Abstract
Camptocormia, also referred to as bent spine syndrome (BSS) is defined as an abnormal flexion of the trunk, appearing in standing position, increasing during walking and abating in supine position. BSS was initially considered, especially in wartime, as a psychogenic disorder. It is now recognized that in addition to psychiatric syndromes, many cases of reducible BSS have a somatic origin related to a number of musculo-skeletal or neurological disorders. The majority of BSS of muscular origin is related to a primary idiopathic axial myopathy of late onset, appearing progressively in elderly patients. Diagnosis of axial myopathy first described by Laroche et al. is based upon CT/MRI examination demonstrating massive fatty infiltration of paravertebral muscles. The non-specific histological aspect includes an extensive endomysial fibrosis and fat tissue with irregular degenerated fibers. Weakness of the paravertebral muscles can be secondary to a wide variety of diseases generating diffuse pathologic changes in the muscular tissue. BSS can be the predominant and sometimes revealing symptom of a more generalized muscular disorder. Causes of secondary BSS are numerous. They must be carefully assessed and ruled out before considering the diagnosis of primary axial myopathy. The principal etiologies include on the one hand inflammatory myopathies, muscular dystrophies of late onset, myotonic myopathies, endocrine and metabolic myopathies, and on the other hand neurological disorders, principally Parkinson’s disease. Camptocormia in Parkinsonism is caused by axial dystonia, which is the hallmark of Parkinson’s disease. There is no specific pharmacologic treatment for primary axial myopathy. General activity, walking with a cane, physiotherapy, and exercises should be encouraged. Treatment of secondary forms of BSS is dependent upon the variety of the disorder generating the muscular pathology. Pharmacologic and general management of camptocormia in Parkinson’s disease merge with that of Parkinsonism. Levodopa treatment, usually active on tumor rigidity and akinesia, has poor or negative effect on BSS.
Keywords: Camptocormia, Bent spine syndrome, Axial myopathy, Muscular dystrophies, Parkinson’s disease
Introduction
For many years camptocormia, designating a forced posture with a forward-bent trunk, has been considered as related to psychiatric manifestations. Times have changed, and it is now well-known that somatic factors are able to produce this chronic abnormal curvature of the lumbar spine in standing position. Since the early wartime literature [16, 40, 41] many developments have taken place, and it is now possible to establish a more precise etiologic diagnosis. However, many aspects concerning nosology, classification, pathogenesis and therapy of this syndrome remain unclear. We have therefore decided to update the current knowledge of this rare but invalidating symptom.
Methods
We carried out a review of the English and French literature on “camptocormia” and “bent spine syndrome” from 1991 to 2009 using PubMed database. This search disclosed a total of 121 articles including 12 reviews. No metanalysis or systematic review was found. Forty-six articles dealt with Parkinson’s disease associated with camptocormia. The other articles concerned the muscular pathology. We analyzed the published materials critically. Among them we selected the most pertinent articles, also taking into account personal communications and our own experience with the disease in order to produce a comprehensive narrative review of a poorly recognized entity.
Definition
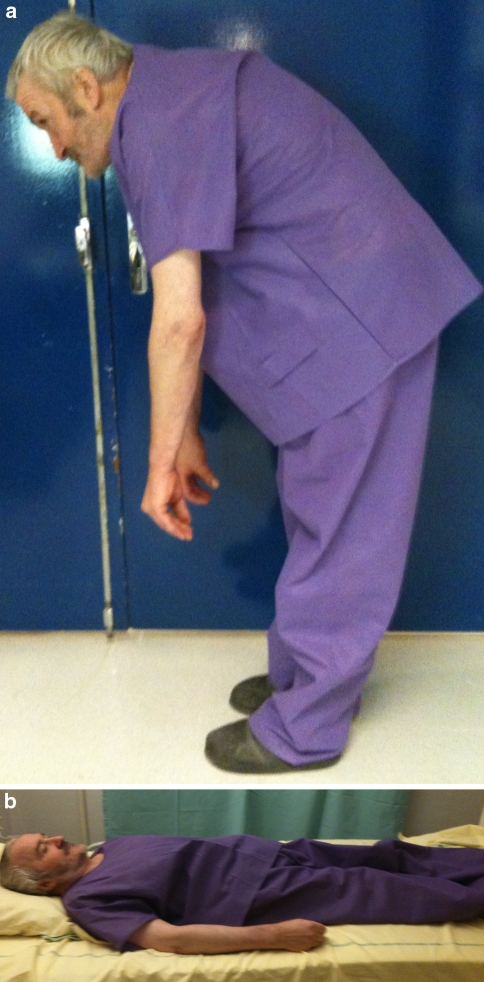
Camptocormia is characterized by an abnormal flexion of the trunk appearing in standing position, increasing while walking and abating in the supine position. The term camptocormia is sometimes referred to as “bent spine syndrome” (BSS). The abnormal curvature must be a lumbar kyphosis. An anterior flexion of 45° or more is required to differentiate BSS from the kyphosis starting from the dorsal spine, frequently observed in elderly individuals (Fig. 1).
Fig. 1.
