Abstract
Repetitive or overuse disorders of the lumbar spine affect the lives of workers and athletes. We hypothesize that repetitive anterior lumbar flexion–extension under low or high load will result in significantly elevated pro-inflammatory cytokines expression several hours post-activity. High loads will exhibit significantly higher expression than low loads. Lumbar spine of in vivo feline was subjected to cyclic loading at 0.25 Hz for six 10-min periods with 10 min of rest in between. One group was subjected to a low peak load of 20 N, whereas the second group to a high peak load of 60 N. Following a 7-h post-loading rest, the supraspinous ligaments of L-3/4, L-4/5 and L-5/6 and the unstimulated T-10/11 were excised for mRNA analysis and IL-1β, IL-6, IL-8, TNFα and TGFβ1 pro-inflammatory cytokines expression. Creep (laxity) developed in the lumbar spine during the loading and the subsequent 7 h of rest was calculated. A two-way mixed model ANOVA was used to assess difference in each cytokines expression between the two groups and control. Tukey HSD post hoc analysis delineated specific significant effects. Significance was set at 0.05. Low and high-load groups exhibited development of creep throughout the cyclic loading period and gradual recovery throughout the 7-h rest period. Residual creep of 24.8 and 30.2% were present in the low and high-load groups, respectively, 7-h post-loading. Significant increases in expression of all cytokines measured relative to control were obtained for supraspinous ligaments from both low and high-load magnitudes. IL-6, IL-8 and TGFβ1 expression in the high-load group were significantly higher relative to the low-load group. Significant increases in cytokines expression indicating tissue inflammation are observed several hours post-repetitive lumbar flexion–extension regardless of the load magnitude applied. Repetitive occupational and athletic activity, regardless of the load applied, may be associated with the potential of developing acute inflammatory conditions that may convert to chronic inflammation if the viscoelastic tissues are further exposed to repetitive activity over long periods. Appropriate rest periods are a relevant preventive measure.
Keywords: Repetitive disorder, Overuse disorder, Ligaments, Spine, Lumbar, Cytokines, Inflammation
Introduction
Many occupational and sports activities require repetitive function of the lumbar spine (rowing, weight training, aerobic exercise, etc.). It is long known that exposure to repetitive (or cyclic) motion under high load and over extended periods results in a musculoskeletal disorder (also known as cumulative or overuse disorder) of the joint. The disorder is associated with chronic pain, weakness, limited range of motion and severe spasms of the relevant muscles. While the sports epidemiology is relatively scarce [27, 36], the occupational epidemiology is rather conclusive that high risk of a disorder is associated with prolonged exposure to repetitive motion of the extremities and spine under high loads [10, 20, 30, 34]. The biomechanical literature provides experimental validation with the demonstration of the development of large values of creep (laxity) in viscoelastic tissues exposed to repetitive loading [6, 7, 11, 13, 18, 19, 22, 38], micro-ruptures in the associated collagen fibers [7, 49, 50] and very long periods required for the recovery of the creep [4, 6, 22, 37]. Neurophysiological evidence also confirms that a neuromuscular disorder is associated with the creep developed in the viscoelastic tissues [40]. Cyclic motion at high loads was shown to elicit spasms and stiffness in the muscles associated with the exposed joint during and several hours to weeks post-loading in experimental animals [40, 48] and in humans [5, 15, 17, 25–27, 32]. Finally, the laxity of ligaments together with decreased muscular activity immediately after cyclic activity leaves the joint exposed to instability on simple, routine, daily tasks and exposure to potential injury [2, 41]. Recent data also demonstrate that the ligaments/tendons associated with the joint performing the prolonged cyclic activity under high loads develop an acute inflammation [1, 12, 40, 43]. The literature does not, however, offer much information on the potential of inflammatory response of ligaments/tendons exposed to prolonged cyclic activity under low loads, in particular, not of the lumbar ligaments. Lumbar ligaments/viscoelastic tissues are selected as their physiologic strain is four to six times larger [29, 48] than the ligaments of the extremity [31] and are scarcely explored, which is the objective of this report.
We hypothesize that prolonged cyclic loading of the lumbar ligaments under low and high loads will result in elevated pro-inflammatory cytokines expression several hours post-loading. We further expect that high loads will exhibit significantly higher cytokines expression when compared to low loads. Such information could provide a new insight into the development of sub-failure and cumulative/overuse ligament/tendon disorders as well as validate the development of exercise/rest preventive protocols.
Methods
Preparation
A total of 26 adult cats, separated into two groups (low load, N = 13, and high load, N = 13) with an average weight of 3.71(±0.78) kg were used in this study. Cats were anesthetized with 60 mg/kg of chloralose, according to a protocol approved by the institutional animal care and use committee. A superficial skin incision overlying the lumbar spine was made to expose the dorso-lumbar fascia, and an S-shaped stainless steel hook made of 1.5-mm diameter rod was applied around the supraspinous ligament half way between the dorsal processes of L-4 and L-5. The preparation was then positioned in a rigid stainless steel frame and the lumbar spine was isolated by means of two external fixators, which were applied to the L-1 and L-7 posterior process (feline animals have seven lumbar vertebrae). The external fixation was intended to limit the elicited flexion to the lumbar spine and to prevent interaction of thoracic and sacral and/or pelvic structures, but not to prevent any motion. A schematic of the setup as well as radiological verification of flexion–extension function resulting from the applied load were published in our previous reports [38, 48].
