Abstract
Diffuse idiopathic skeletal hyperostosis (DISH) is a systemic disorder of the axial and peripheral skeleton in humans and has incidentally been described in dogs. The aims of this retrospective radiographic cohort study were to determine the prevalence of DISH in an outpatient population of skeletally mature dogs and to investigate if dogs can be used as an animal model for DISH. The overall prevalence of canine DISH was 3.8% (78/2041). The prevalence of DISH increased with age and was more frequent in male dogs, similar to findings in human studies. In the Boxer breed the prevalence of DISH was 40.6% (28/69). Dog breeds represent closed gene pools with a high degree of familiar relationship and the high prevalence in the Boxer may be indicative of a genetic origin of DISH. It is concluded that the Boxer breed may serve as an animal model for DISH in humans.
Keywords: DISH, Spine, Dog, Prevalence, Boxer
Introduction
Diffuse idiopathic skeletal hyperostosis (DISH) is a systemic disease of the axial and peripheral skeleton [10]. DISH results in ossification of soft tissues including the longitudinal spinal ligaments and sites of attachment of tendons and capsules to bone. DISH was first described by Forestier and Rotes-Querol in 1950 [10]. In 1976, Resnick and Niwayama [28] postulated three criteria for the diagnosis of DISH which are now widely used in literature and may be helpful in differentiating DISH from other spinal disorders, such as spondylosis deformans, intervertebral osteochondrosis and ankylosing spondylitis. The prevalence of DISH varies with geographic location and ranges from 2.9% in Korea to 25% in the USA [13, 14, 32, 34, 35]. The variation in prevalence for DISH found throughout the world suggests a possible genetic and/or ethnic factor in the pathogenesis of DISH [6, 13, 34]. The etiologic factors are not yet clear but various metabolic, endocrinological, genetic and environmental factors have been suggested to contribute to its development [15, 18, 21]. DISH is associated with obesity and the incidence increases with body weight in both the genders [18, 20, 21]. DISH often remains asymptomatic but can lead to mild back pain, stiffness and restriction in spinal range of motion [3, 23]. In advanced stages of DISH, multiple level spinal ankylosis may lead to spinal cord compression or result in unstable fractures of the spine, even after trivial trauma, often mimicking clinical features of ankylosing spondylitis [4, 32].
DISH has been described in two canine cases, in which both dogs showed orthopedic and neurological abnormalities and were eventually euthanized because of the severity of symptoms [22, 37]. Morgan and Stavenborn suggested that DISH and extensive forms of spondylosis deformans in dogs, a condition frequently described in veterinary literature, have many features in common [5, 17, 22].
For more than 15,000 years dogs have lived with humans in comparable conditions [29, 33]. They share the same environment and are subjected to similar nutritional and toxic substances. The nutrition of dogs on commercial pet food diets has been researched intensively [8]. But yet a growing number of dogs develop obesity and estimations vary between 20 and 40% [11]. Endocrinological syndromes and conditions of the spine show great similarities between humans and dogs, and for specific diseases in humans, dogs may serve as a spontaneous disease animal model [2, 9, 16, 19]. However, no animal model has been described for DISH. Therefore the aims of the present study were: (1) to determine the prevalence and spinal distribution of DISH in an outpatient population of skeletally mature dogs, (2) to study the relation between DISH and age, gender, neutering status and breed and (3) to investigate if dogs could be used as an animal model for DISH.
Materials and methods
Radiographs of dogs older than 1 year, referred to the Department of Clinical Sciences of Companion Animals between February 2003 and January 2008, were retrieved from the hospital database. From this cohort, radiographs were selected when more than 20 dogs of a particular breed were available, leading to a total of 2041 dogs from 33 breeds. All dogs were referred by veterinarians for various medical conditions not necessarily related to the spine.
Radiographs of the thorax, abdomen and cervical and/or thoracolumbar spine were all eligible for review. DISH was confirmed when the criteria of Resnick and Niwayama were met [28]. The radiographs were screened by the principal investigator according to a predefined scoring system: (1) DISH, (2) possible DISH and (3) no DISH. Radiographs marked with score 1 or 2 were re-reviewed by two investigators in a consensus meeting to confirm or refute the diagnosis of DISH. When consensus on the diagnosis was not established, a third investigator was consulted. The indications for radiographic examination were reviewed and categorized in three groups. Suspected thoracic abnormalities and screening for metastatic disease constituted the majority of indications (75%). The group ‘spinal’ included dogs that were examined for neurological or orthopaedic complaints (18%). In 7% the indication for the radiographs was an abdominal problem.
