Abstract
Bone marrow stem cells (BMSCs) can be obtained from the vertebral body (VB) and iliac crest (IC) for augmenting spinal arthrodesis. However, it is still not evaluated, which of the two sites would have a better BMSCs potential on Proliferation and osteoblastic differentiation is still not evaluated. Fourteen patients (10 men and 4 women) undergoing posterolateral lumbar arthrodesis and pedicle screw instrumentation were involved. The mean age was 54.7 years (range 31–75 years). Bone marrow aspirates were obtained from the vertebral body through the bilateral pedicle and were quantified relative to matched, bilateral aspirates from the iliac crest that were obtained from the same patient and at the same time. The mononuclear cell count and concentration of BMSCs were calculated and compared. Proliferation and osteoblastic differentiation of each of the BMSCs were characterized using biochemical and molecular biology techniques. Concentration (cells/mL) of BMSCs from VB and IC were 3.73 × 103 and 3.19 × 103, respectively (P > 0.05). VB and IC exhibited similar proliferation pattern at 3, 5 and 7 days, but BMSCs from the VB exhibited an increased mineralization staining with Alizarin Red S at 14 days. BMSCs from both anatomic sites expressed comparable levels of CD29, CD34, CD44, CD90 and CD105. VB and IC displayed similar levels of expression of ALP, type I collagen and osterix, but VB expressed higher level of osteocalcin and Runx-2, especially at 14 and 21 days. Our studies show that BMSCs from VB have osteogenic differentiation potential similar to IC. Based on these findings, we suggest that BMSCs from VB would be comparable candidates for osseous graft supplementation especially in spinal fusion procedures.
Keywords: Vertebral body, Iliac crest, Bone marrow stem cells, Proliferation, Osteoblastic differentiation
Introduction
Spinal fusion surgery is yet the standard treatment for severe spinal deformity, spinal instability following spinal decompression, spondylolisthesis and some cases of degenerative disease. Autogenous bone graft harvested from iliac crest (IC) generally serves as the preferred method to obtain solid spinal fusion. However, harvesting of autograft is often associated with complications such as increased operative time, blood loss, infection and chronic pain at the donor site [5, 26]. Recently, numerous methods have been developed for substituting autogenous bone graft. Among these, a combination of bone extenders, such as ceramics with bone marrow, has shown significant osteogenic potential in vitro and in vivo [4, 25]. Moreover, there has been a considerable clinical interest in the use of adult mesenchymal stem cells (MSCs) from bone marrow for osseous graft supplementation therapy. Adult MSCs are used to enhance the repair of a wide range of diseased or traumatized human tissues [8, 9, 21]. When cultured at low density in the presence of specific medium supplements, these cells can commit to a number of well-defined lineages such as osteoblasts, chondrocytes, adipocytes or myoblasts [10, 12, 23, 27]; recent work suggests that MSCs may also acquire a neuronal phenotype [24]. Muschler et al. demonstrated that autologous connective-tissue progenitor cells, the osteogenic stem cell precursors, can be harvested in great numbers from the IC marrow and concentrated on allograft cancellous matrix to form a suitable graft substitute [14, 17, 18]. The capacity of this composite material to stimulate arthrodesis has been demonstrated in both animal and clinical models [2, 6, 19]. Several other investigations have shown that vertebral body (VB) bone marrow contains stem cells, which can initiate osteogenesis [1]. The VB bone marrow also has a higher number of colony-forming unit-fibroblastic (CFU-F) and progenitor cell concentration than the IC bone marrow [16, 20]. The purpose of this study was to determine and compare proliferation and osteoblastic differentiation of bone marrow stem cells from VB and IC. This enables us to estimate which of the stem cells are more useful for increasing bone fusion, especially in spinal surgery.
Materials and methods
Subjects
Fourteen adult patients (male = 10; female = 4) older than 21 years of age (mean: 54.7, range: 31–75) were involved in the study. These subjects were diagnosed with spinal stenosis (n = 9), spondylolisthesis (n = 1), recurrent herniated nucleus pulposus (n = 1), posttraumatic kyphosis (n = 1), bursting fracture (n = 1) and adjacent segment degeneration after previous lumbar instrumentation (n = 1). These individuals were scheduled for posterior spinal surgical procedures (n = 14) in which VB and IC bone marrow were harvested with application of spinal instrumentation. The rationale for the investigation and the accompanying risks were discussed with each patient, and an informed consent form approved by the Institutional Review Board of the Kyungpook National University Hospital was signed. Patients with a history of myelogenous disease, or a current history of metastatic neoplasm or infections were excluded from the study. No exclusion criteria regarding gender or race were used.
