Abstract
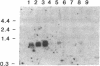
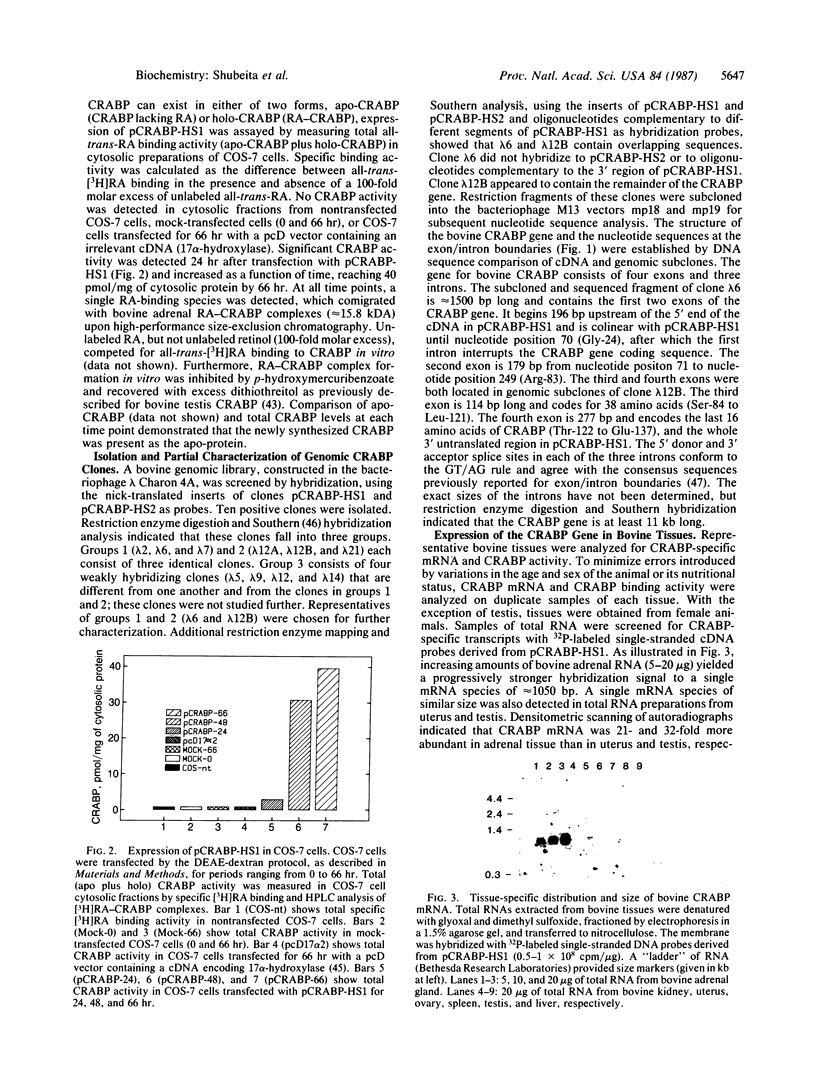
A recombinant cDNA clone, pCRABP-HS1, encoding cellular retinoic acid-binding protein was isolated from a bovine adrenal cDNA library. COS-7 cells transfected with pCRABP-HS1 produced a biologically active retinoic acid-binding protein molecule of the expected molecular mass (15.5 kDa). RNA blot hybridization analysis using pCRABP-HS1 as a probe revealed a single 1050-nucleotide mRNA species in bovine adrenal, uterus, and testis, tissues that contain the highest levels of retinoic acid-binding activity. No hybridization was detected in RNA extracted from ovary, spleen, kidney, or liver, which contain relatively low levels of cellular retinoic acid-binding protein activity. Analysis of genomic clones isolated from an EcoRI bovine genomic library demonstrated that the bovine cellular retinoic acid-binding protein gene is composed of four exons and three introns. Two putative promoter sequences were identified in the cloned 5' sequence of the gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Sullivan W. P., Toft D. O., Birnbaumer M., Cook R. G., Maxwell B. L., Zarucki-Schulz T., Greene G. L., Schrader W. T., O'Malley B. W. Molecular cloning of the chicken progesterone receptor. Science. 1986 Aug 15;233(4765):767–770. doi: 10.1126/science.2426779. [DOI] [PubMed] [Google Scholar]
- Demmer L. A., Birkenmeier E. H., Sweetser D. A., Levin M. S., Zollman S., Sparkes R. S., Mohandas T., Lusis A. J., Gordon J. I. The cellular retinol binding protein II gene. Sequence analysis of the rat gene, chromosomal localization in mice and humans, and documentation of its close linkage to the cellular retinol binding protein gene. J Biol Chem. 1987 Feb 25;262(6):2458–2467. [PubMed] [Google Scholar]
- Dowling J. E., Wald G. THE BIOLOGICAL FUNCTION OF VITAMIN A ACID. Proc Natl Acad Sci U S A. 1960 May;46(5):587–608. doi: 10.1073/pnas.46.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. A superfamily of potentially oncogenic hormone receptors. Nature. 1986 Dec 18;324(6098):615–617. doi: 10.1038/324615a0. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., Spiegelman B. M. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch J. M., Krozowski Z., Quirin-Stricker C., Gronemeyer H., Simpson R. J., Garnier J. M., Krust A., Jacob F., Chambon P. Cloning of the chicken progesterone receptor. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5424–5428. doi: 10.1073/pnas.83.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Possible role of retinoic acid binding protein in retinoid stimulation of embryonal carcinoma cell differentiation. Nature. 1979 Mar 8;278(5700):180–182. doi: 10.1038/278180a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I., Murphy D., Hogan B. L. Expression of c-fos in parietal endoderm, amnion and differentiating F9 teratocarcinoma cells. Differentiation. 1985;30(1):76–81. doi: 10.1111/j.1432-0436.1985.tb00516.x. [DOI] [PubMed] [Google Scholar]
- McCormick A. M., Napoli J. L. Identification of 5,6-epoxyretinoic acid as an endogenous retinol metabolite. J Biol Chem. 1982 Feb 25;257(4):1730–1735. [PubMed] [Google Scholar]