Statistical analysis was performed using SPSS software (version 16.0). The Student’s t-test was performed on the mean ages of dogs with and without DISH. Univariate analysis was performed on age, gender, neutering status, breed in general and in case one or more breed(s) appeared most affected, for a particular breed separately. Variables that were significantly related to DISH in the univariate analysis were subsequently analyzed in a multiple logistic regression model. Significance was set if p < 0.05.
Results
From the total of 2041 dogs, 51.3% were male and 48.7% female. The mean age ± SE of all dogs was 7.2 ± 0.2 years (range 1–17 years). The mean age of dogs with DISH (8.9 ± 1.0 years, range 2–16 years) was significantly (p = 0.00) higher than the mean age of dogs without DISH (7.0 ± 0.2; range 1–17 years). The overall prevalence of DISH in this cohort was 3.8% (78/2041) (95% confidence interval (CI) 3.0–4.7) (Table 1). DISH was present in male dogs more frequently than in female dogs. In male dogs the prevalence of DISH was 4.3%, in female dogs the prevalence was 3.3%. Stratified into three age groups, DISH was more prevalent in older dogs (Table 1).
Table 1.
The prevalence of DISH in 2041 dogs according to gender and age groups: number of dogs (percentage; 95% confidence interval)
| No DISH | DISH | Total | |
|---|---|---|---|
| Gender | |||
| Male dogs | 1,002 (95.7; 94.5–96.9) | 45 (4.3; 3.1–5.5) | 1,047 (100.0) |
| Female dogs | 961 (96.7; 95.6–97.8) | 33 (3.3; 2.2–4.4) | 994 (100.0) |
| Age group (years) | |||
| 1–5 | 736 (98.3; 97.3–99.2) | 13 (1.7; 0.8–2.7) | 749 (100.0) |
| 6–10 | 951 (95.8; 94.5–97.0) | 42 (4.2; 3.0–5.5) | 993 (100.0) |
| 11–17 | 276 (92.3; 89.3–95.3) | 23 (7.7; 4.7–10.7) | 299 (100.0) |
| Total | 1,963 (96.2; 95.4–97.0) | 78 (3.8; 3.0–4.7) | 2,041 (100.0) |
Fifteen breeds were affected by DISH. Among these, only the Boxer breed showed an exceptionally high prevalence and reliable confidence interval: 40.6% (28/69) (95% CI 29.0–52.2). In contrast, two other popular dog breeds, the Labrador Retriever and the Bernese Mountain dog, that were well represented in the cohort, showed a low prevalence for DISH (2.5 and 2.0%, respectively) (Table 2). In dogs of 18 other breeds, including the Beagle (a dog frequently used in experimental studies), DISH was not found.
Table 2.
Prevalence of DISH (number (%; 95% confidence interval) in eight large dog breeds (>25 kg) of which at least 60 dogs were available for screening
| Breed | N | DISH |
|---|---|---|
| Labrador Retriever | 244 | 6 (2.5; 0.5–4.4) |
| Bernese Mountain dog | 199 | 4 (2.0; 0.1–4.0) |
| Golden Retriever | 156 | 6 (3.9; 0.8–6.9) |
| Rottweiler | 120 | 2 (1.7; −0.6–4.0) |
| German Shepherd | 113 | 8 (7.1; 2.4–11.8) |
| Boxer | 69 | 28 (40.6; 29.0–52.2) |
| Bouvier des Flandres | 63 | 4 (6.4; 0.3–12.4) |
| Flatcoated Retriever | 61 | 8 (13.1; 4.6–21.6) |
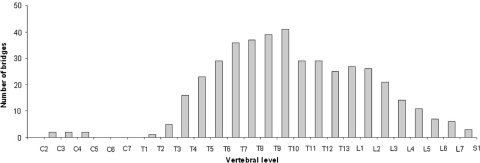
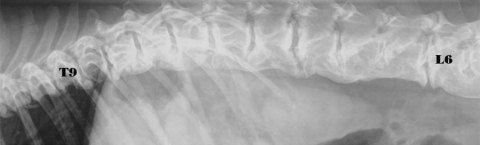
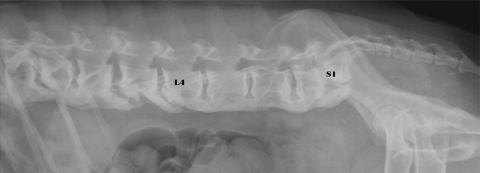
DISH was predominantly localized in the thoracolumbar spine (Fig. 1). Two dogs showed DISH in the cervical spine, 52 dogs in the thoracic spine, 22 dogs in the thoracolumbar spine (Fig. 2) and two dogs in the lumbar spine (Fig. 3).