Clinical photograph showing the flexed posture (a) abating when lying down (b)
History and terminology
History of the syndrome has been extensively reviewed by Karkowski [18]. The word camptocormia was used for the first time by Souques and Rosanoff in 1915 in a meeting of the “Societé de Neurologie de Paris”. When proposing this denomination derived from the Greek words camptos (bent) and kormos (trunk), these authors intended to unify in a unique entity an abnormal curvature of the trunk which was previously designated by various terms. In a consecutive series of articles Souques and collaborators [40, 41] perfectly described the syndrome as it is now recognized and defined. In their report of 16 patients, they drew attention to the fact that a psychogenic origin could generally be identified. The majority of their patients consisted of wounded soldiers who could not cope with more battle stress. They were treated by psychotherapy and electrotherapy, with success for some of them.
Until quite recently, camptocormia was generally considered as a conversion reaction principally observed in soldiers during World Wars I and II. For example Sandler [34] in 1947 reported a series of patients, mostly soldiers, with an acute forward bent which was diagnosed as a hysterical contracture. As emphasized by Karkowski [18] very few papers were published after the Second World War. During that period, camptocormia was still considered as psychogenic. Progressively, in the reports published in the 1990s and later, it became evident that in addition to psychiatric syndromes essentially observed in wartime, many other musculo-skeletal or neurological disorders were associated with this abnormal flexion of the trunk. Because various mechanisms can contribute to the development of this syndrome, the term “bent spine syndrome” was proposed [36], instead of camptocormia, which had kept a strong psychiatric connotation in the medical community. The present authors agree that the term bent spine syndrome (BSS) is preferable.
Etiologic classification
Etiology of BSS can be dichotomized in two main sections: on the one hand a muscular origin; on the other hand, an association with various neurological disorders. As emphasized by Azher et al. [2] distribution of etiologies varies according to the referral pattern. Most patients with muscular disorders are likely to be evaluated in an orthopedic or a rheumatologic clinic. Conversely, patients presenting an association with a neurological disease will be treated in a neurological and movement disorder center. We will discuss in turn these two principal etiologies.
Bent spine of muscular origin
In this category of patients, BSS is caused by weakness of the paravertebral muscles related to degradation of the muscular tissues, independently of their innervation. In standing position the motion segments are subjected to a forward bending moment which must be counterbalanced by the erector spinal muscles. Insufficiency of these muscles generates a rupture of equilibrium between postural dorsal and abdominal muscles, leading to a forward bending of the trunk. Hypotonus of these muscles explains the complete reducibility of the curvature with passive motion and in supine position.
Weakness of the spinal extensor muscles can be secondary to various diseases generating pathologic changes in the anti-gravity muscles involved in trunk extension or primary idiopathic.
Secondary BSS
Secondary BSS has been reported in a wide variety of generalized muscular disorders (Table 1). It can indeed be encountered in polymyositis and dermatomyositis where in a few cases BSS was the revealing symptom [8]. Diagnosis is made by the association of a characteristic EMG pattern, elevation of serum creatine kinase levels, cutaneous lesions, inflammatory syndrome and muscle fiber necrosis with massive perivascular and endomysial inflammatory infiltrates on biopsy.
Table 1.
Secondary bent spine syndrome associated with muscular disorders
| Causes of myopathy |
|---|
| Dystrophy |
| Limb–girdle muscular dystrophy |
| Extensive fascio-scapulo-humeral dystrophy |
| Neuromuscular disorders |
| Myotonic muscular dystrophy |
| Steinert disease |
| Inflammatory |
| Focal myositis |
| Dermatomyositis |
| Polymyositis |
| Inclusion body myositis |
| Endocrine-metabolic |
| Hypothyroidism |
| Osteomalacia |
| Steroid induced |
| Amyloidosis |
| Mitochondrial myopathies |
| Carnitine palmityl-transferase deficiency |
| Respiratory chain complexes deficiency |
BSS has also been reported in “inclusion body myositis”, another inflammatory muscle disease. Muscle biopsy may display several common findings including endomysial inflammatory cells, cytoplasmic vacuolar degeneration, inclusions or plaques of abnormal proteins [15].
BSS can be part of the symptomatology of muscular dystrophies of late onset including limb–girdle and fascio-scapulo-humeral types, further identified by their mode of inheritance, the responsible gene and the deficient protein [12]. Similarly BSS has been reported in the course of myotonic myopathies, including Steinert disease [32] and proximal myotonic myopathy. This latter disorder has recently been identified as a new cause of secondary BSS by Serratrice et al. [37]. First described by Ricker et al. [30], proximal myotonic myopathy is transmitted by autosomal dominant inheritance. Diagnosis of this condition is based upon diffuse pain and myalgias, gait disorders, weakness of the proximal muscles, cataract and electrical myotonia.
Classical etiologies of acquired myopathy can generate abnormal postures and secondary BSS. This is the case of the myopathies disclosed in metabolic or endocrine disorders such as hypothyroidism, osteomalacia, or the muscular dystrophy induced by steroid therapy [8, 14, 38]. Consequently, when faced with a reducible bent spine posture, study of bone metabolism and of skeletal imaging as well as evaluation of the thyroid hormones levels are mandatory. Lastly, two exceptional cases of secondary BSS have been reported [8]. The first concerned an amyloid myopathy recognized by identification on the biopsy of amyloid deposits containing lambda immunoglobulin light chains similar to those detected in blood immunoelectrophoresis. The second was a case of mitochondrial myopathy detected by the presence of “red ragged fibers” on muscle biopsy and by the loss of enzymatic activities of mitochondrial respiratory chains.