Marrow harvest and collection
Bone marrow was aspirated from the lumbar VB and the IC (average volume: VB/IC 4.2/4.6 mL, respectively) of the same patient using a marrow aspiration needle. To maximize the number of harvested osteoprogenitor stem cells, 2-mL samples of marrow were collected at sites of 1 cm distance from each other along the IC [17]. For VB bone marrow aspiration, during posterior spinal procedures, the marrow aspiration needle was used to cannulate the pedicles of accessible lumbar vertebral bodies. In this way, each sample collected from the VB and the IC of the same patients were analyzed in a paired fashion (VB vs. IC). Harvested marrow was collected in polypropylene tubes (Franklin Lakes, NJ, USA) containing dipotassium EDTA as an anticoagulant. Tubes were inverted to mix the marrow with the EDTA. Samples were placed immediately on ice and then processed to isolate BMSCs.
Cell isolation and culture
Bone marrow was spun through histopaque (Sigma; St. Louis, MO, USA) gradient centrifugation. After centrifugation, bone marrow mononuclear cells were collected and then washed twice with phosphate buffered saline (PBS, pH 7.4). The bone marrow mononuclear cells were suspended in α-minimum essential medium (α-MEM) (Gibco-BRL, USA) containing 10% fetal bovine serum (FBS) (Gibco-BRL, USA) and incubated at 37°C in a humidified atmosphere of 5% CO2 for 12 h [14]. Non-adherent cells were removed and adherent cells that contain BMSCs were cultured and expanded for further experiments [11]. To induce osteoblastic differentiation, BMSCs were cultured in an osteogenic media (α-MEM supplemented with 10% FBS, 50 μg/mL α-ascorbic acid, 10 mM β-glycerophosphate and 100 nM dexamethasone and antibiotics). BMSCs under the passage 3 were used in the following experiments. The culture media was changed every 3 days.
Estimation of stem cell concentration
Bone marrow mononuclear cells were suspended in α-minimum essential medium (α-MEM) (Gibco-BRL, USA) containing 10% fetal bovine serum (FBS) (Gibco-BRL, USA) and incubated at 37°C in a humidified atmosphere of 5% CO2 for 12 h. Non-adherent cells were removed and adherent cells that contain BMSCs were cultured until 80–90% confluent (passage 1, culture period average, VB: 8.8 days; IC: 9.4 days). Considering the average population doubling time of 36 h, BMSC concentrations (number of stem cells per 1.0 mL of aspirate) were calculated [28].
Analysis of proliferation of BMSCs
BMSCs were seeded into 96-well plates at a density of 700 cells per well. Cells were cultured in α-MEM supplemented with 10% FBS for 3, 5 and 7 days, and proliferation was measured by proliferation assay kit (Promega, Madison, USA) as described in the manufacture’s instruction. The optical densities were measured at 490 nm using an ELISA reader (BioRad; Hercules, CA, USA) [11].
Flow cytometric analysis
BMSCs were harvested in 0.25% trypsin/EDTA and incubated in 1% BSA containing 100 μL monoclonal antibodies to CD29, CD34, CD44, CD90 and CD105 for 30 min at 4°C. The cells were centrifuged at 1,200×g for 5 min and washed with 1% BSA. The resuspended cells were fixed for 30 min in ice-cold 4% formaldehyde. Following fixation, the cells were washed with 1% BSA [3, 13]. Samples were analyzed using FACScan argon laser cytometer (Becton Dickson, San Jose, CA, USA).
RT-PCR analysis
Briefly, total RNA was extracted from BMSCs cultured for 0, 7, 14 and 21 days in osteogenic medium, using Trizol reagent (Invitrogen); 1 μg of total RNA was reverse transcribed into cDNA using Superscript II RT enzyme (Invitrogen) and random hexamers. For PCR, 1 μL of cDNA template was used for each reaction and sequences were amplified using Taq DNA polymerase (SolGent) [8, 15]. Primers for osteoblastic differentiation-associated genes and GAPDH were custom designed (Table 1) and synthesized by Integrated DNA Technologies (Coralville, IA, USA). Products were electrophoresed on a 1% agarose gel and the image was analyzed by a photodocumentation system (ChemiImager5500, Alpa Innotech, Miami, FL, USA).
Table 1.