Fig. 1.
Distribution of bridging ossifications of the spinal longitudinal ligament in 78 dogs with DISH
Fig. 2.
Radiograph of an 8-year-old neutered female Boxer with DISH at T9-L7
Fig. 3.
Radiograph of an 8-year-old neutered male Boxer with DISH at L4-S1
Univariate analysis on parameters age, gender, neutering status and breed resulted in a statistically significant positive odds ratio of 1.22 for age only (95% CI 1.14–1.31, p = 0.00). The odds ratio’s for gender of 1.31 (95% CI 0.83–2.07, p = 0.25) and neutering status of 1.09 (95% CI 0.69–1.71, p = 0.72) were not significant (p > 0.05). The odds ratio for the most affected dog breeds was investigated and univariate analysis resulted in a significant positive odds ratio for the dog breed Boxer of 26.25 (95% CI 15.05–45.80, p = 0.00).
The parameters significantly related to DISH in the univariate analysis were subsequently analyzed in a multiple logistic regression model. This model showed that age (1.32, 95% CI 1.21–1.44) and dog breed Boxer (51.27, 95% CI 26.57–98.93) were significantly related to the presence of DISH (p = 0.00 for all parameters).
Discussion
Similar to humans, male dogs were more frequently affected by DISH than female dogs and the overall prevalence increased with age. A genetic background of DISH in humans was suggested to underlie the difference of the prevalence in ethnic groups with Caucasians being most frequently affected, compared to native Africans, Afro-Americans and Asians [6, 7, 13, 34]. The Boxer breed, like many inbred dog breeds, represents a closed gene pool with a high degree of familiar relationships [25, 26]. The high prevalence of a specific disease in a certain dog breed and absence of this disease in other breeds, is suggestive of a genetic mechanism [24]. The high prevalence of DISH in the Boxer breed may allow for the study of DISH in a spontaneous disease animal model. With the present knowledge of the sequenced canine (Boxer) genome [19, 25, 26], future studies could focus on the elucidation of the gene or genes involved in the pathogenesis of DISH using association studies with single nucleotide polymorphisms at high density [12].
When using the dog as an animal model for spinal research some biomechanical issues are important to consider. For example the direction of the gravity load on the spine in quadruped dogs is not the same as in bipedal humans and therefore the loading on the human spine is commonly assumed to be larger than that in quadrupeds. However, biomechanical measurements show the contrary. The muscle contraction forces and tension structures such as ligaments and intervertebral discs in the quadruped spine add substantially to the axial loading on the spine [36] which is actually larger in quadrupeds than in bipeds [1, 31]. This may limit how we extrapolate the results of canine experiments to humans [1]. The canine spine is loaded along its long axis just like the human spine [31]. The dog may serve as a valuable animal model for spinal research but, as with all animal models, care must be taken when transferring the results to the human situation [1, 30, 31].
The segmental distribution of DISH in the spine in our cohort of dogs showed great similarities to that in humans. Resnick and Niwayama reported in their study of 215 cadaveric spines and 100 patients with spinal manifestations of DISH that the 7th to 10th thoracic vertebrae were most frequently affected, although lumbar involvement was also present. In addition, they noted a lower incidence in the most cranial thoracic vertebrae [2]. In the present study in dogs, DISH was found in similar locations as in humans. The caudal thoracic vertebrae and, to a lesser degree, the cranial lumbar vertebrae were most frequently affected in dogs.
No studies on DISH in dogs have been reported other than the case reports of Morgan and Stavenborn and Woodard et al. Both dogs were eventually euthanized because of extreme stiffness and pain of the spine and joints refractory to medical treatment [22, 37]. The bone formation along the spine in DISH probably originates from ossification/calcification of the anterior (or, in quadrupeds, ventral) longitudinal ligament whereas spondylosis deformans originates from the endplates of vertebral bodies. Spondylosis deformans is a common disorder in dogs and prevalences of spondylosis deformans ranging from 26 to 50% in Boxers have been published [5, 17]. The radiographic differentiation of both conditions, however, remains difficult in severe cases [27]. Morgan and Stavenborn suggested that DISH in dogs closely resembled extensive spondylosis deformans. In retrospect, it may have been more appropriate to use the term DISH for some of the severe cases of spondylosis deformans reported in dogs [22].