These various causes of secondary BSS must be systematically searched for when faced with an axial myopathy. Their specific clinical and laboratory features allow the differential diagnosis. Similarly, fixed non-reducible bent spines related to vertebral fractures, sequelae of spondylitis and ankylosing spondylitis are excluded by clinical examination and a plain radiograph of the dorsolumbar spine. Because some of these disorders have specific histological features, biopsy is sometimes mandatory to establish the diagnosis firmly.
Primary BSS
Analysis of the literature and of our own cases indicates that idiopathic primary BSS, of myopathic origin, is the most frequently encountered in clinical practice. Primary BSS now appears to be a distinct clinical entity, observed in older individuals who share a strikingly identical clinical picture with a common paravertebral muscle imaging pattern and similar histological findings [20–22, 26, 36].
In 1991, Laroche et al. [22] described for the first time a late-onset myopathy limited to the spinal muscles in 14 patients with a mean age of 66 years. All patients had in common an anterior flexion of the trunk reducible in horizontal position, fatty infiltration of the paravertebral muscles on CT, a common pattern on muscle biopsy, and the existence of a family history. These authors postulated that this acquired lumbar kyphosis was a primary myopathy of late onset localized in the paravertebral extensor muscles. Since this first publication, the initial hypothesis has been supported by other investigators [11, 14, 26, 28, 36] and by further studies of the same group [19, 23].
It was indeed progressively confirmed that idiopathic primary BSS is characterized by a progressive weakness of paravertebral muscles in elderly patients with a female predominance. A frequent family history was also confirmed. For example, there were 2 hereditary cases out of 8 in one report [36] and 12 out of 23 cases in another [31]. Five out of six patients recalled similar family cases in Mahjneh’s series [26]. No associated signs are found. The neurological examination is normal, especially tonus, reflexes and sensory functions. Muscular weakness is strictly localized on the spinal extensor muscles and no other muscular deficit is clinically detectable. BSS is responsible for major functional disability and pain which worsen over time [31] and responds poorly to medical therapy and rehabilitation.
Isokinetic evaluation was performed by Laroche et al. [23] using a cibex device in 23 BSS patients and 15 controls matched for age, sex and build. In the spine, peak torque and power values were much lower in patients than in controls. Interestingly, work during repeated movements at low resistance and fast speed was most affected in the patient group suggesting fatigability in addition to loss of force and power of the erector muscles. This is in keeping with clinical observations of patients where the impossibility to stand upright appears progressively with fatigue during the day or after a certain distance of walking. Shoulder rotators and hip abductors were also evaluated. Work done after a series of 20 rapid movements showed a decrease of force in the gluteus medius and in scapular muscles. The significance of these latter findings will be approached when discussing the nosology of BSS.
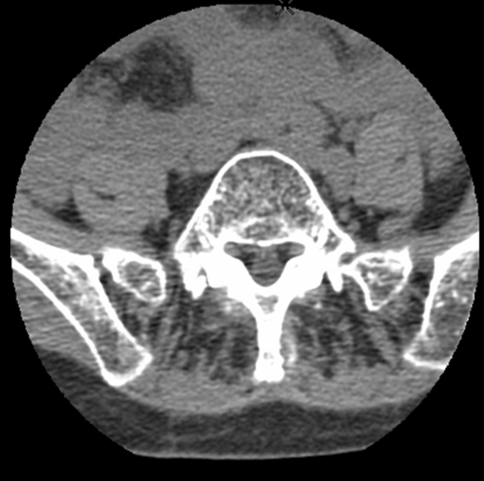
CT scan, including dorsal and lumbar sections of the paravertebral muscles is the most useful diagnostic tool [5, 19, 24, 26, 28]. Similar and characteristic abnormalities are observed in patients with primary BSS [23, 36]. Typical although non-specific pictures include a normal paraspinal outline and a normal volume of the muscles, which appear hypodense, with loss of substance related to a massive fatty infiltration (Fig. 2). This is in contrast to the neurogenic atrophy where the muscle volume decreases, but the density of tissue remains normal [5, 24]. Laroche et al. [23] have compared CT scans of BSS patients with CT scans of control subjects without BSS and with low back pain caused by facet osteoarthritis or lumbar stenosis. In their 43 patients, CT scan disclosed a fatty infiltration of the paravertebral muscle predominating at the distal muscle heads and extending from T4 to L5, whereas in the controls the atrophy and fatty infiltration were centrifugal, predominating posteriorly around the posterior arch and not as extensive as BSS patients. CT scan was repeated for 23 patients after a 3.5-year follow-up [31].The mean loss in muscle density was 6.9 ± 16 HU in the patients. No muscle density difference was observed in the control group. Muscle density was also measured in 43 patients and controls, on the supraspinatus and on the gluteus medius. The CT appearance of the supraspinatus muscles in patients was similar to that of controls but with a significantly decreased density. Three of the 43 patients presented myopathic changes of the gluteus medius similar to those observed on the spinal muscles. Moreover 40% of these patients had a mild fatty involvement of posterior muscles of the thighs and of the calfs. These changes were not accompanied by clinical symptoms or physical signs. These findings highlight the progressive aggravation of the axial muscular disorder. They also indicate the presence of muscular imaging changes in other muscles without muscular weakness at the clinical examination. MRI examination used by other investigators [26, 36] disclosed a similar selective and marked fatty involvement of the paravertebral muscles. The interest of MRI is to obtain coronal sections showing the totality of extensor muscles and the rarefaction of muscles fasciculi [36].