Primers used for RT-PCR analysis
| Gene | Sequence |
|---|---|
| ALP | 5′-GCGAACGTATTTCTCCAGACCCAG-3′ |
| 5′-TCCAAACAGGAGAGTCGCTTCAA-3′ | |
| COL I | 5′-ATCCGCAGTGGCCTCCTAAT-3′ |
| 5′-TCCCCTCACCCTCCCAGTAT-3′ | |
| OC | 5′-CTGGCCCTGACTGCATTCTGC-3′ |
| 5′-AACGGTGGTGCCATAGATGCG-3′ | |
| Ostreix | 5′-GCAGCTAGAAGGGAGTGGTG-3′ |
| 5′-GCAGGCAGGTGAACTTCTTC-3′ | |
| Runx2 | 5′-CCGCACGACAACCGCACCAT-3′ |
| 5′-CGCTCCGGCCCACAAATCTC-3′ | |
| GAPDH | 5′-CCACTGGCGTCTTCACCAC-3′ |
| 5′-CCTGCTTCACCACCTTCTTG-3′ |
Alizarin Red assay
BMSCs were plated at a density of 2 × 104 cells/well in a 24-well plate and cultured in α-MEM containing osteogenic supplements (10 nmol/L dexamethasone, 50 μg/mL ascorbate 2-phosphate, and 10 mmol/L β-glycerophosphate) for 14 days. The medium was changed every 3 days. To detect mineral, cells were fixed with 10% formalin, washed with PBS (pH 7.4) and stained with Alizarin Red S, pH 4.2 for 15 min. Stained cells were photographed. Osteogenic differentiation was quantified by quantitative destaining procedure using 10% (w/v) cetylpyridinium chloride (CPC) in 10 mM sodium phosphate, pH 7.0 for 15 min at room temperature [22]. The Alizarin Red S amount was determined by absorbance measurement at 570 nm using an ELISA reader (BioRad; Hercules, CA, USA).
Statistical analysis
Data were analyzed by Student’s t test using SPSS 13.0. Corresponding P values were considered significant at values less than 0.05.
Results
Mononuclear cell number, concentration of BMSCs and proliferation activity
The number of mononuclear cells per 1.0 mL of aspirates was slightly higher in the IC bone marrow as compared to paired VB bone marrow, but the differences were not significant (P > 0.05). A mean of 3.65 × 107 mononuclear cells (range, 0.4 × 107 to 8.4 × 107 cells) were contained in each 1.0 mL of VB aspirated compared with 3.97 × 107 (range 1.0 × 107 to 9.2 × 107 cells) for IC aspirates (Table 2).
Table 2.
Summary of data from 14 samples of bone marrow: vertebral body (VB) and iliac crest (IC)
| Patients | Sex | Age | Clinical phenotype | BM volume (mL) (VB/IC) | BMM cells/mL of BM (107) (VB/IC) | Total stem cells at P1 (106) (VB/IC) | Culture period for P1 (days) (VB/IC) | Concentration of BMSCs (103/mL) (VB/IC) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 75 | ASD | 4.0/4.0 | 4.5/5.6 | 1.28/0.70 | 10/13 | 2.50/0.34 |
| 2 | M | 52 | SS | 4.5/4.0 | 4.0/4.0 | 0.84/1.20 | 8/9 | 5.83/4.69 |
| 3 | M | 34 | SS | 4.8/4.8 | 8.4/9.2 | 1.00/0.90 | 8/9 | 6.51/2.93 |
| 4 | M | 31 | HNP recur | 5.0/5.5 | 6.8/7.6 | 1.56/1.34 | 8/8 | 9.75/7.61 |
| 5 | F | 40 | PTK | 4.0/5.4 | 5.6/4.4 | 1.00/1.12 | 7/8 | 7.81/6.48 |
| 6 | M | 38 | SS | 4.0/4.0 | 4.5/4.0 | 1.00/1.05 | 8/8 | 5.80/8.20 |
| 7 | M | 72 | SS | 4.0/4.0 | 3.0/2.5 | 0.50/0.60 | 9/12 | 1.95/0.59 |
| 8 | M | 47 | Bursting fracture | 4.0/4.0 | 3.6/2.8 | 0.90/0.90 | 9/8 | 3.51/7.03 |
| 9 | M | 66 | SS | 5.0/6.0 | 0.5/2.3 | 0.75/1.00 | 11/10 | 1.17/1.30 |
| 10 | F | 67 | SS | 4.3/3.3 | 1.0/1.0 | 0.32/0.50 | 11/11 | 0.58/1.18 |
| 11 | M | 66 | SS | 4.3/3.5 | 4.4/3.5 | 1.42/0.80 | 10/14 | 2.58/0.45 |
| 12 | M | 55 | SL | 3.5/5.5 | 0.4/1.5 | 0.01/0.56 | 11/10 | 0.02/0.80 |
| 13 | F | 64 | SS | 4.0/4.0 | 1.9/1.0 | 0.29/0.24 | 10/10 | 0.57/0.47 |
| 14 | M | 58 | SS | 4.0/7.0 | 2.5/6.2 | 0.47/0.58 | 8/8 | 3.67/2.59 |
BM bone marrow, BMM bone marrow mononuclear, BMSCs bone marrow stem cells, ASD adjacent segment degeneration, SS spinal stenosis, HNP herniated nucleus pulposus, PTK posttraumatic kyphosis, P1 passage 1
The concentration of BMSCs (cells/mL) was calculated for each sample as the product of total stem cells at P1 divided by culture period for P1, considering doubling time of 36 h. Aspirates of VB bone marrow demonstrated similar or greater concentrations of BMSCs compared with aspirates from IC. Concentration of BMSCs from VB and IC are 3.73 × 103 (range 0.02 × 103 to 9.75 × 103 cells/mL) and 3.19 × 103(range 0.34 × 103 to 8.20 × 103 cells/mL), respectively (P > 0.05).
