Abstract
Based on our previous work, we expected the Visual Word Form Area (VWFA) in the left ventral visual pathway to be engaged by both whole-word recognition and by serial sublexical coding of letter strings. To examine this double function, a phonological lexical decision task (i.e., “Does xxx sound like an existing word?”) presented short and long letter strings of words, pseudohomophones, and pseudowords (e.g., Taxi, Taksi and Tazi). Main findings were that the length effect for words was limited to occipital regions and absent in the VWFA. In contrast, a marked length effect for pseudowords was found throughout the ventral visual pathway including the VWFA, as well as in regions presumably engaged by visual attention and silent-articulatory processes. The length by lexicality interaction on brain activation corresponds to well-established behavioral findings of a length by lexicality interaction on naming latencies and speaks for the engagement of the VWFA by both lexical and sublexical processes.
Introduction
The work of Cohen, Dehaene and colleagues (Cohen et al., 2000, 2002) has drawn great interest on the role of the left ventral visual “what” pathway for visual word processing. Based on a series of innovative studies, the authors demonstrated that a left occipitotemporal (OT) cortex region at approximately x = −43, y = −54, z = −12 plays an important role in early stages of visual word processing, and they had used the term Visual Word Form Area (VWFA) to refer to this region.
In the original formulation, the VWFA was assumed to be prelexical, that is tuned to letter combinations which are frequently encountered in a writing system (Cohen et al., 2002). Recent evidence for prelexical tuning of the VWFA was provided by Binder et al. (2006), who found that OT cortex activation systematically increased with increasingly higher letter sequence probability (i.e., bigram-frequency) of a nonword. An extension of the VWFA hypothesis by Dehaene et al. (2005) – based on the results of priming studies (Dehaene et al., 2001, Dehaene et al., 2004) – proposed a hierarchy of codings along the ventral visual pathway with case-specific letter shape coding and abstract letter coding posterior to the VWFA and coding of bigrams and short words in the VWFA. Recently, Cohen et al. (2008) extended the original VWFA hypothesis by proposing engagement of the dorsal visual pathway when word processing requires serial shifting of visual attention within the letter string. A similar account supported by MEG data was formulated by Pammer et al. (2006). However, we are aware of the discussion about the specificity of the VWFA for visual word processing (Price & Devlin et al., 2003; Cohen & Dehaene et al., 2004; Devlin et al., 2006). For convenience, in the following we use the term without taking up a position on the issue of specificity.
Our research group questioned the limited function (i.e., computation of prelexical letter string information) attributed to the VWFA in the original formulation by Cohen et al. (2002). In a previous imaging study (Kronbichler et al., 2007), we presented familiar and unfamiliar orthographic forms of the very same phonological words (e.g., Taxi vs. Taksi) together with pseudowords (e.g., Tazi) in a phonological lexical decision task (i.e., “Does xxx sound like an existing word?”) with the results that both familiar and unfamiliar orthographic forms received the same YES response. The critical finding was that familiar compared to unfamiliar orthographic forms led to reduced activation in the left OT cortex corresponding to the VWFA of Cohen et al. (2000, 2002). The very same procedure (a phonological lexical decision task with words, pseudohomophones, and pseudowords) was used in fMRI studies with English speaking adults (Bruno et al., 2008) and German speaking children (van der Mark et al., 2009). Consistent with Kronbichler et al. (2007), both studies found an orthographic familiarity effect in the left OT cortex with reduced activity in response to words compared to pseudohomophones and pseudowords. Reduced VWFA activity for words compared to pseudohomophones was also found in a silent reading task (Kronbichler et al., 2009) and with overt naming tasks (Borowsky et al., 2006, 2007). An EEG-study from our lab (Sauseng et al., 2004) – corresponding to the reduced BOLD response to familiar orthographic forms – found reduced negativity in response to familiar compared to unfamiliar orthographic forms of the same phonological words from about 200 ms onwards. These studies with variation of orthographic familiarity were preceded by an fMRI study (using silent reading) which found a large left OT region including the VWFA to be modulated by the frequency with which the words are encountered in print (Kronbichler et al., 2004). The direction of the modulation corresponded to the orthographic familiarity effect with increasing frequency accompanied by decreasing left OT cortex activation. Similarly, Binder et al. (2003) found that words with a large number of orthographic neighbours (i.e., more familiar words) led to reduced VWFA activation compared to words with less orthographic neighbours. Therefore, we propose that the VWFA is not only involved in the prelexical coding of letter strings, as originally proposed by the VWFA hypothesis (Cohen et al., 2000, 2002), but is also engaged in visual whole-word recognition based on orthographic word representations.
We were led to the interpretation of a double function of the VWFA by the evidence from fMRI studies, which experimentally manipulated the familiarity of pictured faces and objects by repeated presentation before scanning and found reduced OT cortex activation in response to familiarized compared to new objects or faces (Rossion et al., 2003; van Turennout et al., 2003). Obviously, the reduced OT activation in response to familiarized objects and faces corresponds to our finding of reduced VWFA activation in response to familiar orthographic forms. Furthermore, the common activation of OT brain regions by both familiar and unfamiliar objects and faces corresponds to our hypothesis of a double function of the VWFA, that is, whole-word coding in the case of familiar orthographic forms and coding into graphemes or letter patterns in the case of unfamiliar orthographic forms. Obviously, these functions correspond to the distinction of dual-route models of visual word processing between a lexical route (based on an orthographic word lexicon) and a serially operating sublexical route (based on grapheme-phoneme rules) from print to sound (e.g., Coltheart et al., 2001; Perry et al., 2007). They also correspond to the distinction of the Multiple-Trace Memory (MTM) model between a global word recognition procedure and an analytic procedure for unfamiliar orthographic forms (Ans et al., 1998).
Our hypothesis of orthographic whole-word coding in the VWFA differs from the conclusion derived in a meta-analysis of imaging findings by Jobard et al. (2003), which used the well-known dual-route architecture of visual word processing. They concluded, “neuroimaging studies failed so far to uncover a cerebral area that would be the functional equivalent of a written word lexicon” (p. 708). This conclusion was based on the finding that “No cluster of activations has been found more recruited by word than pseudoword reading” (p. 693). An fMRI study by Binder et al. (2005) also directly examined brain activation patterns expected from different cognitive models of visual word processing. In the VWFA, Binder et al. (2005) found higher activation for pseudowords compared to words (with no difference between regular and irregular words) and considered the possibility that “this pattern could represent an assembled phonology (GPC) pathway” (p. 685). However, Binder et al. (2005) finally preferred an interpretation of this pattern in terms of “task difficulty” (measured by response times). The possibility that the orthographic familiarity effect on VWFA activation may reflect whole-word recognition based on orthographic word representations was not considered. Discussing potential evidence for such lexical route processes, Binder et al. (2005) referred to the angular gyrus, which exhibited higher activation for words compared to pseudowords. However, the authors finally opted against this interpretation and concluded, “the available evidence suggests that these “lexical route” regions are modulated by semantic factors and not by lexical neighbourhood size, arguing for a semantic interpretation, though this is clearly an issue warranting further investigation” (p. 687).
One may note that the largely negative conclusions by both Binder et al. (2005) and Jobard et al. (2003) on brain areas hosting an orthographic word lexicon are based on the assumption that such an area responds specifically to words but not as much to pseudowords. However, this assumption is questionable as shown by the mentioned familiarization studies with objects and faces, which found that critical OT regions were engaged by both familiar and unfamiliar items but exhibited higher activation to unfamiliar ones. The alternative assumption says that a brain region responds with less activity when a familiar item is encoded by a specific memory representation compared to when an unfamiliar item is encoded as a new configuration of features. This latter interpretation may be generally useful for identifying the neural correlates of specialization. Pugh et al. (2008) advanced a similar idea by proposing a learning-curve hypothesis based on skill-acquisition studies (e.g. Poldrack & Gabrieli, 2001). According to this hypothesis, initial skill acquisition is associated with increased activation in task-specific cortical areas, whereas continued practice of an acquired skill is associated with task-specific decreases in activation in the same cortical regions.
The present study sought further evidence for the proposal that the VWFA is engaged by both whole-word recognition of familiar orthographic forms and serial sublexical processing of unfamiliar orthographic forms. The main prediction from this assumption is that activation of the VWFA should exhibit a length (number of letters) by lexicality (word vs. pseudoword) interaction. Specifically, VWFA activation should exhibit little or no length effect in the case of words and increased activation in response to long compared to short pseudowords. This expected interaction corresponds to the well-established result pattern for naming latencies in reading aloud tasks, that is, little or no length effect for words and a marked length effect for pseudowords (Weekes et al., 1997; Juphard et al., 2004, 2006; Ziegler et al., 2001). In the mentioned studies, the length by lexicality interaction on naming latencies is interpreted as lexical processing of words and serial analytic processing of pseudowords. Recent simulations by Perry et al. (2007) compared dual-route models of visual word processing with the single route triangle model of Plaut et al. (1996), and found that only dual-route models predicted the length by lexicality interaction on naming latencies.
