Abstract
The second, internet-based multicenter study (MCSII) of the Spine Study Group of the German Association of Trauma Surgery (Deutsche Gesellschaft für Unfallchirurgie) is a representative patient collection of acute traumatic thoracolumbar (T1–L5) injuries. The MCSII results are an update of those obtained with the first multicenter study (MCSI) more than a decade ago. The aim of the study was to assess and bring into focus: the (1) epidemiologic data, (2) surgical and radiological outcome, and (3) 2-year follow-up (FU) results of these injuries. According to the Magerl/AO classification, there were 424 (57.8%) compression fractures (A type), 178 (24.3%) distractions injuries (B type), and 131 (17.9%) rotational injuries (C type). B and C type injuries carried a higher risk for neurological deficits, concomitant injuries, and multiple vertebral fractures. The level of injury was located at the thoracolumbar junction (T11–L2) in 67.0% of the case. 380 (51.8%) patients were operated on by posterior stabilization and instrumentation alone (POSTERIOR), 34 (4.6%) had an anterior procedure (ANTERIOR), and 319 (43.5%) patients were treated with combined posteroanterior surgery (COMBINED). 65% of patients with thoracic (T1–T10) and 57% with lumbar spinal (L3–L5) injuries were treated with a single posterior approach (POSTERIOR). 47% of the patients with thoracolumbar junction (T11–L2) injuries were either operated from posterior or with a combined posterior–anterior surgery (COMBINED) each. Short angular stable implant systems have replaced conventional non-angular stable instrumentation systems to a large extent. The posttraumatic deformity was restored best with COMBINED surgery. T-spine injuries were accompanied by a higher number and more severe neurologic deficits than TL junction or L-spine injuries. At the same time T-spine injuries showed less potential for neurologic recovery especially in paraplegic (Frankel/AISA A) patients. 5% of all patients required revision surgery for perioperative complications. Follow-up data of 558 (76.1%) patients were available and collected during a 30-month period from 1 January 2004 until 31 May 2006. On average, a posterior implant removal was carried out in a total of 382 COMBINED and POSTERIOR patients 12 months after the initial surgery. On average, the rehabilitation process required 3–4 weeks of inpatient treatment, followed by another 4 months of outpatient therapy and was significantly shorter when compared with MCSI in the mid-1990s. From the time of injury until FU, 80 (60.6%) of 132 patients with initial neurological deficits improved at least one grade on the Frankel/ASIA Scale; 8 (1.3%) patients deteriorated. A higher recovery rate was observed for incomplete neurological injuries (73%) than complete neurological injuries (44%). Different surgical approaches did not have a significant influence on the neurologic recovery until FU. Nevertheless, neurological deficits are the most important factors for the functional outcome and prognosis of TL spinal injuries. POSTERIOR patients had a better functional and subjective outcome at FU than COMBINED patients. However, the posttraumatic radiological deformity was best corrected in COMBINED patients and showed significantly less residual kyphotic deformity (biseg GDW −3.8° COMBINED vs. −6.1° POSTERIOR) at FU (p = 0.005). The sagittal spinal alignment was better maintained when using vertebral body replacement implants (cages) in comparison to iliac strut grafts. Additional anterior plate systems did not have a significant influence on the radiological FU results. In conclusion, comprehensive data of a large patient population with acute thoracolumbar spinal injuries has been obtained and analyzed with this prospective internet-based multicenter study. Thus, updated results and the clinical outcome of the current operative treatment strategies in participating German and Austrian trauma centers have been presented. Nevertheless, it was not possible to answer all remaining questions to contradictory findings of the subjective, clinical outcome and corresponding radiological findings between different surgical subgroups. Randomized-controlled long-term investigations seem mandatory and the next step in future clinical research of Spine Study Group of the German Trauma Society.
Keywords: Spinal injuries, Fracture, Treatment, Spine, Prospective, Multicenter study, Online database, Epidemiology, Complications, Spine Study Group (SSG) of the German Association of Trauma Surgery (DGU), Radiological findings, Follow-up, Rehabilitation, Activities of daily living, Outcome
Introduction
There is no consensus on the best treatment for a vast number of different acute traumatic spinal injuries. Therefore, it is desirable to have a better understanding, explicit definitions, clear indications, and “ideal treatment algorithm” for traumatic thoracolumbar (T1–L5) spinal injuries. In the mid-1990s, the results of the first multicenter study (MCSI) of the Spine Study Group (SSG) of the German Association of Trauma Surgery (DGU) showed that there were limitations for isolated posterior instrumentation and fusion techniques in cases with a compromised anterior load-bearing column [41–43]. At the same time, the technically more demanding challenge of combined posterior–anterior surgery was ascertained. During the last decade, the operative treatment of spinal injuries has been advanced considerably: endoscopic and minimally invasive spinal surgery, interbody support implants, and computer-assisted intraoperative navigation have been successfully introduced and are being further developed at a fast pace [7, 30, 71]. In addition, different operative treatments may be applicable to the three different regions of the thoracolumbar spine: thoracic (T) T1–T10; thoracolumbar junction (TL) T11–L2; and lumbar (L) L3–L5. For a better understanding of the current treatment concepts and their clinical results, the SSG initiated its second, prospective, internet-based multicenter study (MCSII). The new study was designed to address the entire scope of operative treatment options. An online database was developed and evaluated for its capability as a tool for data acquisition in the setting of a large clinical prospective multicenter study [45]. In the following, we present the results of MCSII focusing on the epidemiology, operative treatment, and radiological findings of 733 consecutive patients with acute thoracolumbar spinal injuries. The maximum follow-up (FU) period was 30 months.