In only five patients, the concentrations of BMSCs in the IC aspirates were higher than that in the VB aspirates. Interestingly, five patients (patient no 1, 7, 9, 10, 13) who are relatively older (over 65 years) demonstrated very low concentration of BMSCs in both VB and IC bone marrows.
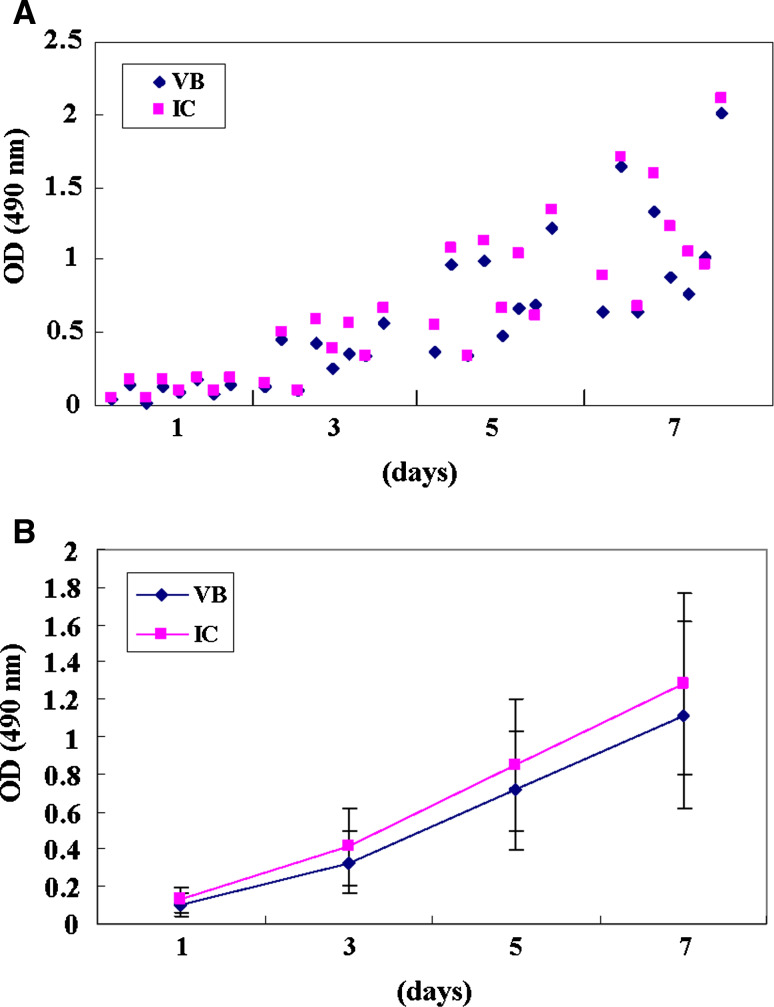
Proliferations of BMSCs at 3, 5 and 7 days were of very similar pattern in both VB and IC bone marrow. The same cell numbers were seeded on 1 day, and OD at 3, 5 and 7 days were nearly of the same absorbance in both groups (Fig. 1).
Fig. 1.
Proliferation analysis of both BMSCs from VB and IC. a Proliferations of BMSCs at 3, 5 and 7 days were of very similar pattern in both VB and IC bone marrow. b OD (VB/IC, mean ± SD) at 1, 3, 5 and 7 days are 0.10 ± 0.06/0.13 ± 0.06, 0.33 ± 0.16/0.41 ± 0.21, 0.71 ± 0.32/0.85 ± 0.35, 1.12 ± 0.50/1.28 ± 0.49, respectively
Expression levels of surface markers of stem cells
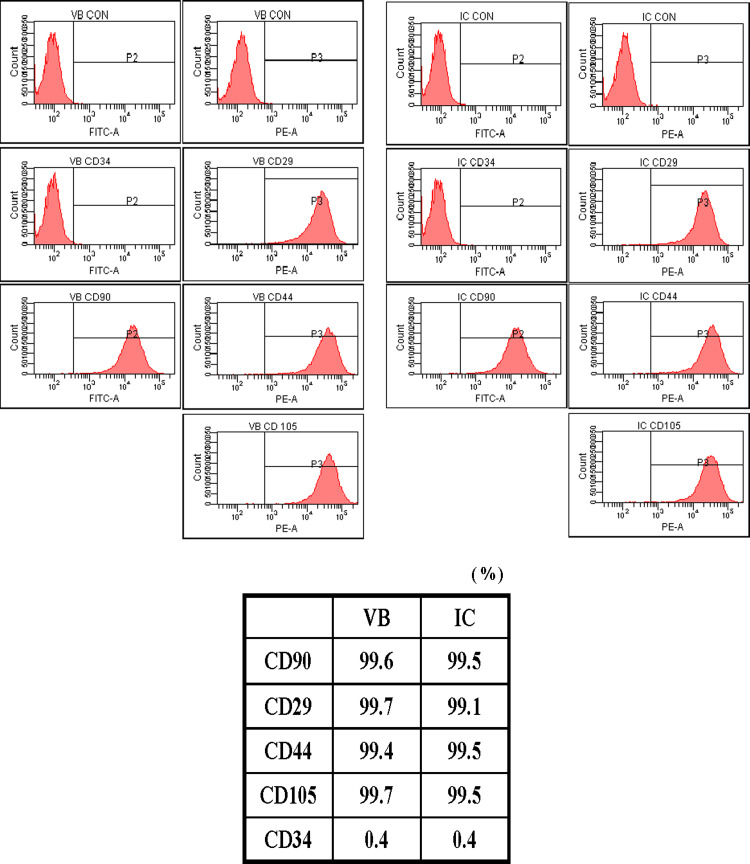
We examined the expression levels of stem cell markers of BMSCs using flow cytometry. Expression of CD29, CD44, CD90 and CD105 of BMSCs from both VB and IC were positive with more than 99% in expression levels (Fig. 2). All groups were CD34 (marker of hematopoietic cells) negative, which precluded contamination by hematopoietic cells. The percentage of BMSCs expressing CD29, CD44, CD90 and CD105 in VB and IC was comparable (n = 14, P > 0.05).