For examining the expected length by lexicality interaction on VWFA activation we again relied on the mentioned phonological lexical decision task and presented words, pseudohomophones and pseudowords. As already noted, this task was used in several recent studies with converging evidence for an orthographic familiarity effect on VWFA activation, that is, reduced activity in response to words compared to pseudohomophones and pseudowords, and little difference between the latter ones (Bruno et al., 2008; Kronbichler et al., 2007; van der Mark et al., 2009). Examining the expected length by lexicality interaction, we manipulated item length with short items consisting of 3-5 letters and long items consisting of 6-10 letters. Obviously, the expectation of a length effect for pseudowords depends on serial sublexical processing. This can be expected from the present task, which asks for a phonological lexical judgement (i.e., “Does xxx sound like an existing word?”) and from the inclusion of pseudohomophones (e.g., Taksi), which discourage simple orthographic judgments. These differences to the standard lexical decision task are critical. The standard task simply asks “Is xxx a word?” and presents only words and pseudowords. Therefore, it induces the strategy to check the letter strings against orthographic word memories. Accordingly, no length by lexicality interaction on response latencies was found in standard lexical decision tasks (Juphard et al., 2004, 2006). As a preview on Behavioral Results, we note that the present phonological lexical decision task, similar to reading aloud tasks, did result in such an interaction on response latencies. Specifically, we found this interaction for words and pseudowords. However, due to specific difficulties posed by short pseudohomophones, we did not find this interaction for words and pseudohomophones.
Recent theorizing on the functional neuroanatomy of visual word processing led to refinements of the VWFA hypothesis, which are of interest for the present study. Dehaene et al. (2005) proposed a hierarchy of codings along the ventral visual pathway. In this account, case-specific letter shape codings and abstract letter codings are located in occipital and OT cortex region posterior to the VWFA. Codings of bigrams and short words are located in the VWFA and more anterior parts of the OT cortex. From this perspective, one would expect that – different from our proposal – not only pseudowords but also words may elicit a length effect in the original VWFA. Furthermore, Cohen et al. (2008) proposed that the dorsal visual pathway is specifically engaged whenever visual word processing requires serial shifting of attention within the letter string. In this case, one would expect that a marked length effect for pseudowords, but not for words, should be found in dorsal parietal regions. A marked length effect for pseudowords, but not for words, should also be found in left temporoparietal and left inferior frontal regions, which are assumed to be engaged by sublexical mapping from orthographic to phonological segments (Pugh et al., 2000; Sandak et al., 2004). However, of main interest here is the expected length by lexicality interaction on the activation of the VWFA, which is based on our previous findings suggesting an engagement of this region by both whole-word recognition of familiar orthographic forms and sublexical processing of unfamiliar orthographic forms. To our knowledge, this prediction was not specifically examined in the few imaging studies which varied length and lexicality (Wydell et al., 2003; Valdois et al., 2006). Actually, there is little convergence between these studies (see Discussion). This is surprising given the convergence between the mentioned behavioral studies with respect to the length by lexicality interaction on naming latencies, and the theoretical importance of the length by lexicality interaction for cognitive models of visual word processing (see Perry et al., 2007).
Methods
Participants
Eighteen German-speaking male adolescent and adult readers aged between 16 and 19 years participated in the present study. They were recruited from two longitudinal studies on reading development (see Wimmer et al., 2000). Reading performance was assessed by a reading fluency test, which is under development in our lab. All participants had a reading speed score above percentile 15 based on a preliminary norm sample of 300 university students. All participants were right-handed and had normal or corrected-to-normal vision. They gave a written informed consent and were paid for their participation. The study was approved by the ethical committee of the University of Salzburg. Informed consent was provided by each participant and, in the case of the adolescents, by a parent as well.
Task and Stimuli
For the in-scanner task, participants were instructed to evaluate each item for correspondence with an existing phonological word (i.e., “Does xxx sound like an existing word?”), and equal proportions of words, pseudohomophones, and pseudowords were presented. Participants responded by button press using the right index finger for YES (in response to words and pseudohomophones) and the right middle finger for NO (in response to pseudowords). As already noted, the presence of pseudohomophones should discourage simple orthographic judgments, which are elicited in the standard lexical decision task (i.e., “Is xxx a word?”). From our previous experience with the phonological lexical decision task (Bergmann & Wimmer, 2008; Kronbichler et al., 2007) we know that participants sometimes find it hard to believe that a “misspelling” (pseudohomophone) sounds exactly like the intended word. Therefore, during the training session of the task, participants were instructed to accept a “misspelling” (such as Draum for Traum – dream) as sounding like the intended word even when they (wrongly) felt that the pronunciation may not be fully correct. However, as evident from Behavioral Results, these attempts met with limited success as evident from a substantial proportion of erroneous NO responses, specifically, to short pseudohomophones. Selection of stimuli started with 180 German words, half of which were short (3 to 5 letters) and the other long (6 to 10 letters). Words were selected in such a way that pseudohomophones could be derived. Most of the items were nouns with capitalized first letter (only 7 adjectives). Short and long words were matched on frequency and number of orthographic neighbours based on the CELEX database (Baayen et al., 1993). For each of these words, a pseudohomophone was created by exchanging one or two letter positions (e.g., Prais for Preis – price vs. Gümnastik for Gymnastik – gymnastics). The construction of pseudohomophones posed difficulties due to the rather high regularity of German. Furthermore, the short words offered fewer possibilities for exchange of homophonic letters (graphemes). For short compared to long pseudohomophones, we had to rely more often (i.e., in 47 vs. 22 of 90 items, respectively) on the exchange of similar stop consonants in consonant clusters in the word initial position (e.g., plau for blau – blue). Overly articulate (silent) pronunciation of such items may have been responsible for the substantial rate of erroneous NO responses to short pseudohomophones and for the comparatively long latencies of the YES responses to short pseudohomophones (see Behavioral Results). The substantial error rate for the short pseudohomophones and the absence of a length effect on response latencies led to the exclusion of the pseudohomophones from the analysis of brain activity.
The construction of pseudowords posed little difficulty compared to the construction of pseudohomophones. We avoided that pseudowords could be distinguished from pseudohomophones by superficial characteristics such as unusual orthographic features or pronunciation difficulties. In the Appendix, all items are listed. Table 1 provides statistical information. Of importance is that the critical length manipulation was close to identical for each of the three item types in terms of number of letters and number of syllables. As shown in Table 1, the number of orthographic neighbours was very small for all three item types, but the mean was higher for pseudohomophones compared to words and pseudowords (Mann-Whitney U tests: z-values > 3.0, ps < .01). Furthermore, for pseudohomophones and pseudowords (but not for words), the number of neighbours was smaller for the long compared to the short items (Mann-Whitney U tests: z-values > 3.85, ps < .01). These differences (about 1 neighbour difference) are small in absolute terms and very small compared to orthographic neighbourhood size differences used in studies which found a neighbourhood size effect on brain activity (e.g., Binder et al., 2003; Fiebach et al., 2007). Apparently, the small differences in the mean number of neighbours between item types and between length levels did not affect phonological lexical judgments as will become evident from the Behavioral Results (Table 2). To illustrate, from the lower number of orthographic neighbours for long compared to short pseudowords (and from orthographic lexical judgements) one would expect faster NO responses to long compared to short pseudowords. This is the opposite of the observed mean latencies in Table 2. Table 1 further shows that the three item types exhibited similar average bigram frequencies with long items exhibiting higher bigram frequencies than short ones. As shown in an ANOVA, pseudohomophones and pseudowords were matched for bigram frequency as only the main effect of length was reliable, F(1,89) = 268.3, p < .01. Importantly, the main effect of item-type (pseudohomophones vs. pseudowords) and the interaction between length and item type were not reliable, F(1, 89) = 1.39, p > .2, and F(1, 89) = 0.89, p > .8, respectively.
Table 1.
Characteristics of the six different item types
| Words |
PH |
PW |
|||||
|---|---|---|---|---|---|---|---|
| Characteristics | short | long | short | long | short | long | |
| Number of letters | M (SD) | 4.5 (0.6) | 7.5 (1.2) | 4.6 (0.7) | 7.5 (1.2) | 4.5 (0.6) | 7.5 (1.1) |
| Number of syllables | M (SD) | 1.3 (0.5) | 2.1 (0.6) | 1.3 (0.5) | 2.2 (0.7) | 1.3 (0.5) | 2.2 (0.6) |
| Frequency (/million) | M (SD) | 1.2 (0.7) | 1.2 (0.6) | - | - | - | - |
| Neighbours | M (SD) | 1.3 (1.1) | 1.3 (1.5) | 2.3 (2.0) | 1.3 (0.9) | 1.3 (1.7) | 0.4 (0.8) |
| Bigram frequency | M (SD) |
18638 (18088) |
79972 (37815) |
18553 (18852) |
71057 (37040) |
15463 (15928) |
66639 (38876) |
Abbreviations: PH = Pseudohomophones, PW = Pseudowords.