Instrumentation
The S-shaped stainless steel hook inserted around the L-4/5 supraspinous ligament was connected to the crosshead of the Bionix 858 Material Testing System (MTS, Minneapolis, MN), in which a load cell and displacement(strain) sensor were located. The load was applied through the MTS actuator with a computer-controlled loading system and monitored continuously along with the vertical displacement of the actuator. The load cell and displacement outputs of the Bionix 858 MTS were also sampled into the computer at 1,000 Hz. Under such loading condition, the lumbar spine underwent anterior flexion–extension while straining the lumbar supraspinous ligaments.
Protocol
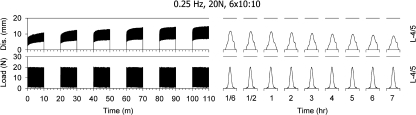
In the low-load group, a pre-load of 1 N was applied just prior to each single period of cyclic loading to produce a standard baseline across all preparations. A set of six 10 min of cyclic (sinusoidal) loading periods at 0.25-Hz (or one cycle every 4 s) and 20-N peak, each followed by 10 min of rest, was applied for a cumulative cyclic loading period of 60 min. The following recovery phase consisted of 7 h of rest at no load, during which single test cycles of 20 N peak load at 0.25 Hz were applied. The single cycles were applied at 10, 30 and 60 min following termination of the cyclic loading period and then once every hour. Overall, nine test cycles were applied during the 7-h recovery period. Figure 1 (bottom trace) shows the schematic of the cyclic loading. Load and displacement were recorded throughout the protocol.
Fig. 1.
A typical recording of the cyclic loading protocol during the six load/rest periods as well as during the single cycle tests during the 7-h rest period is shown on the bottom trace, whereas the associated displacement of the lumbar spine is shown on the top trace
The high-load group was subjected to a cyclic load of 60 N at peak instead of 20 N, while all other loading conditions remained the same as described above. A 20-N peak load was confirmed in our previous reports as a mild load, just above the neuromuscular neutral zone of the lumbar spine of the cat [37]. A 60-N peak load was established by previous study [42] to constitute a non-injurious high load, near the 90th percentile of the maximal load of a specimen of the same age and weight. Loads of 70 N resulted in partial ligament rupture in 10% of the group tested and loads of 80 N ruptured 90% of the ligaments in the group tested. The 60-N load, therefore, was considered as a high, non-injurious load within the physiological range of the ligament. The cyclic frequency of loading was selected to be 0.25 Hz based on prior measurements in normal humans performing deep flexion/extension at a normal, comfortable pace.
At the end of the 7-h recovery period, the supraspinous ligaments of the L-3/4, L-4/5 and L-5/6 were harvested from the specimen for analysis of cytokines. For purposes of comparison, the supraspinous ligament of T-10/11 from each preparation was also removed for analysis as self control, since it was not subjected to the cyclic load or any associated motion. Comparison of lumbar ligaments to unstimulated thoracic ligament as self control was scientifically advantageous. This, however, required confirmation that baseline/levels of cytokine in different unstimulated ligaments of the same specimen were approximately the same. For validation of this assumption, a control group of animals was tested and the validation was described in the appendix of a previous report [12].
Analysis of cytokines
RNA extraction and preparation of cDNA
Ligaments were flash frozen in liquid nitrogen, stored at −80°C and then powdered in a laboratory ball mill (Mikro-Dismembrator S, Sartorious BBI Systems, Inc., Bethlehem, PA). RNA was extracted and purified from the powdered ligaments using the RNeasy Lipid Tissue Mini Kit according to the manufacturer’s directions (QIAGEN, Valencia, CA). The procedure included an on-column DNase step. The average concentration and purity of RNA extracted from all ligaments were 190 ng/μl and 2.16 (λ 260/280 nm), respectively. Complementary DNA (cDNA) was prepared from 1 μg of RNA isolated from each sample using the HC cDNA RT kit with RNase inhibitor (Applied Biosystems, Foster City, CA).
Quantitative real-time PCR
Expression of gene targets was measured using real-time RT-PCR. Primers and probes for IL-1β, IL-6, IL-8, TNFα and TGFβ1 were designed with the assistance of the Primer Express sequence detection software (Applied Biosystems, Foster City, CA).
TaqMan probes (Applied Biosystems, Foster City, CA) were prepared with 5′-label of 6-carboxy fluorescein and 3′-label of 6-carboxy-tetramethylrhodamine. A 50 μl reaction mix containing TaqMan Universal Master Mix, forward and reverse primers, probes, RNase-free water, and 1 μg of the template cDNA was amplified for each target using an ABI prism 7500 sequence detector (Applied Biosystems, Foster City, CA) with an initial melt at 95°C for 10 min followed by 40 cycles of amplification at 95°C for 15 s and 60°C for 1 min. Real-time data acquisition and analysis were performed using Ct values in which mRNA levels for each gene (see Table 1) were normalized to the corresponding expression levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The GAPDH expression of the ligaments was consistent irrespective of the load status. A standard curve was generated for each gene using the fluorescent data from the tenfold serial dilutions of the amplicon that matched the specified primers. The standard curves for each gene did not differ significantly between experiments meaning that normalized values could be compared directly between experiments.
Table 1.