Fig. 2.
CT-scan of an idiopathic bent spine (L5 level): fatty infiltration of spinal muscles with constant muscle volume
The laboratory studies are usually normal. Serum creatine kinase concentration is increased in some cases: from 2 to 5 times the upper normal limit in one study [26] and 1.5- to 2-fold the abnormal levels in 42% in another report [31]. No immunologic abnormalities are found. Electromyography of the spinal muscles is sometimes difficult as complete relaxation of the spinal muscles is not easy to obtain. Predominant findings consisting of a low amplitude pattern during voluntary effort were disclosed in 80% of the patients in one study [19]. In other reports results were not homogenous: myopathic in most cases, neuropathic or normal in others [26, 36]. EMG of the lower limbs as well as conduction velocities is usually normal. In one study however, myopathic patterns were observed in the middle gluteus and in the deltoid of six patients without any clinical symptoms [21].
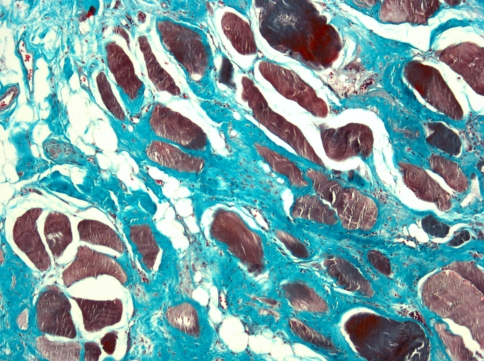
In all studies, muscle biopsies reveal a myopathic pattern [20, 26, 36]. The most striking features are disorganization of the normal architecture with replacement of variable intensity of the muscular tissue by fibrosis and fatty infiltration. There is a marked diminution of the number of fibers with variation in fiber size (Fig. 3). Laroche and Delisle [21] have compared biopsies of BSS paraspinal muscles with muscle specimens from patients with other known spinal disorders (lumbar stenosis, discal herniation). They observed a significant increase of endomysial fibrosis and fat infiltration in the BSS patients compared with the control group. Other abnormalities such as mitochondrial changes found in both groups are considered as being related to old age.
Fig. 3.
Spinal muscle biopsy showing intense endomysial deposit of fibrosis (green) and fatty infiltration. We can also observe irregular distribution of involuted muscle fibers. (Trichrome, original magnification ×100)
The nosology of primary BSS can be discussed in two ways: first, can primary BSS be considered as a progressive muscular dystrophy? Second, can primary BSS be linked with one of the recognized types of the heterogenous group of muscular dystrophies? As defined by Emery [12] “the muscular dystrophies are a group of inherited disorders characterized by progressive muscular wasting and weakness. The unifying feature is the histological analysis of muscle samples, ultimately showing increased amount of fat and connective tissue with marked fiber-size variations and areas of necrosis. The classification of the different varieties of muscular dystrophy depends on the topography of the muscular weakness, on the mode of inheritance and on the genes and protein products identified for the various clinical types of dystrophies.
Following this definition, and analyzing the few papers dealing with primary BSS and including an histological analysis, it seems appropriate to conclude that primary idiopathic BSS can be included in the group of progressive muscular dystrophies of late onset. Indeed in primary idiopathic BSS, electromyographic studies predominantly disclose a myopathic pattern. More importantly, hereditary cases are found in all the studies, but genetic and molecular studies are still lacking in order to appreciate the mode of inheritance and the eventual responsible deficient protein.
In which group of the recognized types of progressive muscular dystrophies could BSS reasonably belong? Late forms of congenital myopathy are not exceptional [36] but they usually do not involve the axial musculature. However, such a case was recently reported [8]. A 66-year-old woman developed a BSS associated with pain and weakness of the pelvic girdle muscles. Biopsy of the paravertebral muscles revealed in addition to the fatty involution, abnormalities of type 1 fibers which on electronic microscopy strongly suggested a congenital myopathy. A similar case has also been reported by Serratrice et al. [36]. In their patient the muscle biopsy showed a paraspinal fingerprint aspect with a predominance of type 1 fibers. On the other hand, as already mentioned, 40% of the 49 patients analyzed in the most important series of the literature [19] had a mild fatty infiltration of the posterior muscles of the thighs on the CT scanning of limb muscles. Three of them had involvement of both gluteus medius muscles. In seven of the eight cases reported by Serratrice et al. [36], a limb muscle biopsy was performed (quadriceps or deltoid muscles) and a myopathic pattern was disclosed without any corresponding clinical symptoms. Taken together, these data indicate that in some cases the predominant axial myopathic lesions extend beyond the spinal extensor muscles particularly in the limb–girdle muscles. It could then be speculated that this late-onset, restricted axial myopathy could be linked with one of the principal types of the muscular dystrophies, possibly the limb–girdle muscular dystrophy group. However, further research is necessary to clarify the nosology as well as the pathogenesis of this syndrome which at the present time remain unknown.