In conclusion, the current radiographic study showed the presence of DISH in some dog breeds and demonstrated that its prevalence increased with age and was higher in male dogs. Considering the unusually high prevalence of DISH in the Boxer breed, this animal may serve as a spontaneous disease animal model for DISH in humans. Furthermore, our results are indicative of a genetic origin of canine DISH. Future research may be directed at the elucidation of the gene(s) involved in the pathogenesis of this disorder.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An HS, Masuda K. Relevance of in vitro and in vivo models for intervertebral disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):88–94. doi: 10.2106/JBJS.E.01272. [DOI] [PubMed] [Google Scholar]
- 3.Belanger TA, Rowe DE. Diffuse idiopathic skeletal hyperostosis: musculoskeletal manifestations. J Am Acad Orthop Surg. 2001;9:258–267. doi: 10.5435/00124635-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Callahan EP, Aguillera H. Complications following minor trauma in a patient with diffuse idiopathic skeletal hyperostosis. Ann Emerg Med. 1993;22:1067–1070. doi: 10.1016/S0196-0644(05)82754-4. [DOI] [PubMed] [Google Scholar]
- 5.Carnier P, Gallo L, Sturaro E, et al. Prevalence of spondylosis deformans and estimates of genetic parameters for the degree of osteophytes development in Italian Boxer dogs. J Anim Sci. 2004;82:85–92. doi: 10.2527/2004.82185x. [DOI] [PubMed] [Google Scholar]
- 6.Cassim B, Mody GM, Rubin DL. The prevalence of diffuse idiopathic skeletal hyperostosis in African blacks. Br J Rheumatol. 1990;29:131–132. doi: 10.1093/rheumatology/29.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Childs SG. Diffuse idiopathic skeletal hyperostosis: Forestier’s disease. Orthop Nurs. 2004;23:375–382. doi: 10.1097/00006416-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council . Ad Hoc Committee on Dog and Cat Nutrition. Nutrient requirements of dogs and cats. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 9.de Bruin C, Meij BP, Kooistra HS, et al. Cushing’s disease in dogs and humans. Horm Res. 2009;71(Suppl 1):140–143. doi: 10.1159/000178058. [DOI] [PubMed] [Google Scholar]
- 10.Forestier J, Rotes-Querol J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis. 1950;9:321–330. doi: 10.1136/ard.9.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.German AJ. The growing problem of obesity in dogs and cats. J Nutr. 2008;136:1940S–1946S. doi: 10.1093/jn/136.7.1940S. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson EK, Lindblad-Toh K. Leader of the pack: gene mapping in dogs and other model organisms. Nat Rev Genet. 2008;9:713–725. doi: 10.1038/nrg2382. [DOI] [PubMed] [Google Scholar]
- 13.Kim SK, Choi BR, Kim CG, et al. The prevalence of diffuse idiopathic skeletal hyperostosis in Korea. J Rheumatol. 2004;31:2032–2035. [PubMed] [Google Scholar]
- 14.Kiss C, O’Neill TW, Mituszova M, et al. The prevalence of diffuse idiopathic skeletal hyperostosis in a population-based study in Hungary. Scand J Rheumatol. 2002;31:226–229. doi: 10.1080/030097402320318422. [DOI] [PubMed] [Google Scholar]
- 15.Kiss C, Szilagyi M, Paksy A, et al. Risk factors for diffuse idiopathic skeletal hyperostosis: a case–control study. Rheumatology (Oxford) 2002;41:27–30. doi: 10.1093/rheumatology/41.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Kooistra HS, Galac S, Buijtels JJ, et al. Endocrine diseases in animals. Horm Res. 2009;71(Suppl 1):144–147. doi: 10.1159/000178059. [DOI] [PubMed] [Google Scholar]