Accession number and sequences for the analyzed cytokines
| Name, accession number | Sequence | Optimized molar concentration (nm) | |
|---|---|---|---|
| GAPDH NM_001009307 | Fwd | TGG AAA GCC CAT CAC CAT CT | 200 |
| Rev | CCA GCA TCA CCC CAT TTG A | 200 | |
| Probe | VICCCA GGA GCG AGA TCC CGC CAATAMRA | 200 | |
| IL-6 NM_001009211 | Fwd | CCC TGT CCC AAC CGT AGA AG | 900 |
| Rev | TTG TTG TGT GCC TCA GCC A | 900 | |
| Probe | 6FAMTGG CCT GCA GCT AAG CTG CAG TCA CTAMRA | 200 | |
| IL-8 NM_001009281 | Fwd | CTC AGA AAT CAT TGT AAA GCT CGT CA | 900 |
| Rev | CCT TCT GCA CCC ACT TTT GC | 300 | |
| Probe | 6FAMTGG AAA AGA GGT GTG CCT GGA CCCTAMRA | 200 | |
| IL-1β NM_001077414 | Fwd | GCT GAT GGC CCC GAA AA | 300 |
| Ref | GCC CTC ATC TCC CAG AAA ACT | 900 | |
| Probe | 6FAMTGA AGG GCA GCC TCC AAA ACC TGATAMRA | 200 | |
| TGF-β AY425617 | Fwd | ACA TCA ACG GGT TCA GTT CCA | 300 |
| Rev | CCG GTT CAT GCC ATG AAT G | 300 | |
| Probe | 6FAMCGC CGA GGT GAC CTG GCC ATAMRA | 200 | |
| TNF-α NM_001009835 | Fwd | CAC ATG GCC TGC AAC TAA TCA | 300 |
| Rev | GCT TGT CAC TCG GAG TTC GAG | 900 | |
| Probe | 6FAMCCC TCT GCC CCA GAC ACT CAG ATC ATCTAMRA | 200 |
Statistical analysis
Normalized cytokine levels (IL-1β, IL-6, IL-8, TNFα, and TGFβ1) were compared between loaded ligaments (L-3/4, L-4/5 and L-5/6) and the unloaded self control reference ligament (T-10/11) from each specimen. Comparison between the 20- and 60-N loading conditions was accomplished using a two-way mixed model ANOVA. Loading condition (20, 60 N) and vertebral level (T-10/11, L-3/4, L-4/5 and L-5/6) were fixed variables and specimen was a random variable. On significant interactions or main effects in both tests, Tukey HSD post hoc analyses were used to delineate specific statistically significant effects. All data were visually inspected for a normal distribution and an appropriate data transformation was applied if necessary. The highest and lowest values from each loading condition × vertebral level grouping were removed before any analyses were performed [3]. The level of significance was set at 0.05.
Results
Figure 1 displays a typical loading sequence with six work/rest phases followed by a 7-h rest period as well as the associated displacement recorded from one preparation.
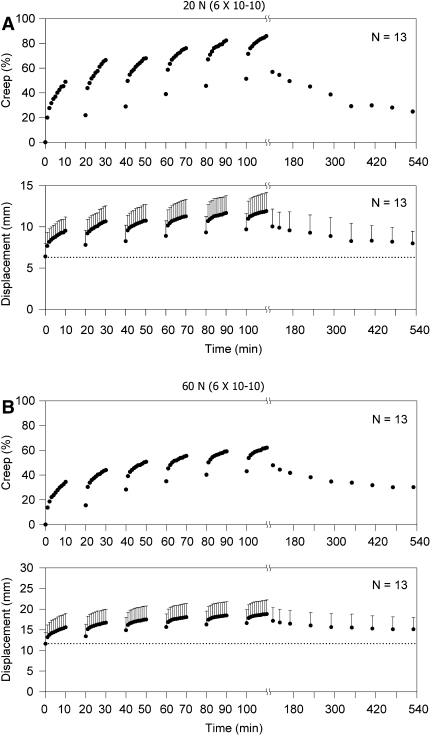
Figure 2a shows the mean (±SD) displacement (bottom trace) and associated creep (top trace) developed during cyclic loading of the preparations subjected to the low load (20 N) as well as the recovery during the following 7-h rest.
Fig. 2.
a The mean displacement (lower trace) and the calculated mean creep (top trace) from the group subjected to a low load of 20 N. b The mean displacement (bottom trace) and the calculated mean creep (top trace) from the group exposed to a high load of 60 N
The initial mean displacement was 6.4 mm and gradually increased during the first 10 min of cyclic loading to 9.5 mm with a corresponding creep of 48.9%. It partially recovered over the first 10 min of rest to 7.8 mm (21.9% creep) and further increased with each of the subsequent five sessions of 10 min of cyclic loading to a final mean value of 11.9 mm corresponding to 85.9% creep. During the following 7-h rest, the mean displacement decreased to a residual of 8.0 mm or 24.8% creep. Complete recovery of the creep was not observed in any animal.
Figure 2b provides the mean (±SD) of the displacement and the associated mean creep from the preparations subjected to high load (60 N) during loading and the following 7-h rest period. The initial mean displacement was 11.6 mm and gradually increased during the first 10 min of cyclic loading to 15.6 mm with a corresponding creep of 34.4%. It partially recovered during the first 10 min of rest to 13.4 mm (15.5% creep) and then further increased during each additional 10 min of loading, reaching a final mean value of 18.8 mm with a corresponding mean creep of 62.1%. During the 7-h rest period, the displacement gradually decreased reaching a residual mean value of 15.1 mm with a corresponding residual mean creep of 30.2%. Full recovery of the displacement or the creep was not observed in any of the preparations.