Bent spine syndrome and neurological disorders
BSS has been reported as an associated sign in a few neurological diseases. They principally include motor neuron disorders and movement and central nervous disorders. A few cases of BSS have been observed in early proximal forms of amyotrophic lateral sclerosis [36].The neurogenic origin of the failure of the extensor spinal muscles is demonstrated by the associated peripheral muscular atrophies and by the other classical signs of dysfunction of the anterior horn cells.
Recent reports have shown that most BSS of somatic neurological origin resulted from lesions and dysfunction of the basal ganglia, a collection of nuclei in the cerebral cortex including caudate, putamen, pallidus and substantia nigra. This complicated system has among other activities the function of coordinating the postural reflexes in flexion and in extension which enable acquisition and maintenance of the erect position [9]. This function is mediated by a neuro-transmitter, dopamine, produced by part of the substantia nigra. The role of dopamine is critical for all the functions of the basal ganglia disorders which comprise the classic form of Parkinson’s disease (PD) and “Parkinson-plus syndromes”, such as multiple system atrophy and supranuclear palsy [1]. The key role of the basal ganglia system in the maintenance of axial posture is strongly suggested by the association of PD and camptocormia with lenticular and putaminal lesions [27].
The propensity to bend the trunk forward is a recognized characteristic of Parkinson’s disease. The postural tonus is deeply modified in PD with a general attitude in flexion. When standing or walking, the patient’s head and trunk are slightly forward inclined. Camptocormia in PD is defined as a major anterior flexion of the trunk of 45° or more [2, 3]. In a sense, BSS in PD is a caricatured exaggeration of the usual bent back related to the axial dystonia which is a hallmark of Parkinsonism [10, 17, 29, 39].
Studies have attempted to describe the clinical characteristics of patients with PD and BSS as well as the characteristics of BSS in PD [2, 3, 25]. Some features are generally recognized: old age, long duration of Parkinsonism, development of the bent posture always following the first symptoms of Parkinsonism. Camptocormia usually appears progressively over a year or more, but sometimes in a few weeks [2, 3, 25]. The trunk flexion is severe and associated with scoliosis in a substantial proportion of patients [42]. It should be noted that BSS in PD can also be detected in a restricted group of patients with a dissociated form of PD in which axial dystonia is the predominant revealing symptom. The present authors have been faced with such patients with normal paravertebral MRI and very subtle signs of Parkinsonism. In the only case–control study of the literature, Bloch et al. [3] have compared a selected sample of patients with PD and BSS with matched PD patients without BSS. The main characteristics disclosed in patients with PD and BSS included axial rigidity, postural instability, and gait disorders all features consistent with axial dystonia of the trunk with excessive activation of the abdominal wall muscles. Levodopa treatment has no effect on camptocormia. However rigidity, tremor, and akinesia, also present in PD patients with BSS, respond at least in part to dopamine therapy [2, 3, 25]. This dichotomized action of dopamine has suggested that PD with BSS represents a specific form of Parkinsonism in which an additional non-dopaminergic neuronal dysfunction occurs in the basal ganglia and brainstem system [3].
The exact prevalence of BSS in PD is not precisely known. Various rates have been estimated in different studies. Lepoutre et al. [25] recruited 23 consecutive cases of BSS out of 700 patients, giving an hypothetic prevalence of about 3%. Azher et al. [2] found 21 cases of BSS out of 164 PD, giving a prevalence of 12%. In a more recent study, Tiple et al. found a prevalence ranging around 6.9%. In this last study, 275 consecutive patients were screened for BSS by clinical evaluation and patients who screened positive for camptocormia were subsequently screened by goniometric analyses. Validity of the clinical examination in assessing camptocormia (anterior flexion > 45°) was evaluated in a sample population using goniometric measurement as a standard. The clinical examination yielded 100% sensitivity and 90% specificity in diagnosing camptocormia [42].
Axial dystonia is considered to be the cause of camptocormia in PD by the majority of investigators [3, 17, 25, 29]. BSS and scoliosis may represent one end of the spectrum of PD abnormal postures, the other end being striatal hand and foot deformities [1, 2]. More recently however, a focal myopathy has been demonstrated in some PD patients by EMG, CT and MRI scans and biopsy of the paraspinal muscles [6, 25, 35, 44]. In 2002, Wunderlich et al. [44] described a case of camptocormia in a PD patient where the clinical signs comprised pain and inflammation of the paravertebral muscles, accompanied by a major elevation of creatine kinase. MRI and biopsy findings were characteristic of a focal myositis. Focal myositis, first described in 1977 by two independent researchers, Cumming and Heffner [7, 13], is a rare disease characterized by hypertrophy and inflammation of one muscle or of one group of muscles. Pathogenesis is not known; evolution is usually self-limited, remaining localized. Extension to polymyositis has also been reported. Characteristic histological findings of focal myositis comprise an important perimysial and endomysial inflammatory infiltrate associated with necrosis, marked size variations of the fibers, and fibrosis [4]. Following Wunderlich’s publication, a few investigators have performed spinal muscle biopsies when signal abnormalities were observed on thoraco-lumbar MRI of PD patients. With the possible exception of the case reported by Charpentier et al. [6], considered by the authors as a true focal myositis, the cases reported by Shabitz and later by Lepoutre were not consistent with the diagnosis of focal myositis [25, 35]. The abnormalities described by Shabitz comprised chronic inflammatory myopathy, non-specific myopathic changes or mitochondrial myopathy. The histological findings reported by Lepoutre mainly showed fibrosis and fatty infiltration. At the present time the prevalence of myopathic lesions in PD camptocormia is not known; documents are scarce and further research is necessary to evaluate the exact role of the muscular changes in the development of camptocormia in PD as well as their pathogenesis.