Table 2.
Behavioral Results
| Error Rate (%) | Reaction Time (ms) | ||||
|---|---|---|---|---|---|
|
|
|||||
| M | SD | M | SD | ||
| Words | short | 1.44 | 1.34 | 848 | 253 |
| long | 1.00 | 1.57 | 894 | 270 | |
| combined | 1.22 | 1.00 | 871 | 261 | |
| PH | short | 18.06 | 12.82 | 1015 | 223 |
| long | 7.67 | 5.40 | 1010 | 237 | |
| combined | 12.86 | 8.95 | 1013 | 228 | |
| PW | short | 4.50 | 7.34 | 1080 | 283 |
| long | 8.56 | 10.43 | 1219 | 283 | |
| combined | 6.53 | 8.69 | 1150 | 281 | |
Abbreviations: PH = Pseudohomophones, PW = Pseudowords.
In order to avoid that a participant had to evaluate both a base-word and its pseudohomophone, participants were presented one of two item sequences, which were designed such that for each word-pseudohomophone pair one sequence contained the base-word and the other its pseudohomophone. Each of these sequences included only 90 items per category (45 short and 45 long ones). Stimuli in yellow on dark grey background were projected on a semitransparent screen by a video projector outside the scanner room. Participants viewed the stimuli via a mirror, which was mounted in front of their eyes. Short items covered a visual angle of roughly 1.5 degrees, and long items covered a visual angle of roughly 2.5 degrees in both directions away from fixation.
Stimulus delivery and response registration was controlled by Presentation (Neurobehavioral Systems Inc., Albany, CA, USA). Presentation of the items was divided into 3 runs (90 items each) with each run additionally containing 25 null-events with a fixation cross in the middle of the screen. The runs were separated by short breaks. A fast event-related design was used to investigate the hemodynamic response to the different types of stimuli. The order of items and null-events within each run was determined by a genetic algorithm (Wager & Nichols, 2003), which selects the most efficient sequence for testing stimulus contrasts. Each item was presented for 1260 ms with an interstimulus interval of 1360 ms during which participants saw a fixation cross. The stimulus onset asynchrony of 2620 ms is not a multiple of the TR of 2200, ms which enhances the efficiency of sampling the hemodynamic response at different time points. The whole imaging task took approximately 16 minutes. Before the experiment started, a training session was used to make participants familiar with the task.
Image acquisition and analysis
Data were obtained with a Philips Gyroscan NT 1.5 Tesla scanner (Philips Medical System Inc., Maastricht, The Netherlands). Functional images sensitive to blood oxygen level dependent (BOLD) contrast were acquired with a T2*weighted gradient echo EPI sequence (TR 2200 ms, TE 45 ms, matrix 64×64 mm, FOV 220 mm, Flip Angle 90°). 25 Slices with a slice thickness of 5 mm and a slice gap of 0.7 mm were acquired within the TR. Scanning proceeded in 3 sessions with 146 scans per session. In addition, a high resolution (1 × 1 × 1.2 mm) structural scan was acquired from each participant with a T1-weighted MPRAGE sequence.
For preprocessing and statistical data analysis, SPM5 software was used (http://www.fil.ion.ucl.ac.uk/spm) running in a MATLAB 6.5 environment (Mathworks Inc., Sherbon MA, USA). Functional images were realigned, unwarped, slice time corrected and then coregistered to the high resolution structural image. The structural image was normalized to the MNI T1 template image, and the resulting parameters were used for normalization of the functional images, which were resampled to isotropic 3 × 3 × 3 mm voxels and smoothed with a 6 mm FWHM Gaussian kernel.
Statistical analysis was performed in a two stage mixed effects model. In the subject-specific first-level model, each stimulus type was modelled by a canonical hemodynamic response function and its temporal derivative. Only correctly answered items were included in the analysis. The incorrect answers and missed items were modelled as covariates of no interest in order to model brain responses independently from the different proportions of incorrect responses to the item types. The functional data in these first-level models were high pass filtered with a cut-off of 128 seconds and corrected for autocorrelation by an AR(1) model (Friston et al., 2002). In these first-level models the parameter estimates reflecting signal change for each item type (short and long items together) vs. baseline were calculated in the context of a GLM (see Henson, 2004). In the baseline condition (interstimulus intervals and null-events) participants viewed a fixation cross. In the same way, we calculated length effect contrasts for each item type, which identify regions exhibiting higher activity in response to long compared to short items. The subject specific contrast images were used for the second-level random effects analyses. Contrasts of interest were examined by t-tests (see below). For all statistical comparisons, we used a voxelwise threshold of p < .001 and a cluster extent threshold of p < 0.05 corrected.
Results
Behavior
Table 2 shows error rates and latencies for the phonological lexical decision task (i.e., “Does xxx sound like an existing word?”) used for measuring brain activation. For errors, statistical analysis was limited to pseudohomophones and pseudowords because the majority of participants was errorless for words (i.e., always responded with YES). An ANOVA of error rates found a marked interaction between item type and length, F(1, 17) = 24.50, p < .01. For pseudohomophones requiring a YES response, short items led to more erroneous NO responses than long items (p < .01), whereas for pseudowords the opposite was the case (p < .01). The higher rate of erroneous NO responses to short compared to long pseudohomophones may reflect the fact that about half of the short pseudohomophones, but only about a quarter of the long pseudohomophones, were constructed by the exchange of similar stop consonants in consonant clusters in word initial position (e.g., plau for blau – blue). Sublexical processing of such pseudohomophones with overly articulate (silent) pronunciation may have led participants to erroneously respond with NO. The specific difficulty of the short pseudohomophones is also evident from the complete absence of a length effect on latencies of correct YES responses as shown in Table 2. Importantly, response latencies to words and pseudowords correspond to the expected length by lexicality interaction, F(1, 17) = 25.19, p < .01. The length effect on response latencies was small for words (about 50 ms, p < .01) and large (about 140 ms, p < .01) for pseudowords.
fMRI
Length effects
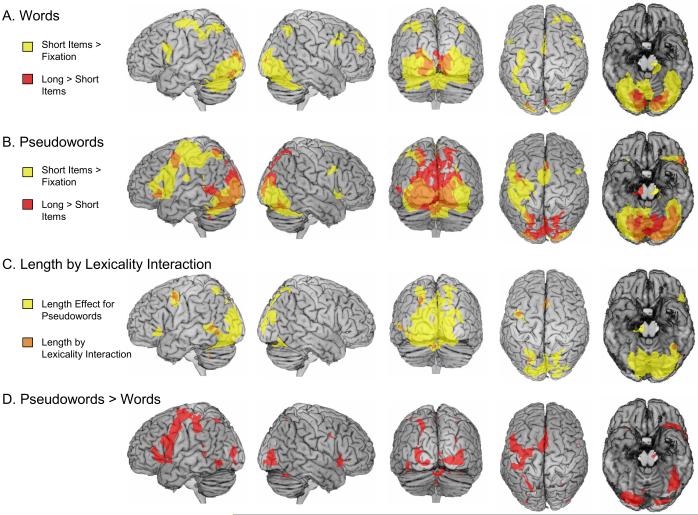
Because of the specific difficulty of the short pseudohomophones and the complete absence of a length effect on response latencies for pseudohomophones, analysis of brain activity is limited to words and pseudowords. Figure 1 shows separately for words and pseudowords, which regions were activated by the short items against baseline. In the baseline condition (interstimulus intervals and null-events), participants viewed a fixation cross. The activations elicited by short items are shown in yellow. In addition, regions which exhibited a length effect (increased activity for long compared to short items) are shown in red. Regions which were activated by short items vs. fixation and by the length effect contrast are coloured orange. Table 3 provides further information on regions identified by the length effect for each item category.
Figure 1.
Regions showing activation for short items against fixation baseline (yellow), and regions showing a length effect (red). Regions which were activated by short items vs. fixation and by the length effect contrast are coloured orange (C) Regions showing a length effect for pseudowords (yellow) serve as mask for regions with a length by lexicality interaction (orange), that is, a stronger length effect for pseudowords than for words. (D) Regions exhibiting activity differences between words and pseudowords. A voxelwise threshold of p < .001, and a threshold for cluster extent of p < .05 corrected were used for all comparisons. For display purposes, no cerebellum is shown on the ventral view (rightmost column) of the renderings. The left side of the brain is shown on the left side of the images, except for ventral view (rightmost column).
Table 3.