- McCue P. A., Matthaei K. I., Taketo M., Sherman M. I. Differentiation-defective mutants of mouse embryonal carcinoma cells: response to hexamethylenebisacetamide and retinoic acid. Dev Biol. 1983 Apr;96(2):416–426. doi: 10.1016/0012-1606(83)90179-3. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Mehta R. G., Cerny W. L., Moon R. C. Nuclear interactions of retinoic acid-binding protein in chemically induced mammary adenocarcinoma. Biochem J. 1982 Dec 15;208(3):731–736. doi: 10.1042/bj2080731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Okret S., Wikström A. C., Wrange O., Gustafsson J. A., Yamamoto K. R. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature. 1984 Dec 20;312(5996):779–781. doi: 10.1038/312779a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. T., Jr, Murtaugh M. P., Davies P. J. Retinoic acid-induced expression of tissue transglutaminase in mouse peritoneal macrophages. J Biol Chem. 1984 Oct 25;259(20):12794–12802. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Cellular retinoic acid-binding protein from rat testis. Purification and characterization. J Biol Chem. 1978 Jul 10;253(13):4551–4554. [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Retinoic acid-binding protein in rat tissue. Partial purification and comparison to rat tissue retinol-binding protein. J Biol Chem. 1975 Aug 10;250(15):6113–6117. [PubMed] [Google Scholar]
- Rainier S., Herrera J. M., McCormick A. M. Rapid characterization of cellular retinoid-binding proteins by high-performance size-exclusion chromatography. Arch Biochem Biophys. 1983 Sep;225(2):818–825. doi: 10.1016/0003-9861(83)90094-2. [DOI] [PubMed] [Google Scholar]
- Rees A. R., Adamson E. D., Graham C. F. Epidermal growth factor receptors increase during the differentiation of embryonal carcinoma cells. Nature. 1979 Sep 27;281(5729):309–311. doi: 10.1038/281309a0. [DOI] [PubMed] [Google Scholar]
- Ross A. C., Adachi N., Goodman D. S. The binding protein for retinoic acid from rat testis cytosol: isolation and partial characterization. J Lipid Res. 1980 Jan;21(1):100–109. [PubMed] [Google Scholar]
- Schindler J., Matthaei K. I., Sherman M. I. Isolation and characterization of mouse mutant embryonal carcinoma cells which fail to differentiate in response to retinoic acid. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1077–1080. doi: 10.1073/pnas.78.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. F., Meyskens F. L., Jr, Russell D. H. Retinoids increase transglutaminase activity and inhibit ornithine decarboxylase activity in Chinese hamster ovary cells and in melanoma cells stimulated to differentiate. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4093–4097. doi: 10.1073/pnas.79.13.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. I., Paternoster M. L., Taketo M. Effects of arotinoids upon murine embryonal carcinoma cells. Cancer Res. 1983 Sep;43(9):4283–4290. [PubMed] [Google Scholar]