Treatment
Education of the patient concerning evolution and therapeutic possibilities of his disease is the first priority.
There is no specific pharmacologic therapy for primary axial myopathy. The use of analgesics depends upon the intensity of back pain. Treatment of osteoporosis, often associated with BSS in these elderly patients, is mandatory to prevent osteoporotic vertebral fractures. General principles of management are important to follow. A balanced diet and loss of weight, if necessary, are recommended. Prolonged bed rest must be avoided. Activity and walking with a cane or a walker must be encouraged. Physiotherapy, massage, exercises and passive motion are an important part of the general management. Appropriate lightweight orthoses can help ambulation. They are not always well tolerated. As already mentioned, response to treatment is generally poor and progressive worsening of the functional disability is usual. Treatment of secondary BSS is dependent upon the variety of the disorder generating the muscular pathology. For example, inflammatory myopathies respond to steroids sometimes associated with cyclosporine or immunoglobulin therapy [8]. Similarly, true hypothyroidism myopathy is corrected by an adequate administration of thyroactive substances. Another example is the osteomalacia proximal myopathy where in addition to calcitriol therapy, treatment must be adapted to the various causes of osteomalacia.
Pharmacologic and general management of camptocormia in PD merge with that of Parkinson’s disease. Unfortunately levodopa treatment usually active on tremor, rigidity and akinesia, has a poor or negative effect on camptocormia. Because an excessive activation of the abdominal wall muscles is clearly observed in some PD with BSS patients, one study [2] reports the effects of botulinum toxin injection (BTI) into the rectus abdominis in an attempt to reduce abdominal muscle hyperactivity. Four of the nine BTI-treated patients had a noticeable improvement in their bent posture lasting for about 3 months after each injection. Because the iliopsoas is traditionally regarded as a flexor of the lumbar spine, a few PD with BSS patients received ultrasound-guided injections of BTI into the iliopsoas with negative results in two studies [33, 43]. There are very few data in the literature about surgical treatment. A thoracolumbosacral fixation was performed in our institution for a BSS related to an axial myopathy. The patient who had begged us to be operated finally achieved a successful clinical result with an adequate correction and bony fusion at 1 year follow-up but at the expense of a difficult, complicated and prolonged rehabilitation period. This case indicates that this procedure can be an alternative to a conservative treatment. However, most BSS patients are elderly with co-morbidities which exclude the possibility of surgical treatment for the majority of them. In our opinion, if surgery is discussed, it should be restricted to patients who are strongly motivated with good general health and informed that it is a major type of operation with long post-operative period and a risk of local and general complications. These recommendations can be also apply to BSS associated with PD. Bilateral subthalamic nucleus stimulation has been reported in PD with BSS patients [2, 25, 45] with varying results, sometimes with a remarkable improvement [45].
Conclusions
It is now accepted that axial myopathy is a separate clinical entity and is the most frequent cause of camptocormia in elderly patients [19]. When faced with a patient progressively developing a bent spine, an algorithm of clinical, imaging, laboratory and electrophysiological investigations should be undertaken to eliminate the other possible etiologies of camptocormia. Clinical examination and history-taking verify the absence of associated signs, particularly of neurological symptoms and signs. Imaging studies include on the first line plain X-rays to exclude bone lesions or systemic diseases such as ankylosing spondylitis, responsible for non-reducible bent spine. CT scan and preferably MRI of the paravertebral muscles are the most useful diagnostic tools demonstrating the characteristic abnormalities of primary axial myopathy, as opposed to the muscular atrophy seen in elderly patients with spine osteoarthritis or stenosis. CT or MRI scanning should also explore the shoulder and pelvic girdle muscles and the limb muscles in order to assess an eventual extension of the myopathy. EMG of paraspinal muscles and of limbs are part of the muscular and neurological evaluation. Laboratory examinations include serum creatine kinase, sedimentation and protein-C reactive rates, acetylcholine receptor antibody, thyroid function studies, as well as other more specific investigations in order to exclude myopathies secondary to various diseases generating pathologic muscular changes. In dubious cases, in order to exclude any other muscular disorder, biopsy of the paravertebral muscles should be done at a site designated by CT or MRI scanning where the abnormal muscle is not completely invaded by fat tissue.
Acknowledgments
We would like to thank Maryse Lesage for her excellent secretarial work.