Brain regions showing length effects
| MNI Coordinates |
|||||
|---|---|---|---|---|---|
| Region | x | y | z | t | Voxel Extent |
| Length Effects for Words | |||||
| L lingual | −22 | −82 | −12 | 7.83 | 2111 |
| R lingual | 14 | −80 | −10 | 7.12 | - |
| Length Effects for Pseudowords | |||||
| R Lingual | 14 | −82 | −16 | 9.16 | 9423 |
| L Lingual | −16 | −80 | −12 | 9.16 | - |
| L Middle Temporal | −54 | −52 | 2 | 5.11 | - |
| L Cerebellum | −40 | −50 | −38 | 4.86 | - |
| L VWFA | −38 | −58 | −12 | 3.65 | - |
| L Superior Parietal | −24 | −70 | 42 | 5.08 | 509 |
| L Superior Occipital | −24 | −66 | 32 | 4.97 | - |
| L Precentral | −38 | 0 | 46 | 5.54 | 384 |
| L Inferior Frontal, Orbital | −52 | 26 | −4 | 4.60 | 97 |
| R Superior Parietal | 18 | −66 | 62 | 4.93 | 288 |
| R Thalamus | 12 | −18 | −10 | 4.74 | 124 |
| R SMA | 6 | 22 | 46 | 4.72 | 197 |
| Lentgh by Lexicality Interaction (Words and Pseudowords) | |||||
| L Vermis | 2 | −80 | −24 | 3.45 | 20 |
| L Superior Parietal | −24 | −68 | 40 | 4.40 | 94 |
| L Middle Temporal | −54 | −54 | 4 | 4.39 | 30 |
| L Middle Temporal | −46 | −64 | −2 | 3.78 | 25 |
| L Cerebellum | −38 | −50 | −38 | 4.92 | 77 |
| L VWFA | −38 | −50 | −16 | 3.91 | - |
| L Precentral | −40 | 0 | 44 | 4.66 | 79 |
| R SMA | 6 | 22 | 46 | 5.16 | 141 |
| L SMA | −2 | 16 | 54 | 4.33 | - |
Figure 1A shows the results for words. The important observation here is that a large reading network was activated by short words against baseline, but that the length effect for words was limited to a relatively small occipital cluster with activation maxima in the lingual gyri (see Table 3). An inverse length effect (higher activation for short compared to long items) was not found in any brain region for words. The network of regions active for short words against fixation included occipital, OT and parietal regions of both hemispheres and inferior frontal, precentral and postcentral regions predominantly in the left hemisphere. Activation was also found in subcortical structures (right putamen, left caudate).
Figure 1B shows rather different length effects for pseudowords. Here the length effect was not limited to lingual gyrus regions but was also found in large occipital, OT and parietal regions of both hemispheres. Additional regions were identified in the left frontal cortex (orbital part of the inferior frontal gyrus, precentral gyrus), in bilateral supplementary motor area (SMA), right thalamus, and in the left cerebellum (see Table 3). Again, no inverse length effect (higher activation for short compared to long items) was found in any brain region for pseudowords. The contrast of short pseudowords against fixation identified the same brain areas as short words against fixation, however activations were much more extended.
Regions exhibiting a reliable length by lexicality interaction on brain activity (i.e., a stronger length effect for pseudowords than for words) are shown in Figure 1C coloured in orange. The coordinates are provided in Table 3. The search for such regions was limited by a mask. This mask consisted of regions with a reliable length effect for pseudowords, shown in Figure 1C coloured in yellow. Because of this mask, a more liberal cluster extent correction was applied and all clusters larger than 10 voxels are reported. Within this mask, a length by lexicality interaction was found in a left OT region with activity extending ventrally into the cerebellum. Within this region, a maximum roughly corresponding to the VWFA was identified. A length by lexicality interaction was also identified in several left hemisphere regions (superior parietal, posterior middle temporal, precentral), bilateral SMA regions and two cerebellar regions.
Item type effects
The comparison between words and pseudowords was restricted to regions which exhibited reliable activation against baseline for at least one of the item types. This mask excludes brain activity differences which result only from deactivations against baseline. No single brain area was identified by the contrast of words minus pseudowords. Figure 1D shows that the pseudoword minus word contrast identified a large OT cluster with an activity maximum at x = −44, y = −60, z = −10 (t = 5.89). This contrast also identified bilateral occipital, parietal and frontal brain areas, a left middle temporal region, the SMA and a region in the thalamus. The largest brain areas showing higher activation for pseudowords compared to words were found in the SMA, and in the left frontal cortex.
Several brain regions exhibited deactivation compared to baseline in response to both words and pseudowords (precuneus, right middle frontal, left superior frontal) with more extended deactivations for pseudowords than for words. In addition, pseudowords elicited deactivation in the left angular gyrus. Such deactivations do not necessarily reflect reading related processes, but rather suppression of spontaneous “resting-state” activity by stimulus-driven processes (Gusnard & Raichle, 2001).
Regions of Interest (ROIs)
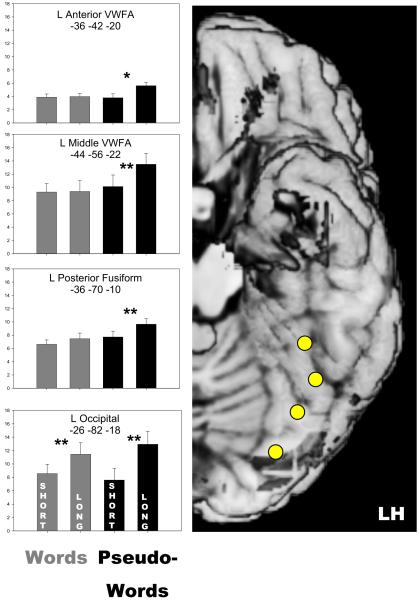
The differential length effects in response to words and pseudowords in the left OT cortex – evident from the whole-brain analysis - were further examined by ROI-based analysis. Of main interest was to specify the disappearance of the length effect for words along the ventral visual pathway. Accordingly, as shown in Figure 2, four ROIs along this pathway were selected. The coordinates of the specific ROIs were based on activity maxima of an “effects of interest” contrast (i.e., comparing activity to both words and pseudowords against baseline). This contrast was restricted to a small volume including the left fusiform, left inferior temporal and left inferior occipital gyrus (see Tzourio-Mazoyer et al., 2002). The ROI at y = −52 corresponds to the center of the original VWFA (Cohen et al., 2002). According to the coding hierarchy for visual word processing of Dehaene et al. (2005) it is engaged in bigram (i.e., sequences of two letters) coding. In this model, the more anterior ROI at y = −42 is engaged by small word and recurrent substring coding. The posterior fusiform ROI at around y = −70 is supposed to code abstract letter identities. The occipital ROI at around y = −82 is involved in low-level visual feature processing (local feature and shape detection).
Figure 2.
Right Panel: Approximate locations of Regions of Interest, indicated on a standard brain template.
Left Panel: Exact locations of Regions of Interest (given in MNI space coordinates) and brain activity estimates (given in arbitrary units) in response to short and long words and pseudowords. * p < .05; ** p < .01.
Figure 2 shows the means of the signal change scores for these ROIs and the results of the t-test comparisons of the means. As evident from Figure 2, the length of pseudowords affected brain activity at each ROI. Although, the length effect decreased in the posterior to anterior direction. The length by location (posterior to anterior) interaction was found reliable for pseudowords, F(3,51) = 11.38, p < .01. In contrast to the pervasive length effect for pseudowords, the length effect for words was clearly limited to the two posterior ROIs and was significant only for the occipital ROI. Again the length by location interaction was reliable, F(3,51) = 5.54, p < .01. In addition, for each ROI separately, reliability of the length by lexicality interaction was examined. This interaction was found reliable for the two anterior ROIs, Fs(1,17) > 5.40, ps < .05, but not for the posterior fusiform and the occipital ROIs, Fs < 4.2.
Discussion
Response Latencies: Length by lexicality interaction
In the present imaging study, we used a phonological lexical decision task with the intention to examine processes similar to those, which are assumed to mediate between the visual information of a letter string and the overt response in reading aloud tasks. In this respect, the finding of a length by lexicality interaction on response latencies is of specific importance. This interaction is a well-established finding in studies which used reading aloud tasks and measured the onset-latency of the naming response (Weekes et al., 1997; Juphard et al., 2004, 2006; Ziegler et al., 2001). For serial sublexical processing, the systematic increase of naming latencies with the number of letters of a pseudoword is of specific importance (e.g. Weekes et al., 1997). The present response latencies for words and pseudowords correspond to the general result pattern of the naming studies, as we found an only small length effect on response latencies for words (50 ms) and a substantial effect for pseudowords (140 ms). As outlined in the Introduction, the length by lexicality interaction on naming latencies – in dual-route models of visual word processing – is taken to reflect different pathways from vision to phonology, that is, a dominance of a lexical pathway to phonology (via orthographic lexicon entries) in the case of words and a dominance of a serially operating sublexical pathway in the case of pseudowords (e.g. Coltheart et al., 2001; Perry et al., 2007). The length by lexicality interaction also corresponds to the distinction between a global (whole word) and an analytical (sublexical syllabic) processing mode in the MTM model of visual word processing by Ans et al. (1998).
