. C., Banks G. R., Lehman I. R. Association of DNA primase with the beta/gamma subunits of DNA polymerase alpha from Drosophila melanogaster embryos. J Biol Chem. 1983 Aug 10;258(15):9037–9039. [PubMed] [Google Scholar]
- Kaguni L. S., Rossignol J. M., Conaway R. C., Lehman I. R. Isolation of an intact DNA polymerase-primase from embryos of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2221–2225. doi: 10.1073/pnas.80.8.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A., Goodman M. F. On the fidelity of DNA replication. The accuracy of T4 DNA polymerases in copying phi X174 DNA in vitro. J Biol Chem. 1984 Feb 10;259(3):1539–1545. [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Further studies on calf thymus DNA polymerase delta purified to homogeneity by a new procedure. Biochemistry. 1984 Apr 24;23(9):1906–1913. doi: 10.1021/bi00304a003. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Ottiger H. P., Hübscher U. Mammalian DNA polymerase alpha holoenzymes with possible functions at the leading and lagging strand of the replication fork. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3993–3997. doi: 10.1073/pnas.81.13.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speyer J. F. Mutagenic DNA polymerase. Biochem Biophys Res Commun. 1965 Oct 8;21(1):6–8. doi: 10.1016/0006-291x(65)90417-1. [DOI] [PubMed] [Google Scholar]
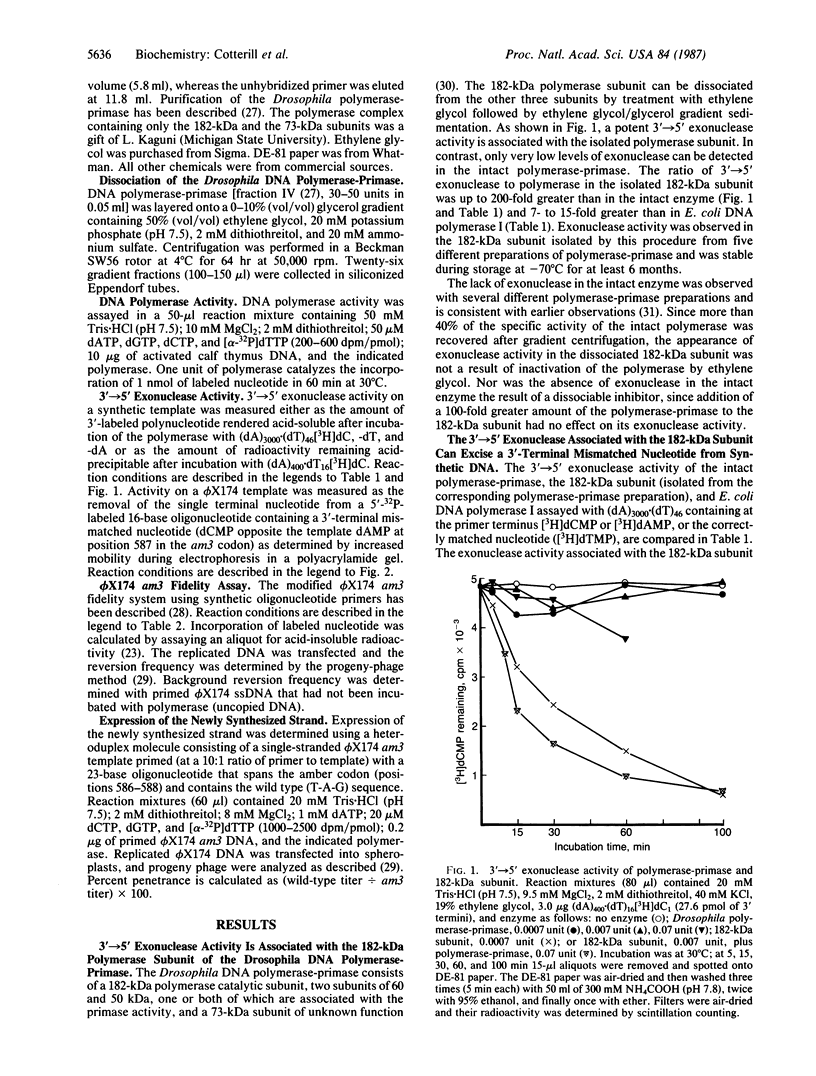
- Szopa J., Rose K. M. Cleavage of the 190-kDa subunit of DNA-dependent RNA polymerase I yields small polypeptides capable of degrading DNA. J Biol Chem. 1986 Jul 5;261(19):9022–9028. [PubMed] [Google Scholar]
- Vishwanatha J. K., Coughlin S. A., Wesolowski-Owen M., Baril E. F. A multiprotein form of DNA polymerase alpha from HeLa cells. Resolution of its associated catalytic activities. J Biol Chem. 1986 May 15;261(14):6619–6628. [PubMed] [Google Scholar]
- Von Borstel R. C., Cain K. T., Steinberg C. M. Inheritance of spontaneous mutability in yeast. Genetics. 1971 Sep;69(1):17–27. doi: 10.1093/genetics/69.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymouth L. A., Loeb L. A. Mutagenesis during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1924–1928. doi: 10.1073/pnas.75.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintersberger U., Wintersberger E. Studies on deoxyribonucleic acid polymerases from yeast. 1. Parial purification and properties of two DNA polymerases from mitochondria-free cell extracts. Eur J Biochem. 1970 Mar 1;13(1):11–19. doi: 10.1111/j.1432-1033.1970.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Wright G. E., Baril E. F., Brown N. C. Butylanilinouracil: a selective inhibitor of HeLa cell DNA synthesis and HeLa cell DNA polymerase alpha. Nucleic Acids Res. 1980 Jan 11;8(1):99–109. doi: 10.1093/nar/8.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]