Fig. 2.
Flow cytometric analysis of BMSCs isolated from the marrow of VB and IC. Expression of CD29, CD44, CD90 and CD105 of BMSCs from both VB and IC were positive with more than 99% of expression levels. The majority of cells (>99%) were CD34 negative. Note that BMSCs from VB exhibit cell surface markers similar to those of IC cells (P > 0.05)
Evidence of mineral formation
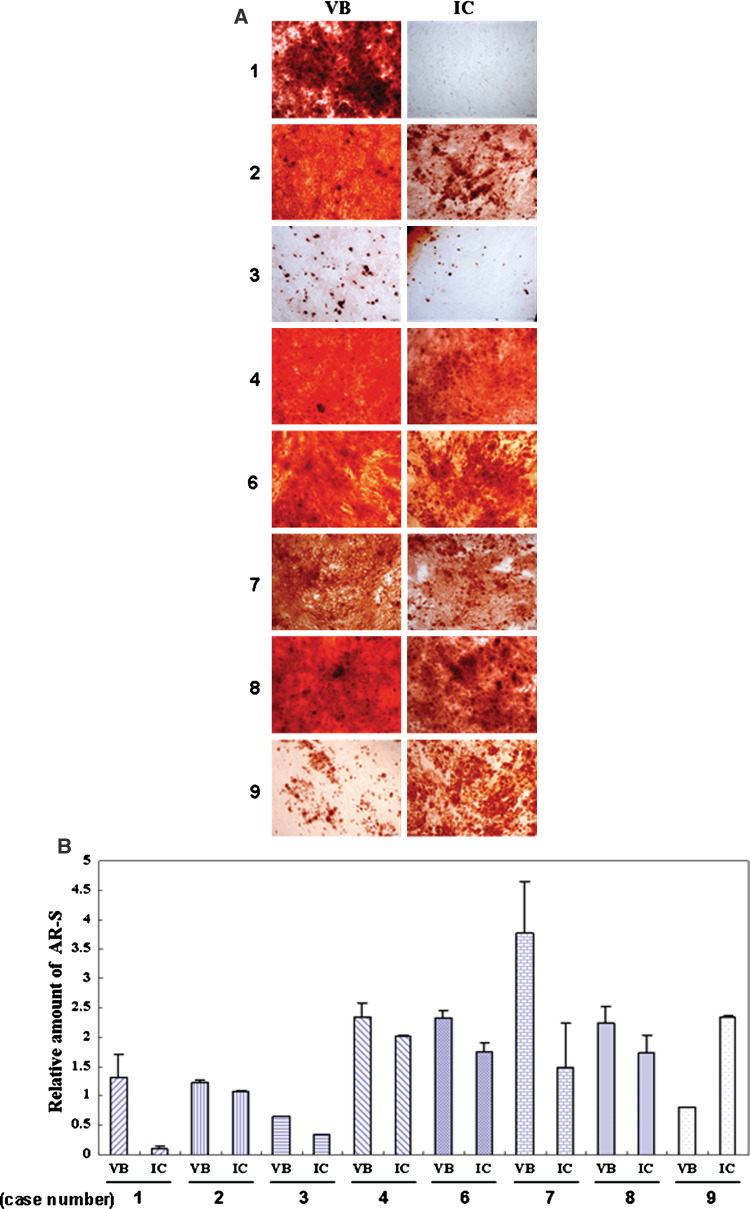
BMSCs isolated from the VB and the IC were maintained in culture supplemented with dexamethasone, β-glycerophosphate and ascorbic acid for 14 days. When stained with Alizarin Red S, positive mineral deposits were apparent. BMSCs from VB bone marrow showed higher mineral accumulation compared with BMSCs from the IC (Fig. 3, P = 0.001).