Obviously, the present phonological lexical decision task requires additional processing compared to arriving at a naming response via the lexical or the sublexical reading route. This is evident from a comparison of latencies. In the present study, response latencies were about 870 ms for words and about 1150 ms for pseudowords. In a representative single word reading study (Ziegler et al., 2001), naming latencies were about 570 ms for words and about 670 ms for pseudowords. However, despite this generally prolonged processing, the correspondence of the latency patterns (words < pseudowords, length effect limited to pseudowords) suggests that the present responses to words reflect largely lexical processing and the responses to pseudowords reflect largely serial sublexical processing. For discussion, a comparison with the standard lexical decision task is instructive. The standard task simply asks “Is xxx a word?” and presents only words and pseudowords. The present task asks “Does xxx sound like an existing word?” and presents words, pseudohomophones, and pseudowords. Importantly, in the standard task, different from naming tasks and the present task, no length effect on the response latencies for pseudowords is found (Juphard et al., 2004, 2006). This absence of a length effect is critical as it suggests that, in the standard lexical decision task, pseudowords are not processed by the serially operating sublexical route, but by the lexical route. This means that in the standard lexical decision task, whole letter strings of both words and pseudowords are matched against stored orthographic word representations (see Coltheart et al., 1977). In this account, prolonged latencies of the NO response to pseudowords (with no length effect) compared to the latencies of the YES response to words do not imply serial sublexical processing of pseudowords but prolonged search of the orthographic word lexicon. The present phonological lexical decision task does not encourage lexical processing of pseudowords and pseudohomophones as this would result in erroneous NO responses to the pseudohomophones. Still, one may speculate that our participants first matched letter strings against orthographic lexicon entries and, in case of no match for pseudohomophones and pseudowords, resorted to serial sublexical processing which led to a YES response for pseudohomophones and to a NO response for pseudowords (see Borowsky et al., 2002). This account is unproblematic for the present study as it still assumes that the length by lexicality (word vs. pseudoword) interaction on response times reflects reliance on lexical processing in the case of words and reliance on serial sublexical processing for pseudowords. We certainly acknowledge that the present latency findings cannot adjudicate between cognitive models of visual word processing and do not allow to evaluate complex explanations of pseudohomophone processing (e.g., Borowsky et al., 2002; Reynolds & Besner, 2005).
Lexical and sublexical processing along the ventral visual pathway
From the hypothesis that the VWFA is engaged by both orthographic whole-word coding and serial sublexical letter string processing, we expected to find a length by lexicality interaction on activation of the VWFA, that is, little or no length effect in response to the orthographically familiar forms of words and a marked length effect in response to the unfamiliar orthographic forms of pseudowords. Indeed, a region roughly corresponding to the VWFA of Cohen et al. (2002) was identified by the search for regions which exhibit a length by lexicality (word vs. pseudoword) interaction. The activation maximum of this region at x = −38, y = −50, z = −16 is in close spatial correspondence to the original coordinates of the VWFA reported by Cohen et al. (2002) at x = −43, y = −54, z = −12. In further correspondence with the original VWFA coordinates is the finding that the pseudoword minus word contrast identified a large OT cluster with a maximum at x = −44, y = −60, z = −10. This finding corresponds to the orthographic familiarity effect on VWFA activation (i.e., words < pseudohomophones = pseudowords), which was found in previous studies with the phonological lexical decision task (Kronbichler et al., 2007; Bruno et al., 2008; van der Mark et al., 2009).
The ROI analysis provided additional evidence for a differential length effect for words and pseudowords along the left ventral visual pathway. The main finding was that for the familiar orthographic forms of words, the length effect on activation was limited to occipital regions, whereas for pseudowords an effect of length was present throughout the ventral visual pathway. For our most posterior ROI in the left occipital cortex (y = −82), there was a substantial length effect on activation for both words and pseudowords. In the left posterior fusiform gyrus (y = −70), the length effect for words was already decreased and no longer reliable, and it was completely absent for the ROI corresponding to the classical VWFA location (y = −52). This early disappearance of a word-length effect on activation along the ventral visual pathway is not an isolated finding and was also found by Mechelli et al. (2000), who systematically varied word-length from 3 to 9 letters. In correspondence with the present finding, word-length was found to have a monotonic positive effect on neural activity only in bilateral lingual and posterior fusiform gyri.
The early disappearance of the length effect for words is astonishing in relation to the recent extension of the VWFA hypothesis of Dehaene et al. (2005), which posits a hierarchy of increasingly abstract and more complex codings along the ventral visual pathway. In this scheme, the classical VWFA (y = −54) codes for bigrams (i.e., two-letters) and a more anterior segment (y = −48) codes for small words and recurrent substrings within words. Hence, one would expect a length effect for words even at these levels of the coding hierarchy. Obviously, this was not the case as the word length effect ceased to be reliable in the posterior fusiform ROI. This absence of a word-length effect is suggestive for orthographic whole-word recognition, but we cannot rule out that the early disappearance of a word-length effect along the ventral visual pathway is due to inhibitory feed-back processes from word-level coding in anterior OT regions to lower-level codings in posterior regions. Such inhibitory effects may not be present in the priming studies (Dehaene et al., 2001; 2004) on which the Dehaene et al. (2005) model is based. The disappearance of the word length effect along the left ventral visual pathway is in general agreement with a number of studies which found that posterior OT regions are engaged by sublexical letter string processing, while anterior OT regions are more engaged by lexico-semantic processing (Dietz et al., 2005; James et al., 2005; Jobard et al., 2003; Kronbichler et al., 2009; Levy et al., 2008; Mechelli et al., 2005; Seghier et al., 2008; Vigneau et al., 2005; Vinckier et al., 2007).
Length by lexicality interaction in other brain regions
Dorsal visual pathway
Cohen et al. (2008) recently proposed that bilateral dorsal visual pathways in parietal regions are engaged when visual word processing requires serial guidance of visual attention within the letter string. Cohen et al. (2008) induced serial letter processing by presenting the familiar orthographic forms of words in increasingly distorted formats (e.g., by increasing letter spacing) and found increased format distortion to be associated with both increased response latencies and with increased activation in bilateral superior parietal regions and left posterior OT regions. The parietal activations were interpreted as reflecting serial movement of visual attention over the distorted letter strings. Consistent with Cohen et al.’s (2008) hypothesis, long compared to short pseudowords led to increased activation in bilateral occipitoparietal and parietal regions. Importantly, these regions were not affected by the length of the familiar letter strings of words. Furthermore, a length by lexicality interaction was identified in the left superior parietal lobe with an activity maximum at x = −24, y = −68, z = 40. This location closely corresponds to the left intraparietal cortex (x = −22, y = −72, z = 48), which Cohen et al. (2008) found to be involved in all modes of format distortions. A novel finding with respect to Cohen et al.’s (2008) study is that we found a length effect for unfamiliar orthographic forms of pseudowords on activation in the classical VWFA (at y = −52) and also in a more anterior region (y = −42). Cohen et al. (2008) found a format distortion effect in the ventral visual pathway only in a more posterior region (at y = −70). A main difference between the present study and the study by Cohen et al. (2008) is that the latter presented familiar orthographic forms of words in distorted formats, while we presented unfamiliar orthographic forms of pseudowords in undistorted format. Accordingly, one may reason that orthographically familiar words in distorted format affect only posterior OT regions, whereas orthographically unfamiliar pseudowords also affect the VWFA along the ventral visual pathway.
Frontal and temporal regions
The length by lexicality interaction also identified clusters in bilateral SMA, in the dorsal left precentral gyrus, and in the cerebellum. The specific effect of pseudowords in these regions may reflect the higher demands caused by the silent assembly of new pronunciations, when long compared to short pseudowords have to be processed. In contrast, long compared to short words do not cause such increased demands as existing phonological word entries are accessed. This interpretation finds specific support by the findings of a recent fMRI study by Alario et al. (2006). In contrast to the present phonological lexical decision, Alario et al. (2006) asked participants to read aloud words and pseudowords and to repeat them. In both conditions, activity in the pre-SMA was found to exhibit a length by lexicality interaction, that is, little length effect for words and a marked effect for pseudowords. In the present study, further clusters with a length by lexicality interaction were identified in a region between the posterior inferior temporal and the posterior middle temporal gyrus in the left hemisphere. In language studies, this region is assumed to be engaged by lexical semantic processes (Hickock & Poeppel, 2004, 2007). This assumption cannot be applied easily to the present finding of a marked length effect for pseudowords. In general, the length by lexicality interactions in left temporal and frontal regions are broadly consistent with the position of several authors that the sublexical “phonological” reading route poses specific demands to language regions of the left hemisphere (Borowsky et al., 2006; Pugh et al., 2000; Sandak et al., 2004).
There are two other studies which specifically searched for a length by lexicality interaction on brain activity (Valdois et al., 2006; Wydell et al., 2003). Surprisingly, neither one of these studies identified the VWFA as exhibiting such an interaction effect. The MEG study by Wydell et al. (2003) presented short and long words and pseudowords. At about 100 ms after presentation, activation around the occipital midline was affected by string length independent of lexicality. Around 200-600 ms after presentation, a tendency towards a length by lexicality interaction was observed in a posterior region of the left superior temporal gyrus. In correspondence with the present study, the fMRI study by Valdois et al. (2006) did find a length by lexicality interaction in the left superior parietal lobe, the posterior aspect of the middle temporal gyrus, and the left cerebellum.