References
- 1.Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. Mov Disord. 2006;21:1856–1863. doi: 10.1002/mds.21058. [DOI] [PubMed] [Google Scholar]
- 2.Azher SN, Jankovic J. Camptocormia: pathogenesis, classification and response to therapy. Neurology. 2005;65:355–359. doi: 10.1212/01.wnl.0000171857.09079.9f. [DOI] [PubMed] [Google Scholar]
- 3.Bloch F, Houeto JL, Tezena du Montcel S, et al. Parkinson’s disease with camptocormia. J Neurol Neurosurg Psychiatry. 2006;77:1223–1228. doi: 10.1136/jnnp.2006.087908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchaud-Chabot A, Sicre J, Bardin Th, Kahn MF (1996). Myosites focales. In: Kahn MF, Kuntz et al (eds) L’actualité rhumatologique. Expansion scientifique Française. pp 55–64
- 5.Bulke JA, Crolla D, Termote JL, et al. Computed tomography of the muscles. Muscle Nerve. 1981;4:67–72. doi: 10.1002/mus.880040112. [DOI] [PubMed] [Google Scholar]
- 6.Charpentier P, Dauphin A, Stojkovic T, et al. Maladie de Parkinson, camptocormie et myosite focale paraspinale. Rev. Neurol. 2005;161:459–463. doi: 10.1016/S0035-3787(05)85077-5. [DOI] [PubMed] [Google Scholar]
- 7.Cumming W, Weiser R, Teoh R, et al. Localized nodular myositis: a clinical and pathological variant of polymyositis. Q J Med. 1977;46:531–546. [PubMed] [Google Scholar]
- 8.Delcey V, Hachulla E, Michon-Pasturel U, et al. La camptocormie: un signe de myopathie axiale. A propos de sept observations. Rev Med Interne. 2002;23:144–154. doi: 10.1016/S0248-8663(01)00530-6. [DOI] [PubMed] [Google Scholar]
- 9.Dietz V, Zylstra W, Assaiante C, et al. Balance control in Parkinson disease. Gait Posture. 1993;1:77–84. doi: 10.1016/0966-6362(93)90018-V. [DOI] [Google Scholar]
- 10.Djaldetti R, Mosber G, Galili R, Sroka H, et al. Camptocormia in patients with Parkinson’s disease: characterization and possible pathogenesis of an unusual phenomenon. Mov Disord. 1999;14:443–447. doi: 10.1002/1531-8257(199905)14:3<443::AID-MDS1009>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenstein MR, Stoll T, Edwards JCW. Not all stoops are due to osteoporosis. Ann Rhum Dis. 1996;55:21–28. doi: 10.1136/ard.55.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emery AEH. The muscular dystrophies. BMJ. 1998;317:991–995. doi: 10.1136/bmj.317.7164.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heffner R, Armbrustmacher V, Eanle K. Focal myositis. Cancer. 1977;40:301–306. doi: 10.1002/1097-0142(197707)40:1<301::AID-CNCR2820400142>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Hilliquin P, Menkes CJ, Laoussadi S, et al. Camptocormie du sujet âgé: Une nouvelle entité par atteinte des muscles paravertébraux. Rev Rhum Mal Osteoartic. 1992;59:169–175. [PubMed] [Google Scholar]
- 15.Hund E, Heckl R, Goebel H, et al. Inclusion body myositis presenting with isolated erected spinal paresis. Neurology. 1995;45:993–994. doi: 10.1212/wnl.45.5.993. [DOI] [PubMed] [Google Scholar]
- 16.Hurst AF. The bent back of soldiers. BMJ. 1918;2:621–623. doi: 10.1136/bmj.2.3023.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic J, Tintner R. Dystonia and Parkinsonism. Parkinson Dis Relat Disord. 2001;8:109–121. doi: 10.1016/S1353-8020(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 18.Karkowski K. The old and new camptocormia. Spine. 1999;24:1494–1498. doi: 10.1097/00007632-199907150-00017. [DOI] [PubMed] [Google Scholar]
- 19.Laroche M, Curtas P (2008) Usefullness of CT scan in bent spine syndrome (personnal communication)
- 20.Laroche M, Delisle MB. La camptocormie primitive est une myopathie para-vertébrale. Rev Rhum Mal osteoartic. 1994;61:481–484. [PubMed] [Google Scholar]
- 21.Laroche M, Delisle MB, Aziza R, et al. Is camptocormia a primary muscular disease? Spine. 1995;20:1011–1016. doi: 10.1097/00007632-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Laroche M, Delisle MB, Mazieres B, et al. Myopathic localisée aux muscles spinaux: une cause de cyphose lombaire acquise de l’adulte. Rev Rhum Mal osteoartic. 1991;58:829–838. [PubMed] [Google Scholar]
- 23.Laroche M, Ricq G, Delisle MB. Bent spine syndrome: computed tomography study and isokinetic evaluation. Muscle Nerve. 2002;25:189–193. doi: 10.1002/mus.10016. [DOI] [PubMed] [Google Scholar]