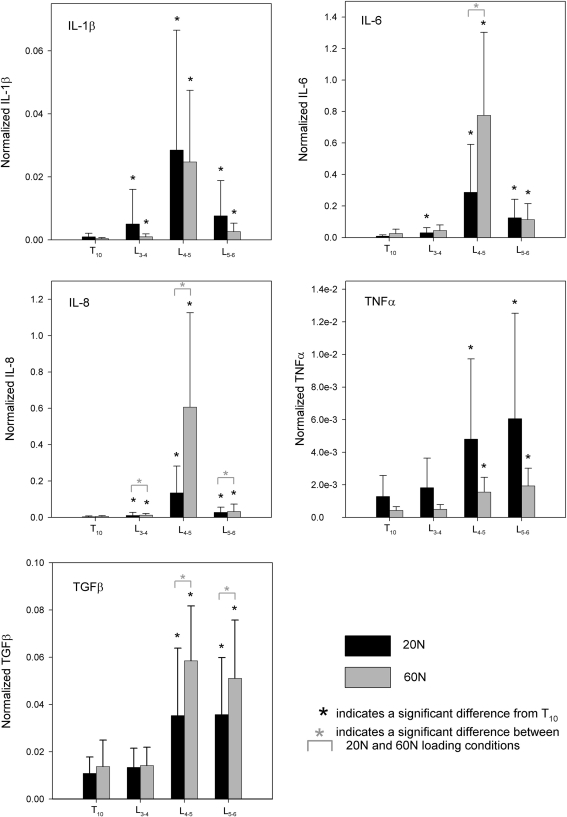
Histograms of each cytokine demonstrated a clear right-tailed distribution; therefore, the log of each cytokine value was calculated before statistical analyses. All plots displaying normalized cytokine levels represent the untransformed data. Each cytokine demonstrated significant differences between the loaded vertebral ligaments (L-3/4, L-4/5 and L-5/6) and the self control reference ligament (T-10/11). Figure 3 and Table 2 provide graphical and tabular representation of these specific data, respectively.
Fig. 3.
Histograms of the expression of the five pro-inflammatory cytokines (IL-1β, IL-6, IL-8, TNFα and TGFβ1) from each of the lumbar levels (L-3/4, L-4/5 and L-5/6) and from the groups subjected to high and low loads as compared to their self control ligament, T-10/11
Table 2.
Statistical results of load condition and vertebral level analysis
| Cytokine | Statistical effect | ||
|---|---|---|---|
| Interaction | Load condition | Vertebral level | |
| IL-1β | 0.270 | 0.694 | <0.001* |
| IL-6 | 0.019* | – | – |
| IL-8 | 0.115 | 0.028* | <0.001* |
| TGFβ1 | 0.422 | 0.017* | <0.001* |
| TNFα | 0.743 | 0.379 | <0.001* |
* Significant effect, P < 0.05
Three of the cytokines (IL-6, IL-8 and TGFβ1) demonstrated both effect of vertebral ligament level and effect of loading level. Analysis of normalized IL-6 revealed an interaction effect (P = 0.019). When compared to reference ligaments, the 20 N loading condition yielded significantly greater gene expression of IL-6 in all loaded ligaments, while the 60 N loading condition yielded greater gene expression of IL-6 in L-4/5 and L-5/6. In addition, the IL-6 expression with 60 N was significantly larger than with 20 N in L-4/5. Normalized IL-8 demonstrated significant effects of both load (P = 0.028) and ligament level (P < 0.001). Both loading conditions caused significantly greater gene expression of IL-8 than the reference ligament in all vertebral levels. The loading effect revealed greater gene expression of IL-8 in 60 N than in 20 N in these levels. Likewise, normalized TGFβ1 demonstrated significant effects of both load (P = 0.017) and ligament level (P < 0.001). Post hoc analyses revealed significantly greater gene expression of TGFβ1 than the reference ligament in vertebral levels L-4/5 and L-5/6. The effect of loading indicated that the 60 N loading condition caused greater expression of TGFβ1 in 60 N than in 20 N.
However, normalized IL-1β and normalized TNFα demonstrated significant effects only between vertebral level (P < 0.001 for both cytokines), but did not yield an effect of loading condition (P = 0.694 and P = 0.379, respectively). Specifically, the expression of IL-1β was significantly larger in all loaded vertebral levels when compared to the reference ligaments, and the expression of TNFα was significantly larger than the reference ligaments in L-4/5 and L-5/6 only.
Discussion
The results of this study demonstrate that prolonged performance of repetitive/cyclic movement triggers significantly elevated pro-inflammatory cytokines expression in the associated ligaments regardless of the load magnitude. Furthermore, repetitive exposure to high loads triggers significantly higher expression in several (IL-6, IL-8 and TGFβ1), but not all, cytokines than exposure to low loads. Elevated cytokines expression indicates that significant tissue degradation, consistent with an acute inflammation, occurred post-loading. Low cyclic loads, therefore, should not be simply dismissed as a no-risk activity.
Cytokines are soluble proteins that respond to ligament injury by binding to their receptors and initiating a cellular response [35]. Interleukin-1 (IL-1) is a strong pro-inflammatory cytokine that works both directly and indirectly. IL-1 induces B-cell differentiation and acute phase proteins [8]. It also drives extracellular matrix destruction by increasing degradative enzymes such as matrix metalloproteinases [44]. In addition, IL-1 stimulates fibroblasts to express and secrete other inflammatory cytokines including IL-6 [9, 45]. The action of IL-6 is typically pro-inflammatory during the acute phase by inducing the production of acute phase proteins (some distinct from those produced by IL-1 and TNFα) and can also promote B- and T-cell functions and stimulate the secretion of immunoglobulin [9, 16]. IL-8 is considered a chemokine because it attracts neutrophils to the site of inflammation [23], for example, in inflammatory joint disease [46]. The action of TNFα is similar to that of IL-1, but is thought to be less potent in most tissues. The remaining cytokine measured in this study, TGFβ, is a multifunctional cytokine that acts in normal physiology and pathology. It may act in potentially contradictory ways depending on tissue and pathology [21]. In inflammation, TGFβ can recruit B and T cells, but can also lower the immune response and promote extracellular matrix production.