Converging evidence for a double function of the VWFA in visual word processing
Lexicality effects
The present activation pattern in left OT regions, that is, lower activation to words compared to pseudowords, adds to the results from imaging studies which examined lexicality effects (i.e., words vs. pseudowords) on VWFA activation. As already noted, there are three preceding studies which used the present phonological lexical decision task and found higher activation of the VWFA in response to pseudowords and pseudohomophones compared to words (Kronbichler et al., 2007; Bruno et al., 2008; van der Mark et al., 2009). Furthermore, three studies using silent reading found higher VWFA activation to pseudohomophones compared to words (Kronbichler et al., 2009) or to exception words (Borowsky et al., 2006, 2007). A lexicality effect with similar direction (i.e., pseudowords > words) in left OT cortex regions was found in 8 of 13 imaging studies which relied on silent reading or reading aloud (of alphabetic scripts). Presence of this effect was reported in the reading studies of Binder et al., 2005; Fiez et al., 1999; Hagoort et al., 1999; Kronbichler et al., 2004; Mechelli et al., 2003, 2005; Paulesu et al., 2000; Vigneau et al., 2005. No reliable lexicality effect was found by Carreiras et al., 2007; Dietz et al., 2005; Herbster et al., 1997; Ingvar et al., 2002; Joubert et al., 2004. A quite different pattern of findings is typically observed in studies which relied on standard lexical decision task. With this task, there is only one study with higher OT cortex activation in response to pseudowords compared to words (Kuchinke et al., 2005) and further 6 studies did not report a reliable difference (Binder et al., 2003; Carreiras et al., 2007; Fiebach et al., 2005, 2007; Heim et al., 2005, 2009). This review suggests that a lexicality effect on VWFA activity is observed with some consistency when processing of unfamiliar orthographic forms of pseudowords requires serial processing and assembly of a pronunciation. Apparently, a lexicality effect on VWFA activity is infrequently observed when a standard lexical decision task does not require serial processing and pronunciation assembly. The present finding of a length effect on left OT activation for pseudowords, but not for words, provides specific support for this interpretation. In the discussion of the length by lexicality interaction on reaction time, we noted that the length effect for pseudowords is consistently found in reading tasks but not in lexical decision tasks.
There is a single study which, different from the mentioned studies, found higher VWFA activation in response to pseudowords compared to words (Vinckier et al.; 2007). This opposite finding may reflect theoretically interesting task differences. Vinckier et al. (2007) used an implicit reading task (i.e., hashmark detection) and presented stimuli for only 100 ms in immediate sequence. One may hypothesize, that under these conditions, it was still possible to process the familiar letter strings of words but not the increasingly unfamiliar letter strings. In other words, the experimental set-up of Vinckier et al. (2007) may have led to a word-superiority effect (e.g., Reicher, 1969). The procedure of the present study stands in marked contrast to that of the Vinkier et al. (2007) study. Presumably, the present phonological lexical decision task (i.e., “Does xxx sound like an existing word?”) induced sublexical processing of pseudohomophones and pseudowords. Furthermore, stimuli were presented for a comparatively long time, 1260 ms, with an interstimulus interval of 1360 ms to allow full processing of the unfamiliar orthographic forms.
Priming, repetition and adaptation studies
Support for orthographic whole-word coding in the VWFA comes from recent subliminal priming studies (Dehaene et al., 2004; Devlin et al., 2006). Dehaene et al. (2004) subliminally presented cross-case primes consisting of the same words (e.g., prime: ANGER, target: anger) compared to anagram primes consisting of the same letters in different order (e.g., prime: RANGE, target: anger). The critical finding was that in an anterior portion of the left OT cortex (y=−48) same word primes elicited a slightly stronger priming effect than anagram primes. This pattern suggests that subliminal word primes but not anagram primes preactivate existing orthographic word representations. Similarly, Devlin et al. (2006) showed that the VWFA responded with reduced activity to subliminal cross-case priming of words (e.g., prime: cabin, target: CABIN) but not to subliminal priming of pseudowords (e.g., prime: solst, target: SOLST). Consistent with subliminal priming, studies of repetition priming (Pugh et al., 2008; Fiebach et al., 2005), and studies in which words were trained before scanning (Katz et al., 2005) showed that previously encountered compared to novel words lead to reduced activation in the VWFA. Of further interest is a recent fMRI-adaptation study by Glezer et al (2009). This study found that, in the case of adaptation (decreasing activation) to repeatedly presented visual words, exchange of a single letter (e.g., coat instead of boat) led to the same increase of VWFA activation as the change of 4 letters (e.g, fish instead of boat). This result pattern is expected when the change of a single letter activates a different orthographic word representation in the VWFA. The activation pattern in response to pseudowords was different. Here, exchange of a single letter (e.g., soat instead of poat) led to a significantly smaller increase of activation than exchange of 4 letters (e.g., hime instead of poat). This result pattern is expected when the adapted pseudoword is not represented as a single orthographic word coding but as a string of letter or graphemes. In this case, the change of one letter constitutes less deviance from the representation than the change of four letters.
Summary and Relation to Cognitive Models of Visual Word Processing
Based on our previous work, we expected that the VWFA of Cohen et al. (2000, 2002) is engaged by both orthographic whole word recognition and serial sublexical coding of unfamiliar orthographic forms. With respect to this double function of the VWFA, we expected to find a length by lexicality interaction on activity in the VWFA, that is, little or no effect of length (number of letters) for familiar orthographic forms of words and presence of a marked length effect for unfamiliar orthographic forms of pseudowords. This expected interaction was indeed found. For words, the length effect was limited to occipital and posterior fusiform regions and completely absent in the VWFA. In contrast, for pseudowords, the length effect was found throughout the ventral visual pathway, present in the VWFA as well as a more anterior OT cortex segment. A length by lexicality interaction was also found in a left superior parietal region, presumably engaged by serial visual attention (Cohen et al., 2008), and in left frontal (SMA, precentral) and cerebellar regions, presumably engaged by silent articulatory processes. We again note that these length by lexicality interaction effects were found in a phonological lexical decision task, which – different from a standard reading task – requires phonological decision processes in addition to silent reading processes.
Although the conceptual relationship between cognitive models and brain activation findings is complex, we do think that the present imaging findings provide support for basic tenets of the well-known dual-route model of visual word processing (Colheart et al., 2001; Perry et al., 2007) and for central assumptions of the dual-process model of Ans et al. (1998). A main component of dual-route models is an orthographic word lexicon, which is critically involved in orthographic whole-word recognition of the lexical route from vision to phonology. This orthographic word lexicon and the distinction between lexical and sublexical route processes are explicitly denied by single route connectionist models (Seidenberg & McClelland, 1989; Plaut et al., 1996; Harm & Seidenberg, 2004). The finding of an orthographic familiarity effect on activation of the VWFA and the complete absence of a word length effect on VWFA activation seems broadly consistent with lexical route processing based on an orthographic lexicon. From a dual-route perspective, the absent length effect in the VWFA reflects instantiation of an orthographic lexicon entry, while the length effect for pseudowords reflects serial conversion of the letter strings into graphemes or sublexical orthographic segments required for the assembly of a coherent pronunciation. However, the complete absence of a length effect for words in regions outside of the occipital cortex cannot be immediately reconciled with the assumption of parallel lexical and sublexical processing which are proposed by the computational versions of the dual route model (Coltheart et al., 2001; Perry et al., 2007). If serial sublexical processing operated simultaneously with lexical processing, then a small length effect for words would be expected in the VWFA (presumably also engaged by sublexical coding) and in frontal regions (presumably engaged by sublexical phonological coding). This was not the case. One may reason that complete absence of such a word length effect is due to fast operation of the lexical route. The assumption would be that an orthographic lexicon entry reaches a critical threshold very quickly, and this results in early shut-down of the serially operating sublexical route (for details on time-course assumptions, see Coltheart et al., 2001). The dual-process model of Ans et al. (1998) has no problem to account for the complete absence of a word length effect as it assumes that a global recognition mode always precedes a serial analytical mode. In the case of words, success of the global mode prevents the start of the serial analytical mode. We are aware of the tentative nature of the suggested correspondences between cognitive dual-route processes and the present brain activation findings. Though, we think that the establishment of such correspondences is major goal on the neurocognitive research agenda.
Acknowledgements
This research was supported by grant of the Austrian Science Foundation to Heinz Wimmer and Gunther Ladurner (Grant Number P18832-B02). We are grateful to the members of the Department of Radiology for their assistance. We would also like to thank Johannes Klackl for help with data analysis, and Andy Fugard and Julia Sophia Crone for reading the manuscript. The comments of our reviewers greatly improved the paper.