- 17.Langeland M, Lingaas F. Spondylosis deformans in the boxer: estimates of heritability. J Small Anim Pract. 1995;36:166–169. doi: 10.1111/j.1748-5827.1995.tb02872.x. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Jiang LS, Dai LY. Hormones and growth factors in the pathogenesis of spinal ligament ossification. Eur Spine J. 2007;16:1075–1084. doi: 10.1007/s00586-007-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 20.Mader R, Dubenski N, Lavi I. Morbidity and mortality of hospitalized patients with diffuse idiopathic skeletal hyperostosis. Rheumatol Int. 2005;26:132–136. doi: 10.1007/s00296-004-0529-y. [DOI] [PubMed] [Google Scholar]
- 21.Mata S, Fortin PR, Fitzcharles MA, et al. A controlled study of diffuse idiopathic skeletal hyperostosis. Clinical features and functional status. Medicine (Baltimore) 1997;76:104–117. doi: 10.1097/00005792-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Morgan JP, Stavenborn M. Disseminated idiopathic skeletal hyperostosis (DISH) in a dog. Vet Radiol Ultrasound. 1991;32:65–70. doi: 10.1111/j.1740-8261.1991.tb00082.x. [DOI] [Google Scholar]
- 23.Olivieri I, D’Angelo S, Cutro MS, et al. Diffuse idiopathic skeletal hyperostosis may give the typical postural abnormalities of advanced ankylosing spondylitis. Rheumatology (Oxford) 2007;46:1709–1711. doi: 10.1093/rheumatology/kem227. [DOI] [PubMed] [Google Scholar]
- 24.Ostrander EA, Galibert F, Patterson DF. Canine genetics comes of age. Trends Genet. 2000;16:117–124. doi: 10.1016/S0168-9525(99)01958-7. [DOI] [PubMed] [Google Scholar]
- 25.Ostrander EA, Wayne RK. The canine genome. Genome Res. 2005;15:1706–1716. doi: 10.1101/gr.3736605. [DOI] [PubMed] [Google Scholar]
- 26.Parker HG, Ostrander EA. Canine genomics and genetics: running with the pack. PLoS Genet. 2005;1:e58. doi: 10.1371/journal.pgen.0010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resnick D. Degenerative diseases of the vertebral column. Radiology. 1985;156:3–14. doi: 10.1148/radiology.156.1.3923556. [DOI] [PubMed] [Google Scholar]
- 28.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH) Radiology. 1976;119:559–568. doi: 10.1148/119.3.559. [DOI] [PubMed] [Google Scholar]
- 29.Savolainen P, Zhang Y, Luo J, et al. Genetic evidence for an East Asian origin of domestic dogs. Science. 2002;298:1610–1613. doi: 10.1126/science.1073906. [DOI] [PubMed] [Google Scholar]
- 30.Schimandle JH, Boden SD. Spine update. Animal use in spinal research. Spine. 1994;19:2474–2477. doi: 10.1097/00007632-199411000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Smit TH. The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J. 2002;11:137–144. doi: 10.1007/s005860100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sreedharan S, Li YH. Diffuse idiopathic skeletal hyperostosis with cervical spinal cord injury—a report of 3 cases and a literature review. Ann Acad Med Singapore. 2005;34:257–261. [PubMed] [Google Scholar]
- 33.Vilà C, Savolainen P, Maldonado J, et al. Multiple and ancient origins of the domestic dog. Science. 1997;276:1687–1689. doi: 10.1126/science.276.5319.1687. [DOI] [PubMed] [Google Scholar]
- 34.Weinfeld RM, Olson PN, Maki DD, et al. The prevalence of diffuse idiopathic skeletal hyperostosis (DISH) in two large American Midwest metropolitan hospital populations. Skeletal Radiol. 1997;26:222–225. doi: 10.1007/s002560050225. [DOI] [PubMed] [Google Scholar]
- 35.Westerveld LA, van Ufford HM, Verlaan JJ, et al. The prevalence of diffuse idiopathic skeletal hyperostosis in an outpatient population in The Netherlands. J Rheumatol. 2008;35:1635–1638. [PubMed] [Google Scholar]
- 36.Wilke H, Rohlmann A, Neller S, et al. ISSLS prize winner: a novel approach to determine trunk muscle forces during flexion and extension: a comparison of data from an in vitro experiment and in vivo measurements. Spine. 2003;28:2585–2593. doi: 10.1097/01.BRS.0000096673.16363.C7. [DOI] [PubMed] [Google Scholar]
- 37.Woodard JC, Poulos PW, Jr, Parker RB, et al. Canine diffuse idiopathic skeletal hyperostosis. Vet Pathol. 1985;22:317–326. doi: 10.1177/030098588502200404. [DOI] [PubMed] [Google Scholar]