Fig. 3.
a Mineral deposition by BMSCs derived from the VB and the IC (8 cases). BMSCs isolated from the VB and the IC were maintained in culture supplemented with dexamethasone, β-glycerophosphate and ascorbic acid for 14 days. b When stained with Alizarin Red S, BMSCs from VB bone marrow showed higher mineral accumulation compared with BMSCs from the IC (case number 1, 2, 3, 4, 6, 7, 8). Original magnification ×100. (AR-S; Alizarin Red S)
Measurement of osteogenic gene expression
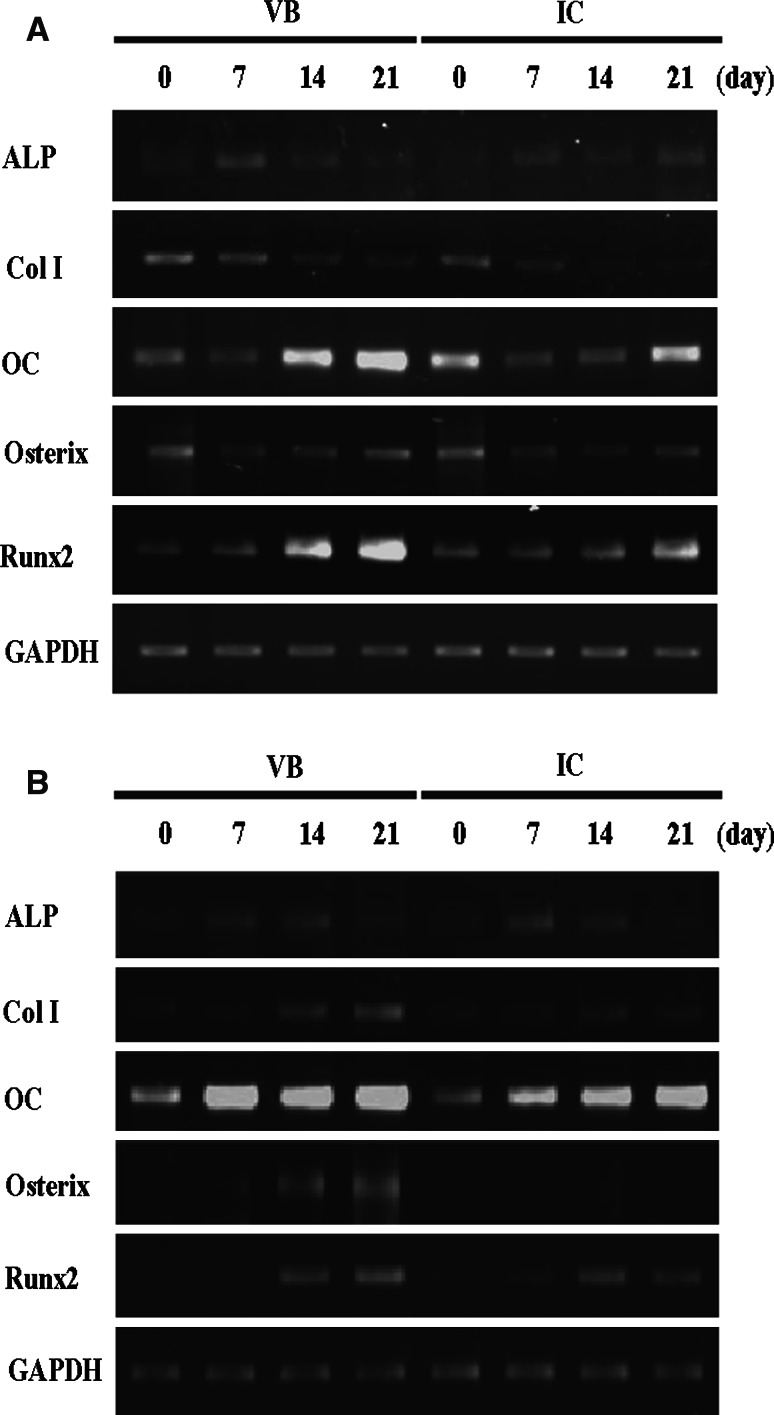
RT-PCR analysis was conducted on representative two cases (case number 2 and 8) of selected genes expressed by VB and IC BMSCs under osteogenic conditions. The expression of ALP, type I collagen and osterix in BMSCs isolated from lumbar VB was similar to IC under basal as well as osteogenic conditions. The osteogenic medium supplementation enhanced higher expression levels of osteocalcin and Runx-2 in BMSCs derived from VB, especially at 14 and 21 days, compared with those of IC (Fig. 4).