Anterior flexion executed to a pre-determined angle should manifest with an identical strain (elongation %) regardless of the load applied. Why, then, a significantly higher pro-inflammatory cytokines expression is exhibited following flexion under high loads? It is well established that viscoelastic tissues (such as ligaments, facet capsules, dorsolumbar fascia, etc., in this case) are highly sensitive to the load application rate. In the group subjected to low loads, the peak load of 20 N was reached within 2 s. The load application rate, in this case, was 10 N/s. Conversely, in the group subjected to high loads, the peak load of 60 N was also reached within 2 s. Thus, the load application rate was 30 N/s, or three times (3×) the rate of the low-load group. Viscoelastic tissues can adapt to slow application of load with larger strain and minimal development of micro-fractures within its collagen fibers. Fast application of loads, however, results in the inability of the tissue to strain fast enough to accommodate the load rate, development of high tension [28, 39] and substantial development of micro-ruptures within its collagen fibers [7, 49, 50]. Overall, high rates of loading result in substantially larger damage within the collagen fibers and the expected larger inflammatory response.
Indeed, the creep recorded at the end of the first 10 min of cyclic loading was 48.9% for the low-load group, but was 34.4% for the high-load group, demonstrating the ability of the tissue to strain more in the low-load group and the inability to strain fast enough in the high-load group. Similarly, at the end of the six loading/rest periods, the final creep in the low-load group was 85.9%, but only 62.1% in the high-load group.
The final, or residual, creep at the end of the 7-h rest period is most illuminating. It was 24.8% for the low-load group, but a much higher 30.2% in the high-load group. Similar results were obtained throughout our long investigation of cyclic loading [2, 11, 13, 24, 41], and the residual creep at the end of 7-h recovery was always higher in the high-load condition. Finally, our study assessing the direct impact of increased frequency of the cyclic load at the same load [18, 19] confirm the increased impact of frequency on the elicited neuromuscular disorder, leaving the assessment of the change in cytokines expression to the next study on our agenda. Preliminary data confirm that significant increase in cytokines is observed at high-frequency loading.
During the cyclic loading periods, the lumbar viscoelastic tissues of the group subjected to high load continuously incurs micro-damage to more and more collagen fibers. At the end of the loading periods, large numbers of fibers are damaged and are not functional. Therefore, at the end of the rest period, the viscoelastic tissues exhibit the recovery of the remaining undamaged, functional collagen fibers together with the laxity induced by the dysfunctional, damaged fibers. The overall higher residual creep, therefore, represents the larger damage inflicted by the high-load rate. Since significantly higher pro-inflammatory cytokines expression elicits inflammatory tissue degradation, more pronounced degradation is expected to be observed in the group exposed to higher load.
Although a significant increase in cytokines expression was observed in the group subjected to low loads, the epidemiology, biomechanics and neuromuscular findings do not support the existence of a repetitive disorder. The epidemiology does not describe a pronounced increase in reports of disability associated with repetitive activity at low loads [10, 20, 30, 34]. The biomechanical and neurophysiological evidence also show minimal spasms during loading, absence of muscular spasms/hyperexcitability post-loading and fair recovery of lumbar laxity [11, 13, 24]. With the lack of supporting evidence for a disorder, one is left to conclude that the cytokines expression level recorded in the low-load group did not exceed a certain physiological threshold required to trigger a disorder directly or indirectly.
Significantly greater expression of IL-6, IL-8 and TGFβ1 were observed in the ligaments harvested from the group exposed to high load relative to the group exposed to low loads. The remaining cytokines, IL-1β and TNFα, were not significantly different in the high-load group. The specific cytokines that registered elevated expression in the high-load group may lead us to gain insight into the threshold discussed above. Indeed, a recent report points out that upregulation of IL-6 and IL-8 are each positively correlated with pain intensity in humans with an acute inflammation [47]. The authors went onto conclude that increased IL-6 and IL-8 expression contributed to the development of acute inflammation and inflammatory pain. Considering their conclusion in the context of our data, the significantly lower levels of these two cytokines in the low-load group are probably below the threshold of triggering an acute inflammation and the associated pain. Another possibility is that a mild acute inflammation was present in the low-load group, but the pain level was not sufficient to trigger a neuromuscular disorder. A third possibility is that regardless of the presence or level of the acute inflammation, the pain level may be the trigger of the neuromuscular disorder. Unfortunately, we could not assess pain in anesthetized animals. It is obvious that more research is required to reveal the details of this important and fascinating issue.
The significant increase in the expression of all five pro-inflammatory cytokines tested in the ligaments from the group exposed to low loads indicates that repetitive activity under such conditions cannot be designated as “no risk.” Possibly, with higher number of repetitions [24] and with less intermittent rest [11], low load can become problematic as the amount of damage to the collagen fibers may exceed a certain threshold. It may be more appropriate to designate such category as “low risk”.