Appendix
| Short Words | |||||||||
| Arzt | Blatt | blau | Blech | Blei | Blick | blond | Blume | Blut | Brand |
| Brett | Brief | Brot | Bruch | Bühne | Chor | Dämon | Dom | Draht | Drama |
| Dreck | drei | Droge | Gel | Gips | Glanz | glatt | Glück | Glut | Groll |
| Größe | Grund | Gruß | Juli | Keks | Kerze | Klage | Kleid | Klima | Klo |
| Kluft | König | Kraft | Krähe | Krimi | Krug | Latz | Luxus | Malz | Mehl |
| Naht | Pelz | Pfand | Pfau | Platz | Pony | Preis | prima | Prinz | Probe |
| Profi | Reue | Rohr | Rotz | Saal | Saure | Stand | Stuhl | Taxi | Text |
| Tiger | Träne | Traum | treu | trub | Truhe | Typ | Vater | Vieh | Vogel |
| Volk | vorne | Wärme | weich | Wespe | Zelt | Zeug | Zimt | Zoo | Zug |
| Short Pseudohomophones | |||||||||
| Artst | Bladt | plau | Pläch | Blai | Plick | plond | Plume | Plut | Prand |
| Prett | Brihf | Prot | Pruch | Büne | Kor | Demon | Dohm | Traht | Trama |
| Treck | trei | Troge | Gehl | Gibs | Klanz | klatt | Klück | Klut | Kroll |
| Kröße | Krund | Kruß | Juhli | Keex | Kertse | Glage | Klaid | Glima | Glo |
| Gluft | Köhnig | Graft | Krehe | Grimi | Grug | Lats | Luksus | Malts | Mel |
| Nahd | Pelts | Pfant | Bfau | Blatz | Ponni | Prais | brima | Prints | Brobe |
| Brofi | Räue | Ror | Rots | Sahl | Seure | Stant | Stul | Taksi | Tekst |
| Tieger | Dräne | Draum | dreu | drüb | Druhe | Tüp | Fater | Fieh | Fogel |
| Folk | forne | Werme | waich | Wesbe | Zeld | Zäug | Zimd | Tso | Zuhg |
| Short Pseudowords | |||||||||
| Äbu | Bana | Bfei | Bfind | blünt | Brebe | bremu | Breus | Brifo | Bruf |
| Diman | Dreim | Drihe | dröb | Dühm | Fagul | Ferta | Feuh | Filk | förni |
| Füpp | Glaht | Glift | Gligo | Gloid | Gluma | Göobs | Gräg | Grime | Grüft |
| Irts | Jahla | Klaa | Klinz | Klöck | klütt | Köhr | Körte | Kränd | Krißa |
| Kruf | Krufe | Krull | Kunag | Leex | Liksas | Luts | Mil | Mults | Noit |
| Pinni | Plie | Plih | Plime | Plit | ploh | Plött | Pluk | Pluts | Pölts |
| Prants | Prich | Prid | Prin | Prüt | Rihr | Ruts | Sereu | Söhl | Stel |
| Stönt | Tigo | Tiksa | Tokst | trah | Triga | Trine | Track | Tru | Trut |
| Tsag | Tsul | Tsüm | Tuma | Wehl | Wime | wösch | Wusbe | Zäub | Zei |
| Long Words | |||||||||
| Anliegen | Anspielung | Bedrängnis | Begehren | Beispiel | Belohnung | Beziehung | Brandung | Brauerei | Broschüre |
| Brutalität | dringend | fällig | Fehler | Fetzen | Fleisch | Flieger | Freiheit | Freizeit | freundlich |
| Frieden | Fühler | Gefängnis | Glauben | Gleichnis | Gletscher | grandios | Groschen | Gründung | Gymnasium |
| Gymnastik | Häufchen | Heilung | Heirat | Heizung | hygienisch | jährlich | Kaffee | Kerker | Ketzer |
| Klassiker | klinisch | Klinke | kräftig | Kreuzung | Kristall | Lähmung | Leistung | Leiter | Meinung |
| Papier | Pfleger | Pflicht | Prägung | Problem | Produktion | Räuber | Reichtum | Satzung | säubern |
| Schatz | Schätzung | Scheich | Schein | Scherz | Schiene | Schild | Schreiber | Schürze | Schutz |
| schwarz | Schweigen | Sektion | Sendung | Siedler | Steuer | Streit | Teilchen | typisch | Verführer |
| Verkehr | Verleger | vermuten | Vorgehen | Wächter | Währung | Wechsel | Wendung | Zahlung | Zeugnis |
| Long Pseudohomophones | |||||||||
| Anligen | Anspihlung | Bedrengnis | Begeren | Baispiel | Belonung | Bezihung | Prandung | Prauerei | Proschüre |
| Prutalitet | tringend | fällik | Feler | Fetsen | Flaisch | Flihger | Fraiheit | Fraizeit | fräundlich |
| Friden | Füler | Gefengnis | Klauben | Kleichnis | Kletscher | krandios | Kroschen | Kründung | Gümnasium |
| Gümnastik | Heufchen | Hailung | Hairat | Haizung | hügienisch | jehrlich | Kaffeh | Kärker | Kätzer |
| Glassiker | glinisch | Klincke | gräftig | Kräuzung | Gristall | Lämung | Laistung | Laiter | Mainung |
| Papir | Pflehger | Bflicht | Pregung | Broblem | Broduktion | Reuber | Raichtum | Satsung | seubern |
| Schats | Schätsung | Scheisch | Schain | Schärz | Schine | Schildt | Schraiber | Schürtze | Schuts |
| schwarts | Schwaigen | Säktion | Sändung | Sietler | Stäuer | Strait | Tailchen | tüpisch | Ferführer |
| Verkähr | Ferleger | fermuten | Forgehen | Wechter | Wehrung | Wächsel | Wändung | Zalung | Zäugnis |
| Long Pseudowords | |||||||||
| Angenung | Baizieh | Bauspil | Belahnis | Breinetik | Brisäm | Broheit | Bruler | dringtal | Eindung |
| fäferen | Fehgeke | Fehklasen | fehreich | fehrhaul | Fetsität | Fleusch | Fliewach | fraifeng | Frändung |
| Freinis | Freistum | Frieglauch | Fröhden | Füberung | Fürleger | Gehrnis | Glauzeit | Goschwider | Grandium |
| Groschnis | grundios | Gümfel | gümlich | Gürnikat | haizlich | Heilsiker | Heizal | Heufnas | hüregig |
| kafgenisch | Kehrbetz | Kettum | Kletnas | Klinung | Kräuzlon | Kristnis | Krustill | Läustigung | Lemion |
| Lihmang | Lühtor | Meindukt | Mutseller | Pablem | Pflegal | Pfocht | Proklinom | Proleugen | Pubith |
| Reuger | Reutik | Satzeng | Schauch | Schaule | Schäun | Scheld | Scherwend | Schipf | Schirz |
| Schits | schotzig | schürisch | Schwaiben | schwirts | Sendwehr | Seubeil | Sierat | steubern | Straut |
| teileibe | Tüppir | Veräfung | Vergiren | Verkauter | Verleist | Verzeug | Vorschür | Wechze | Zerfekung |
References
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Research. 2006;1076:129–43. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Ans B, Carbonnel S, Valdois S. A connectionist multiple-trace memory model for polysyllabic word reading. Psychological Review. 1998;105:678–723. doi: 10.1037/0033-295x.105.4.678-723. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX lexical database (CD-ROM) Linguistic Data Consortium, University of Pennsylvania; Philadelphia, PA: 1993. [Google Scholar]
- Bergmann J, Wimmer H. A dual-route perspective on poor reading in a regular orthography: Evidence from phonological and orthographic lexical decisions. Cognitive Neuropsychology. 2008;25:653–676. doi: 10.1080/02643290802221404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons M, Westbury CF, Possing ET, Kaufman JN, et al. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. NeuroImage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. NeuroImage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky R, Owen WJ, Masson ME. Diagnostics of phonological lexical processing: Pseudohomophone naming advantages, disadvantages, and base-word frequency effects. Memory & Cognition. 2002;30:969–987. doi: 10.3758/bf03195781. [DOI] [PubMed] [Google Scholar]
- Borowsky R, Cummine J, Owen WJ, Friesen CK, Shih F, Sarty GE. FMRI of ventral and dorsal processing streams in basic reading processes: Insular sensitivity to phonology. Brain Topography. 2006;18:233–239. doi: 10.1007/s10548-006-0001-2. [DOI] [PubMed] [Google Scholar]
- Borowsky R, Esopenko C, Cummine J, Sarty GE. Neural representations of visual words and objects: A functional MRI study on the modularity of reading and object processing. Brain Topography. 2007;20:89–96. doi: 10.1007/s10548-007-0034-1. [DOI] [PubMed] [Google Scholar]
- Bruno JL, Zumberge A, Manis F, Lu Z, Goldman JG. Sensitivity to orthographic familiarity in the occipito-temporal region. NeuroImage. 2008;39:1988–2001. doi: 10.1016/j.neuroimage.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Estévez A, Price CJ. Brain activation for lexical decision and reading aloud: Two sides of the same coin? Journal of Cognitive Neuroscience. 2007;19:433–444. doi: 10.1162/jocn.2007.19.3.433. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff M, et al. The visual word form area. Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Vinckier F, Jobert A, Montavont A. Reading normal and degraded words: Contribution of the dorsal and ventral visual pathways. NeuroImage. 2008;40:353–366. doi: 10.1016/j.neuroimage.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lerner C, Rivaud S, Dehaene S. Language-specific tuning of the visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Davelaar E, Jonasson JT, Besner D. Access to the internal lexicon. In: Dornic S, editor. Attention and performance VI. Erlbaum; Hillsdale, NJ: 1977. pp. 535–555. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler JC. DRC: A dual-route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the left posterior fusiform gyrus in reading. Journal of Cognitive Neuroscience. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: A proposal. Trends in Cognitive Sciences. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Le Bihan D, Mangin J-F, Poline J-B, Rivière D. Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline J-B, Le Bihan B, et al. Letter Binding and Invariant Recognition of Masked Words. Behavioral and Neuroimaging Evidence. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dietz NA, Jones KM, Gareau L, Zeffiro TA, Eden GF. Phonological decoding involves left posterior fusiform gyrus. Human Brain Mapping. 2005;26:81–93. doi: 10.1002/hbm.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Gruber T, Supp G. Neuronal mechanisms of repetition priming in occipitotemporal cortex: Spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. Journal of Neuroscience. 2005;25:3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Ricker B, Friederici AD, Jacobs AM. Inhibition and facilitation in visual word recognition: Prefrontal contribution to the orthographic neighbourhood size effect. NeuroImage. 2007;36:901–911. doi: 10.1016/j.neuroimage.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RNA, Kiebel S, Phillips C, Ashburner J. Classical and bayesian inference in neuroimaging: Applications. NeuroImage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the ”Visual Word Form Area“. Neuron. 2009;62:199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D, Raichle M. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ. The neural circuitry involved in the reading of German words and pseudowords: A PET study. Journal of Cognitive Neuroscience. 1999;11:383–398. doi: 10.1162/089892999563490. [DOI] [PubMed] [Google Scholar]