Fig. 4.
RT-PCR analysis (representative 2 cases, case number 2 and 8) of selected genes expressed by VB and IC BMSCs under osteogenic conditions. The osteogenic medium supplementation enhanced higher expression levels of osteocalcin and Runx-2 in BMSCs derived from VB, especially at 14 and 21 days, compared to those of IC
Discussion
The purpose of this investigation was to compare proliferation and osteoblastic differentiation potential of VB BMSCs with those of IC. Characteristics of VB bone marrow have not been well quantified and have not previously been considered to be a routine source of augmenting bone graft material. Muschler et al. reported that aspiration volume affects the number and concentration of osteoblast progenitor cells in marrow aspirates [17]. They also reported that an aspiration volume of 2 mL allows the highest concentration of osteoblast progenitor cell. Therefore, we tried to collect a 2-mL bone marrow aspiration volume for each of the aspiration sites. Moreover, determining the capacity of the bone marrow to augment bone fusion is the richness of BMSCs among mononuclear cells, which can be differentiated into osteoblasts, because not all mononuclear cells have the capability of differentiating into osteoblast.
Our studies show that the VB bone marrow contains a lower number of mononuclear cells on average, but a slightly higher concentration of BMSCs. This result is in agreement with recent studies that show a higher prevalence of CFU-F in VB marrow than IC or peripheral blood, suggesting a higher frequency of progenitor cells [1, 20]. But, we cannot find any significant statistical differences regarding concentration and proliferation potential of BMSCs at 3, 5 and 7 days (P > 0.05).
We also examined the osteogenic characteristics of VB BMSCs cultured in the presence of dexamethasone, β-glycerophosphate and ascorbic acid. Surprisingly, we found equivalent volumes of unprocessed VB marrow in culture, this provided an elevated number of plastic adherent cells reached early confluence and higher mineralization with Alizarin Red S at 14 days. It is possible that the higher mineralization of VB BMSCs under osteogenic media may be the result of rapid differentiation of VB marrow cells into osteoblast. The apparent increase in staining of the VB cell cultures also supports that these cells would undergo more rapid osteogenesis in vivo. If this is the case, it would give us strong support to the clinical concept that cells derived from lumbar VB could be effectively used to augment bone fusion, especially during spinal surgery, as much as IC.
Flow cytometric analysis revealed that BMSCs from VB and IC had similar profile as surface markers of stem cells. Expression of CD29, CD44, CD90 and CD105 of BMSCs from both VB and IC were positive with more than 99% of expression levels. A representative flow cytometric analysis of VB and IC BMSCs is presented in Fig. 2. All groups were CD34 negative, which precluded contamination by hematopoietic cells. The percentage of BMSCs expressing CD29, CD44, CD90 and CD105 in VB and IC was comparable (n = 14, P > 0.05). However, because surface antigen expression is influenced by the number of variables, functional in vitro and in vivo assays for BMSCs will be required to confirm that VB BMSCs have some differences compared to other BMSCs.
To define the differences of osteoblastic differential potential of both BMSCs, osteogenic gene expression was studied using RT-PCR. VB BMSCs proved the expression of ALP, type I collagen and osterix and was similar to that of IC under basal as well as osteogenic conditions. Interestingly, the osteogenic medium supplementation enhanced higher expression levels of osteocalcin and Runx-2 in BMSCs derived from VB, especially at 14 and 21 days, compared to those of IC. Since osteocalcin is an important regulator of the mineralization process [7] and Runx-2 serves as a critical bone cell transcription factor, higher expression of these genes seems to be related with higher mineralization and VB cells have more likelihood for osteogenic potential.
In conclusion, VB bone marrow could provide sufficient BMSC volumes and comparable osteoblastic differentiation potential compared with IC, thus effectively providing an additional source of stem cells for graft augmentation during spinal fusion surgery. The potential benefit of this finding would be to eliminate the need for IC harvest in many cases and to provide an additional source of BMSCs both for patients requiring extensive grafting and for those with limited cancellous volume within the IC. Moreover, the operations that naturally provide access to the vertebral bone marrow, especially spinal instrumentation using pedicle screws, depend on fusion for clinical success, so VB BMSCs would be of great clinical interest to spine surgeons.
Acknowledgments
This research was supported by Musculoskeletal Bioorgans Center (0405-B001-0204-0006) of the Ministry of Health and Welfare, the Ministry of Knowledge Economy (MKE), Korea Institute for Advancement of Technology (KIAT) and Dae-Gyeong Leading Industry Office through the Leading Industry Development for Economic Region (2009-T-2-A-Y0-A-05) and a grant (SC4170) from Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea.