- Shubeita H. E., Patel M. D., McCormick A. M. Determination of apo and holo retinoic acid-binding protein levels in retinoid-responsive transformed cells by high-performance size-exclusion chromatography. Arch Biochem Biophys. 1986 Jun;247(2):280–288. doi: 10.1016/0003-9861(86)90585-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Strickland S., Breitman T. R., Frickel F., Nürrenbach A., Hädicke E., Sporn M. B. Structure-activity relationships of a new series of retinoidal benzoic acid derivatives as measured by induction of differentiation of murine F9 teratocarcinoma cells and human HL-60 promyelocytic leukemia cells. Cancer Res. 1983 Nov;43(11):5268–5272. [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Sundelin J., Das S. R., Eriksson U., Rask L., Peterson P. A. The primary structure of bovine cellular retinoic acid-binding protein. J Biol Chem. 1985 May 25;260(10):6494–6499. [PubMed] [Google Scholar]
- Sweetser D. A., Lowe J. B., Gordon J. I. The nucleotide sequence of the rat liver fatty acid-binding protein gene. Evidence that exon 1 encodes an oligopeptide domain shared by a family of proteins which bind hydrophobic ligands. J Biol Chem. 1986 Apr 25;261(12):5553–5561. [PubMed] [Google Scholar]
- Takase S., Ong D. E., Chytil F. Transfer of retinoic acid from its complex with cellular retinoic acid-binding protein to the nucleus. Arch Biochem Biophys. 1986 Jun;247(2):328–334. doi: 10.1016/0003-9861(86)90591-6. [DOI] [PubMed] [Google Scholar]
- Trown P. W., Palleroni A. V., Bohoslawec O., Richelo B. N., Halpern J. M., Gizzi N., Geiger R., Lewinski C., Machlin L. J., Jetten A. Relationship between binding affinities to cellular retinoic acid-binding protein and in vivo and in vitro properties for 18 retinoids. Cancer Res. 1980 Feb;40(2):212–220. [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Selection and characterization of F9 teratocarcinoma stem cell mutants with altered responses to retinoic acid. J Biol Chem. 1984 May 10;259(9):5899–5906. [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggert B., Russell P., Lewis M., Chader G. Differential binding to soluble nuclear receptors and effects on cell viability of retinol and retinoic acid in cultured retinoblastoma cells. Biochem Biophys Res Commun. 1977 Nov 7;79(1):218–225. doi: 10.1016/0006-291x(77)90083-3. [DOI] [PubMed] [Google Scholar]
- Williams J. B., Napoli J. L. Metabolism of retinoic acid and retinol during differentiation of F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4658–4662. doi: 10.1073/pnas.82.14.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbach S. B., Howe P. R. TISSUE CHANGES FOLLOWING DEPRIVATION OF FAT-SOLUBLE A VITAMIN. J Exp Med. 1925 Nov 30;42(6):753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Steinert P. Retinoic acid induces transglutaminase activity but inhibits cornification of cultured epidermal cells. J Biol Chem. 1982 Sep 10;257(17):9906–9908. [PubMed] [Google Scholar]
- Zuber M. X., John M. E., Okamura T., Simpson E. R., Waterman M. R. Bovine adrenocortical cytochrome P-450(17 alpha). Regulation of gene expression by ACTH and elucidation of primary sequence. J Biol Chem. 1986 Feb 15;261(5):2475–2482. [PubMed] [Google Scholar]