- Harm WM, Seidenberg MS. Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychological Review. 2004;111:662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Heim S, Alter K, Ischebeck AK, Amunts K, Eickhoff SB, Mohlberg H, et al. The role of the left Brodmann’s areas 44 and 45 in reading words and pseudowords. Cognitive Brain Research. 2005;25:982–993. doi: 10.1016/j.cogbrainres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan KE, Amunts K. Effective connectivity of the left BA 44, BA 45, and the inferior temporal gyrus during lexical and phonological decisions identifed with DCM. Human Brain Mapping. 2009;30:392–402. doi: 10.1002/hbm.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Human Brain Mapping. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Analysis of fMRI time series: Linear time-invariant models, event-related fMRI and optimal experimental design. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, et al., editors. Human brain function. 2nd ed. Academic Press; London: 2004. pp. 793–822. [Google Scholar]
- Ingvar M, af Trampe P, Greitz T, Eriksson L, Stone-Elander S, von Euler K. Residual differences in language processing in compensated dyslexics revealed in simple word reading tasks. Brain and Language. 2002;83:249–267. doi: 10.1016/s0093-934x(02)00055-x. [DOI] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong AC, Gauthier I. Letter processing in the visual system: Different activation patterns for single letters and strings. Cognitive, Affective & Behavioral Neuroscience. 2005;5:452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Joubert S, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux J-M, et al. Neural correlates of lexical and sublexical processes in reading. Brain and Language. 2004;89:9–20. doi: 10.1016/S0093-934X(03)00403-6. [DOI] [PubMed] [Google Scholar]
- Juphard A, Serge C, Valdios S. Length effect in reading and lexical decision: Evidence from skilled readers and a developmental dyslexic participant. Brain and Cognition. 2004;55:332–340. doi: 10.1016/j.bandc.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Juphard A, Carbonnel S, Ans B, Valdois S. Length effect in naming and lexical decision: the multitrace memory model’s account. Current Psychology Letters. 2006;19:2–13. [Google Scholar]
- Katz L, Lee CH, Tabor W, Frost SJ, Mencl WE, Sandak R, et al. Behavioral and neurobiological effects of printed word repetition in lexical decision and naming. Neuropsychologia. 2005;43:2068–2083. doi: 10.1016/j.neuropsychologia.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, et al. Taxi vs. Taksi: Orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2007;19:1584–1594. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: Evidence from a parametric fMRI study. NeuroImage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Klackl J, Richlan F, Schurz M, Staffen W, Ladurner G, Wimmer H. On the functional neuroanatomy of visual word processing: Effects of case and letter deviance. Journal of Cognitive Neuroscience. 2009;211:1–8. doi: 10.1162/jocn.2009.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo ML-H, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. NeuroImage. 2005;28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Levy J, Pernet C, Treserras S, Boulanouar K, Berry I, Aubry F, et al. Piecemeal recruitment of left-lateralized brain areas during reading: A spatio-functional account. NeuroImage. 2008;43:581–591. doi: 10.1016/j.neuroimage.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, et al. Dissociating reading processes on the basis of neuronal interactions. Journal of Cognitive Neuroscience. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Humphreys GW, Mayall K, Olson A, Price CJ. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proceedings of the Royal Society of London, Series B. 2000;267:1909–1913. doi: 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Pammer K, Hansen P, Holliday I, Cornelissen P. Attentional shifting and the role of the dorsal pathway in visual word recognition. Neuropsychologia. 2006;44:2926–2936. doi: 10.1016/j.neuropsychologia.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, et al. A cultural effect on brain function. Nature Neuroscience. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Perry C, Ziegler JC, Zorzi M. Nested incremental modeling in the development of computational theories: The CDP+ model of reading aloud. Psychological Review. 2007;1142:273–315. doi: 10.1037/0033-295X.114.2.273. [DOI] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: Computational principles in quasi-regular domains. Psychological Review. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JDE. Characterizing the neural mechanisms of skill learning and repetition priming. Evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. NeuroImage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Frost SJ, Sandak R, Landi N, Rueckl JG, Constable RT, et al. Effects of stimulus difficulty and repetition on printed word identification: A functional magnetic resonance imaging comparison of nonimpaired and reading disabled adolescent cohorts. Journal of Cognitive Neuroscience. 2008;20:1–15. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Reicher GM. Perceptual recognition as a function of meaningfulness of stimulus material. Journal of Experimental Psychology. 1969;81:275–280. doi: 10.1037/h0027768. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Besner D. Basic processes in reading: A critical review of pseudohomophone effects in reading aloud and a new computational account. Psychonomic Bulletin & Review. 2005;12:622–646. doi: 10.3758/bf03196752. [DOI] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Crommelinck M. The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. NeuroImage. 2003;19:877–883. doi: 10.1016/s1053-8119(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Pugh KR. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Scientific Studies of Reading. 2004;8:273–292. [Google Scholar]
- Sauseng P, Bergmann J, Wimmer H. When does the brain register deviances from standard word spelling? - An ERP study. Cognitive Brain Research. 2004;20:529–532. doi: 10.1016/j.cogbrainres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. NeuroImage. 2008;42:1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychological Review. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Valdois S, Carbonnel S, Juphard A, Baciu M, Ans B, Peyrin C, et al. Polysyllabic pseudo-word processing in reading and lexical decision: converging evidence from behavioral data, connectionist simulations and functional MRI. Brain Research. 2006;1085:149–162. doi: 10.1016/j.brainres.2006.02.049. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmüller J, et al. Dyslexia in children: Dysfunction of specialization within the Visual Word-Form (VWF) System. NeuroImage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Bielamowicz L, Martin A. Modulation of Neural Activity during Object Naming: Effects of Time and Practice. Cerebral Cortex. 2003;13:381–391. doi: 10.1093/cercor/13.4.381. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: what role for the visual word form area? NeuroImage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaehe S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical Coding of Letter Strings in the Ventral Stream: Dissecting the Inner Organization of the Visual Word-Form System. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: A general framework using a genetic algorithm. NeuroImage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Weekes B. Differential effects of number of letters on word and nonword naming latency. The Quarterly Journal of Experimental Psychology. 1997;50A:439–456. [Google Scholar]
- Wimmer H, Mayringer H, Landerl K. The Double-Deficit Hypothesis and Difficulties in Learning to Read a Regular Orthography. Journal of Educational Psychology. 2000;924:668–680. [Google Scholar]
- Wydell TN, Vuorinen T, Helenius P, Salmelin R. Neural Correlates of Letter-String Length and Lexicality during Reading in a Regular Orthography. Journal of Cognitive Neuroscience. 2003;157:1052–1062. doi: 10.1162/089892903770007434. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Perry C, Jacobs AM, Braun M. Identical words are read differently in different languages. Psychological Science. 2001;12:379–384. doi: 10.1111/1467-9280.00370. [DOI] [PubMed] [Google Scholar]