Inflammation of damaged viscoelastic tissue such as tendons and ligament is biphasic. Once the damage in the tissue is detected, pro-inflammatory substances and cells invade the affected tissue and mediate the removal of the damaged molecules, passing them to the circulatory system [14]. Once this first phase is complete, a second phase is initiated during which a reconstruction of the damaged collagen matrix is performed. Therefore, a minor acute inflammation due to repetitive physical activity can be spontaneously resolved if sufficient rest is allowed. Often, 24–72 h are sufficient to spontaneously resolve most repetitive exercise-related damage [14]. Indeed, a model based on animal work [37] shows that creep/laxity in ligaments together with a neuromuscular disorder will not fully recover within 7–8 h of rest post-cyclic exercise, as it leaves a large residual laxity [6, 11, 13, 24] probably due to the lost tension from the damaged collagen fibers [7, 49, 50]. Measurements performed in humans confirm this model and show that 24-h post-exercise rest resolves the residual laxity/creep of the tissue [4]. Most likely, the second phase of the inflammation had been completed by then, restoring the lost viscoelastic properties of the damaged fibers. The major factor associated with a successful healing by the inflammation seems to be rest. Therefore, any moderate repetitive/cyclic exercise can benefit from at least 24 h of rest, and 48 h may be required for exercise under high loads. Understanding of the healing process provides justification for taking a 48-h rest between exercise sessions of the same joints. For example, exercise of a specific joint performed at 3:00 p.m. of 1 day should not be repeated the next day and preferably addressed again the second day, at about the same time. Such rest practice is already common in the athletic circles, but is validated here based on the inflammatory process.
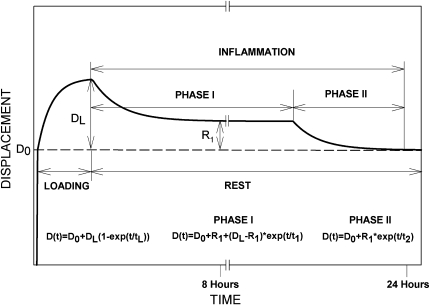
Based on the literature reviewed above, we can propose a biphasic recovery theory of viscoelastic tissues exposed to repetitive exercise. The theory is summarized in Fig. 4 and shows the initial displacement, Do, associated with load application as well as the exponentially increasing laxity during the exercise period reaching a maximum of DL at the end of loading. DL can be expressed as creep when calculated as a percentage of Do. As the post-exercise rest begins, Phase I of the inflammation is initiated, during which pro-inflammatory cytokines begin to be accumulated in the ligaments and direct the removal of the damaged molecules into the circulatory system. The duration of Phase I is not documented yet and may depend on the amount of damage present in the tissue, which is further associated with the load magnitude, frequency and duration of the physical activity. So far, the longest duration of Phase I available in the literature is 7–8 h. At the end of Phase I, a residual laxity, R1, is present, and is at an asymptotic saturation of its exponential decay, indicating that further significant recovery is not expected in this phase. As mentioned above, the residual laxity, R1, is due to the lost viscoelastic properties of the damaged collagen fibers. As Phase II begins, a new exponentially decaying equation describes the gain in the viscoelastic tension in the ligament as the reconstruction/repair of the damaged fibers is underway. This phase also reaches asymptotic saturation near baseline, signifying a full recovery to normal operating functionality. While literature fully supports and validates the different components, Phase II is a highly educated speculation and in need of experimental validation.
Fig. 4.
A schematic of ligament elongation during loading and the biphasic recovery to baseline over the following rest. Note the large residual laxity at the end of the first phase and the second exponential decrease to baseline at the end of the second phase
The balance of pro- and anti-inflammatory mediators determines the final tissue response. The limitation of most studies on this topic is that one cannot quantify all possible mediators. Furthermore, some cytokines, such as TGFb for example, exist as either pro- or anti-inflammatory mediators depending on the context and the circumstances (tissue type, injury status, repair status, etc.). Nevertheless, a significant elevated level of the major pro-inflammatory cytokines and especially of the pain-inducing IL-8 suggest a significant tissue injury and inflammatory response.
In conclusion, low-load activities such as aerobics, for example, should not be exempt from such work/rest protocols if overuse/cumulative disorder are to be prevented. Two types of rest, therefore, are available as a preventive measure for pro-inflammatory activity: an inter-session rest during the performance of exercise as an attenuation measure [33] and an inter-day rest as a preventive measure from propagation of the acute phase into chronic inflammation. Repeating exercise of the same ligament/tendon on a daily basis for prolonged periods (weeks/months) can further aggravate a minor inflammation and convert it into a chronic inflammation: an irreversible and degenerative disease called overuse or cumulative disorder.
Acknowledgments
This work was supported in part by grant RO1-OH-07622 from NIOSH and by the Academic Enrichment Fund from the UC Denver, School of Medicine. Peter D’Ambrosia, MD was a research Orthopedic resident supported by the Department of Orthopedics.