Abbreviations
- BMSCs
Bone marrow stem cells
- VB
Vertebral body
- IC
Iliac crest
- CD
Clust designation
- ALP
Alkaline phosphatase
- CFU-F
Colony-forming unit-fibroblastic
- DNA
Deoxynucleic acid
- RNA
Ribonucleic acid
- cDNA
Complementary DNA
- cRNA
Complementary RNA
- MEM
Minimum essential medium
- FBS
Fetal bovine serum
- FACS
Flow cytometric analysis scan
- ELISA
Enzyme-linked immunosorbent assay
- ASD
Adjacent segment degeneration
- SS
Spinal stenosis
- HNP
Herniated nucleus pulposus
- PTK
Posttraumatic kyphosis
References
- 1.Ahrens N, Tormin A, Paulus M, Roosterman D, Salama A, Krenn V, Neumann U, Scheding S. Mesenchymal stem cell content of human vertebral bone marrow. Transplantation. 2004;78:925–929. doi: 10.1097/01.TP.0000133305.81823.2A. [DOI] [PubMed] [Google Scholar]
- 2.Connolly JF, Guse R, Tiedeman J, Dehne R (1991) Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res 259–270 [PubMed]
- 3.Deschaseaux F, Gindraux F, Saadi R, Obert L, Chalmers D, Herve P. Direct selection of human bone marrow mesenchymal stem cells using an anti-CD49a antibody reveals their CD45med, low phenotype. Br J Haematol. 2003;122:506–517. doi: 10.1046/j.1365-2141.2003.04469.x. [DOI] [PubMed] [Google Scholar]
- 4.Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77:940–956. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Heary RF, Schlenk RP, Sacchieri TA, Barone D, Brotea C. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery. 2002;50:510–516. doi: 10.1097/00006123-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 7.Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317(Pt 1):59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. doi: 10.1002/(SICI)1097-4644(199702)64:2<295::AID-JCB12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. Curr Opin Biotechnol. 2004;15:406–410. doi: 10.1016/j.copbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Joyner CJ, Bennett A, Triffitt JT. Identification and enrichment of human osteoprogenitor cells by using differentiation stage-specific monoclonal antibodies. Bone. 1997;21:1–6. doi: 10.1016/S8756-3282(97)00074-4. [DOI] [PubMed] [Google Scholar]
- 11.Kafienach W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006;24:1113–1120. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Huang GT, Chiang H, Chiou LL, Chen MH, Hsieh CH, Jiang CC. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells. 2003;21:190–199. doi: 10.1634/stemcells.21-2-190. [DOI] [PubMed] [Google Scholar]
- 13.Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 14.Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546–557. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 15.Malaval L, Modrowski D, Gupta AK, Aubin JE. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994;158:555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- 16.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655–2661. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 19.Muschler GF, Nitto H, Matsukura Y, Boehm C, Valdevit A, Kambic H, Davros W, Powell K, Easley K (2003) Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res 102–118 [DOI] [PMC free article] [PubMed]
- 20.Risbud MV, Shapiro IM, Guttapalli A, Di Martino A, Danielson KG, Beiner JM, Hillibrand A, Albert TJ, Anderson DG, Vaccaro AR. Osteogenic potential of adult human stem cells of the lumbar vertebral body and the iliac crest. Spine. 2006;31:83–89. doi: 10.1097/01.brs.0000193891.71672.e4. [DOI] [PubMed] [Google Scholar]
- 21.Risbud MV, Sittinger M. Tissue engineering: advances in in vitro cartilage generation. Trends Biotechnol. 2002;20:351–356. doi: 10.1016/S0167-7799(02)02016-4. [DOI] [PubMed] [Google Scholar]
- 22.Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP) J Biol Chem. 1995;270:9420–9428. doi: 10.1074/jbc.270.16.9420. [DOI] [PubMed] [Google Scholar]
- 23.Stewart K, Monk P, Walsh S, Jefferiss CM, Letchford J, Beresford JN. STRO-1, HOP-26 (CD63), CD49a and SB-10 (CD166) as markers of primitive human marrow stromal cells and their more differentiated progeny: a comparative investigation in vitro. Cell Tissue Res. 2003;313:281–290. doi: 10.1007/s00441-003-0762-9. [DOI] [PubMed] [Google Scholar]
- 24.Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–203. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 25.Toquet J, Rohanizadeh R, Guicheux J, Couillaud S, Passuti N, Daculsi G, Heymann D. Osteogenic potential in vitro of human bone marrow cells cultured on macroporous biphasic calcium phosphate ceramic. J Biomed Mater Res. 1999;44:98–108. doi: 10.1002/(SICI)1097-4636(199901)44:1<98::AID-JBM11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 26.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Zannettino AC, Harrison K, Joyner CJ, Triffitt JT, Simmons PJ. Molecular cloning of the cell surface antigen identified by the osteoprogenitor-specific monoclonal antibody, HOP-26. J Cell Biochem. 2003;89:56–66. doi: 10.1002/jcb.10481. [DOI] [PubMed] [Google Scholar]
- 28.Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–2419. doi: 10.1634/stemcells.2006-0006. [DOI] [PubMed] [Google Scholar]