- 24.Laroche M, Rousseau H, Mazieres B, et al. Intérêt de la tomodensitométrie dans la pathologie musculaire. Rev Rhum Mal Osteoartic. 1989;56:433–439. [PubMed] [Google Scholar]
- 25.Lepoutre AC, Devos D, Blanchard-Dauphin A. A specific clinical pattern of camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1229–1234. doi: 10.1136/jnnp.2005.083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahjneh I, Marconi G, Paetau A, et al. Axial myopathy—an unrecognized entity. J Neurol. 2002;249:730–734. doi: 10.1007/s00415-002-0701-9. [DOI] [PubMed] [Google Scholar]
- 27.Nieves AV, Miyasaki JM, Lang AE. Acute onset dystonic camptocormia caused by lenticular lesions. Mov Disord. 2001;16:177–180. doi: 10.1002/1531-8257(200101)16:1<177::AID-MDS1035>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Poullin P, Daumen-Legre V, Serratrice G. Camptocormie du sujet âgé. Rev Rhum Mal Osteoartic. 1993;60:159–1661. [PubMed] [Google Scholar]
- 29.Reichel G, Kirchhofer U, Stenner A. Camptocormia-segmental dystonia. Proposal of a new definition for an old disease. Nervenarzt. 2001;72:281–285. doi: 10.1007/s001150050751. [DOI] [PubMed] [Google Scholar]
- 30.Ricker K, Koch MC, Lehmann-Horn F, et al. Proximal myotonic myopathy. Clinical features of a multisystem disorder similar to myotonic dystrophy. Arch Neurol. 1995;52:25–31. doi: 10.1001/archneur.1995.00540250029009. [DOI] [PubMed] [Google Scholar]
- 31.Ricq G, Laroche M. Cyphose lombaire acquise de l’adulte par myopathie primitive des muscles paravertébraux. Aspects épidémiologiques tomodensitométriques et évolutifs. A propos d’une cohorte de 23 patients. Rev Rhum Mal Osteoartic. 2000;67:908–913. doi: 10.1016/S1169-8330(00)00034-X. [DOI] [Google Scholar]
- 32.Rolland Y, Laroche M (1997) Cyphoses lombaires acquises révélatrices d’une maladie de Steinert. Rachis 9:115–118
- 33.Salvatori FM. Injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord. 2009;24:316. doi: 10.1002/mds.22249. [DOI] [PubMed] [Google Scholar]
- 34.Sandler SA. Camptocormia or the functional bent back. Psychosomat Med. 1947;9:197–204. doi: 10.1097/00006842-194705000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Schabitz WR, Glatz K, Schuhan C, et al. Severe flexion forward of the trunk in Parkinson’s disease: focal myopathy of the paraspinal muscles mimicking camptocormia. Mov Disord. 2003;18:408–414. doi: 10.1002/mds.10385. [DOI] [PubMed] [Google Scholar]
- 36.Serratrice G, Poujet J, Pelissier JF. Bent spine syndrome. J Neurol Neurosurg Psychiatry. 1996;60:51–54. doi: 10.1136/jnnp.60.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serratrice J, Weiler PJ, Pouget J, Serratrice G. Une cause méconnue de camptocormie: la myopathie myotonique proximale. Presse Med. 2000;29:1121–1123. [PubMed] [Google Scholar]
- 38.Serratrice G, Pelissier JF, Cros J. Les atteintes musculaires des osteomalacies. Rev Rhum Mal Osteoartic. 1978;45:621–630. [PubMed] [Google Scholar]
- 39.Slawek J, Derejko M, Lass P. Camptocormia as a form of dystonia in Parkinson’s disease. Eur J Neurol. 2003;10:107–108. doi: 10.1046/j.1468-1331.2003.00503_1.x. [DOI] [PubMed] [Google Scholar]
- 40.Souques A (1914–1915) Contractures ou pseudo-contractures hystero-traumatiques. Rev Neurol 28:430–431
- 41.Souques A, Rosanoff-Saloff B (1914–1915). La camptocormie, incurvation du tronc, consecutive aux traumatismes du tronc et des lombes, considérations morphologiques. Rev Neurol 28:937–939
- 42.Tiple D, Fabbrini G, Ottaviani D, et al. Camptocormia in Parkinson’s disease: an epidemiological and clinical study. J Neurol Neurosurg Psychiatry. 2009;80:145–148. doi: 10.1136/jnnp.2008.150011. [DOI] [PubMed] [Google Scholar]
- 43.Coelin R, Raible A, Gasser T, et al. Ultrasound-guided injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord. 2008;23:889–892. doi: 10.1002/mds.21967. [DOI] [PubMed] [Google Scholar]
- 44.Wunderlich S, Csoti I, Bliners K, et al. Camptocormia in Parkinson’s disease mimicked by focal myositis of the paraspinal muscles. Mov Disord. 2002;17:598–600. doi: 10.1002/mds.10110. [DOI] [PubMed] [Google Scholar]
- 45.Yamada K, Goto S, Matsuzaki K, et al. Alleviation of camptocormia by bilateral subthalamic nucleus stimulation in a patient with Parkinson’s disease. Parkinsonism Relat Disord. 2006;12(6):372–375. doi: 10.1016/j.parkreldis.2006.02.003. [DOI] [PubMed] [Google Scholar]