References
- 1.Barbe M, Barr A, Gorzelany I, et al. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in rat model of MSD. J Orthop Res. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Masaud A, Solomonow D, Davidson B, Zhou B, Lu Y, Patel V, Solomonow M. Motor control of lumbar instability following exposure to various cyclic load magnitudes. Eur Spine J. 2009;18:1022–1034. doi: 10.1007/s00586-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Routledge Academic press, London
- 4.Crisco J, Chelikani S, Brown R, Wolfe S. The effect of exercise on ligamentous stiffness in the wrist. J Hand Surg. 1997;22A:44–48. doi: 10.1016/S0363-5023(05)80178-9. [DOI] [PubMed] [Google Scholar]
- 5.Dickey J, McNorton S, Potvin J. Repeated spinal flexion modulates the flexion–relaxation phenomenon. Clin Biomech. 2003;18:783–789. doi: 10.1016/S0268-0033(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 6.Duenwald S, Vanderby R, Lakes R. Viscoelastic relaxation and recovery of tendons. Ann Biomed Eng. 2009;37:1131–1140. doi: 10.1007/s10439-009-9687-0. [DOI] [PubMed] [Google Scholar]
- 7.Fung D, Wang V, Laudier D, Shine J, Basta-Pljakic J, Jepesen K, Schaffler M, Flatow E. Subrupture tendon fatigue damage. J Orthopedic Res. 2009;27:264–273. doi: 10.1002/jor.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauldie J, Sauder DN, McAdam KP, Dinarello CA. Purified interleukin-1 (IL-1) from human monocytes stimulates acute-phase protein synthesis by rodent hepatocytes in vitro. Immunology. 1987;60(2):203–207. [PMC free article] [PubMed] [Google Scholar]
- 9.Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83(2):585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogendoorn WE, Bongers PM, Vet HC, et al. Flexion and rotation of the trunk and lifting at work are risk factors for low back pain: results of a prospective cohort study. Spine. 2000;25:3087–3092. doi: 10.1097/00007632-200012010-00018. [DOI] [PubMed] [Google Scholar]
- 11.Hoops H, Zhou B, Lu Y, Solomonow M, Patel V. Short rest between cyclic flexion periods is a risk factor for lumbar disorder. Clin Biomech. 2007;22:745–757. doi: 10.1016/j.clinbiomech.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 12.King K, Davidson B, Zhou B, Lu Y, Patel V, Solomonow M. High magnitude cyclic load triggers inflammatory response in lumbar ligaments. Clin Biomech. 2009;24:792–798. doi: 10.1016/j.clinbiomech.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Le P, Solomonow M, Zhou B, Lu Y, Patel V. Cyclic load magnitude is a risk factor for cumulative low back disorder. J Occup Environ Med. 2007;49:375–387. doi: 10.1097/JOM.0b013e318046eb0b. [DOI] [PubMed] [Google Scholar]
- 14.Leadbetter W. An introduction to sports induced soft tissue inflammation. In: Leadbetter W, Buckwalter J, Gordon S, editors. Sports induced inflammation; clinical and basic science concepts. Park Ridge: Am. Acad. Orthopedic Surgeons; 1990. [Google Scholar]
- 15.Li L, Patel N, Solomonow D, Le P, Hoops H, Gerhardt D, Johnson K, Zhou B, Lu Y, Solomonow M. Neuromuscular response to cyclic lumbar twisting. Hum Factors. 2007;49:820–829. doi: 10.1518/001872007X230190. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky PE (2006) Interleukin-6 and rheumatic diseases. Arthritis Res Ther 8 (suppl 2):S4 [DOI] [PMC free article] [PubMed]
- 17.Little J, Khalsa P. Human lumbar spine creep during cyclic and static flexion: creep rate, biomechanics, and facet joint capsule strain. Ann Biomed Eng. 2005;33:391–401. doi: 10.1007/s10439-005-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu D, Solomonow M, Zhou B, Baratta R, Li L. Frequency dependent changes in neuromuscular response to cyclic lumbar flexion. J Biomech. 2004;37:845–855. doi: 10.1016/j.jbiomech.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Le P, Davidson B, Zhou B, Lu Y, Patel V, Solomonow M. High frequency cyclic flexion is a risk factor for a lumbar disorder. Muscle Nerve. 2008;38:867–874. doi: 10.1002/mus.21019. [DOI] [PubMed] [Google Scholar]
- 20.Marras WS. Occupational low back disorder causation and control. Ergonomics. 2000;43:880–902. doi: 10.1080/001401300409080. [DOI] [PubMed] [Google Scholar]
- 21.Massague J, Gomis RR. The logic of TGF[beta] signaling. FEBS Lett. 2006;580(12):2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 22.McGill S, Brown S. Creep response of the lumbar spine to prolonged full flexion. Clin Biomech. 1992;17:43–46. doi: 10.1016/0268-0033(92)90007-Q. [DOI] [PubMed] [Google Scholar]
- 23.Namen A, Schmierer A, March C, Overell R, Park L, Urdal D, Mochizuki D. B cell precursor growth-promoting activity purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navar D, Zhou B, Lu Y, Solomonow M. High repetition of cyclic loading is a risk factor for lumbar disorders. Muscle Nerve. 2006;34:614–622. doi: 10.1002/mus.20629. [DOI] [PubMed] [Google Scholar]
- 25.Olson M, Li L, Solomonow M. Flexion–relaxation response to cyclic lumbar flexion. Clin Biomech. 2004;19:769–776. doi: 10.1016/j.clinbiomech.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Olson M, Li L, Solomonow M. Interaction of viscoelastic tissue compliance with lumbar muscles during passive cyclic flexion–extension. J Electromyogr Kinesiol. 2009;19:30–38. doi: 10.1016/j.jelekin.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Orchard J, James T, Portus M, Kountouris, Dennis R (2009) Fast bowlers in cricket demonstrate up to 3–4 weeks delay between high workloads and increased risk of injury. Am J Sports Med 37:1186–1192 [DOI] [PubMed]
- 28.Panjabi M, Courtney T. High speed sub failure stretch of rabbit ACL: changes in elastic failure and viscoelastic characteristics. Clin Biomech. 2001;16:334–340. doi: 10.1016/S0268-0033(01)00007-9. [DOI] [PubMed] [Google Scholar]
- 29.Panjabi M, Goel V, Takata K. Physiologic strains in the lumbar spinal ligaments. Spine. 1982;7:192–203. doi: 10.1097/00007632-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Punnett L, Wegeman D. Work related musculoskeletal disorders; the epidemiologic evidence and the debate. J Electromyogr Kinesiol. 2004;14:13–23. doi: 10.1016/j.jelekin.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Renstrom R, Arms S, Stanwyck T, et al. Strains within the anterior cruciate ligament during hamstring and quadriceps activity. Am J Sports Med. 1986;14:83–87. doi: 10.1177/036354658601400114. [DOI] [PubMed] [Google Scholar]
- 32.Sbriccoli P, Solomonow M, Zhou B, Lu Y, Sellards R. Neuromuscular response to cyclic loading of the anterior cruciate ligament. Am J Sports Med. 2005;33:543–551. doi: 10.1177/0363546504268408. [DOI] [PubMed] [Google Scholar]
- 33.Sbriccoli P, Solomonow M, Zhou B, Lu Y. Work to rest duration ratios exceeding unity are a risk factor for low back disorder. J Electromyogr Kinesiol. 2007;17:142–152. doi: 10.1016/j.jelekin.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein B, Fine L, Armstrong T. Hand wrist cumulative trauma disorders in industry. Br J Ind Med. 1986;43:779–784. doi: 10.1136/oem.43.11.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith AJ, Humphries SE. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20:43–59. doi: 10.1016/j.cytogfr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Smoljanovic T, Bojnic I, Hannafin J, Hren D, Delimar D, Pecina M. Traumatic and overuse injuries among international elite junior rowers. Am J Sports Med. 2009;37:1193–1199. doi: 10.1177/0363546508331205. [DOI] [PubMed] [Google Scholar]
- 37.Solomonow M, Zhou B, Baratta R, Lu Y, Zhu M, Harris M. Bi-exponential recovery model of lumbar viscoelastic creep and reflexive muscular activity after prolonged cyclic loading. Clin Biomech. 2000;15:167–175. doi: 10.1016/S0268-0033(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 38.Solomonow M, Zhou B, Baratta R, Lu Y, Harris M. Biomechanics of increased exposure to lumbar injury due to cyclic loading: I loss of reflexive muscular stabilization. Spine. 1999;24:2426–2434. doi: 10.1097/00007632-199912010-00003. [DOI] [PubMed] [Google Scholar]
- 39.Solomonow M. Ligaments: a source of work related musculoskeletal disorder. J Electromyogr Kinesiol. 2004;14:49–60. doi: 10.1016/j.jelekin.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Solomonow M, Baratta R, Zhou B, Burger E, Zieske A, Gedalia A. Muscular dysfunction elicited by creep of lumbar viscoelastic tissues. J Electromyogr Kinesiol. 2003;13:381–396. doi: 10.1016/S1050-6411(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 41.Solomonow D, Davidson B, Zhou B, Lu Y, Patel V, Solomonow M. Neuromuscular neutral zones response to cyclic lumbar flexion. J Biomechan. 2008;41:2821–2828. doi: 10.1016/j.jbiomech.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Solomonow M, Zhou B, Harris M, Lu Y, Baratta R. The ligamento-muscular stabilizing system of the spine. Spine. 1998;23:2552–2562. doi: 10.1097/00007632-199812010-00010. [DOI] [PubMed] [Google Scholar]
- 43.Soslowsky L, Thomopoulos S, Tun S, et al. Overuse activity injures the supraspinatous tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. doi: 10.1016/S1058-2746(00)90033-8. [DOI] [PubMed] [Google Scholar]
- 44.Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21(2):256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 45.Van Damme J, Cayphas S, Opdenakker G, Billiau A, Van Snick J (1987) Interleukin 1 and poly(rI).poly(rC) induce production of a hybridoma growth factor by human fibroblasts. Eur J Immunol 17(1):1–7 [DOI] [PubMed]
- 46.Verburgh CA, Hart MH, Aarden LA, Swaak AJ. Interleukin-8 (IL-8) in synovial fluid of rheumatoid and nonrheumatoid joint effusions. Clin Rheumatol. 1993;12(4):494–499. doi: 10.1007/BF02231778. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Hamza M, Wu T, Dionne R. Upregulation of IL-6, IL-8 and CCL2 gene expression after acute inflammation: correlation to clinical pain. Pain. 2009;142:275–283. doi: 10.1016/j.pain.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams M, Solomonow M, Zhou B, Baratta V, Harris M. Multifidus spasms elicited by prolonged lumbar flexion. Spine. 2000;25:2916–2924. doi: 10.1097/00007632-200011150-00014. [DOI] [PubMed] [Google Scholar]
- 49.Woo S, Gomez M, Woo Y, Akeson W. Mechanical properties of tendons and ligaments: the relationships of exercise in tissue remodeling. Biorheology. 1982;19:379–408. doi: 10.3233/bir-1982-19302. [DOI] [PubMed] [Google Scholar]
- 50.Woo S, Gomez M, Amiel D, Akeson W. The effect of exercise on the biomechanical and biochemical properties of swine digital flexor tendon. J Biomechan Eng. 1981;103:51–56. doi: 10.1115/1.3138246. [DOI] [PubMed] [Google Scholar]