Aim of the study
The specific aims of this study were
To gather epidemiological data regarding the cause of injury, to calculate the incidence of thoracolumbar spinal injuries, and to collect specific comorbidities and neurological deficits.
To analyze treatments and radiological findings with regard to: the frequency of different operative treatments in the three spinal regions (T, TL, and L); the frequency and advantages and disadvantages of different surgical approaches, implants, and operative techniques; and the frequency of perioperative complications.
To collect FU data of specific treatment subgroups (POSTERIOR, ANTERIOR, and COMBINED) for a better understanding of the objective and subjective outcome.
Patients and methods
Primary data from a prospective consecutive case series of 733 patients from eight German and Austrian trauma centers covering the period from 1 January 2002 until 31 December 2003 were included.
Inclusion criteria
We included operatively treated (OP) patients with acute (<3 weeks after the time of injury), traumatic thoracolumbar lesions (T1–L5). We divided these patients into three subgroups based on the surgical procedure: isolated posterior procedure (POSTERIOR); isolated anterior procedure (ANTERIOR), and combined anterior–posterior surgery (COMBINED.)
Exclusion criteria
We excluded pediatric (≤16 years of age) and non-operatively treated patients.
Follow-up criteria
A FU examination was scheduled 6 months or later for patients undergoing implant removal. Patients without implant removal should have been examined 1 year after the time of injury.
Data collection
The “MEMdoc system (http://www.memdoc.org)” is an online database system developed by the Institute for Evaluative Research in Orthopaedic Surgery at the University of Bern (former Department of Education and Documentation of the Maurice E. Mueller Foundation). It is based on the principle of an application service provider (ASP,) which means a centrally controlled application that is made available to all users via the Internet. The system handles different case report forms as online questionnaires with various sub-forms for every relevant step throughout the course of treatment and FU. We collected our data using the “MEMdoc system.”
Hospital course
After admission to the hospital, an informed consent was obtained from patients and the following data collected:
- Admission data
- Date of injury, admission, surgery and revision procedures
- Cause of injury, initial neurological status (Frankel/ASIA Score), VAS score before the time of injury. The modified Frankel/ASIA Scale [3] was used to grade the neurological deficits into five categories from A (complete neurologic deficit) to E (without neurologic deficits).
- Type of injury
- Type of spinal injury, classified according to the AO/Magerl classification, a comprehensive classification for thoracic and lumbar spine injuries [54]. In cases with multiple spinal injuries, the most severe level of injury was determined.
- Level of injury
- T1–L5
-
Analysis of pre- and postoperative radiographic and CT images:
The following radiological parameters were measured:- Vertebral body (VB) height: anterior and posterior vertebral wall of the most severely injured VB (mm), sagittal index (SI): the ratio of posterior and anterior vertebral wall height; posterior VB height of the adjacent cranial and caudal VB (mm)
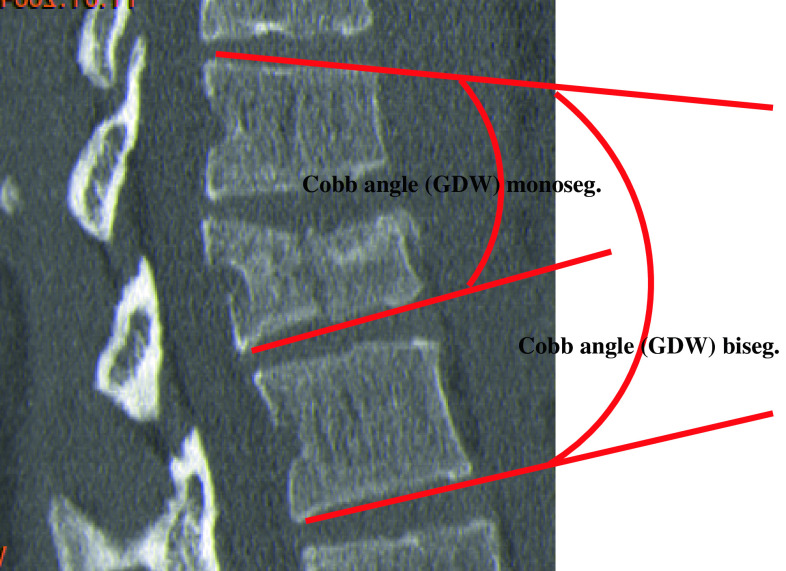
- Monosegmental Cobb angle between VB endplates (monoseg GDW) (°) (Fig. 3)
- Bisegmental Cobb angle between VB endplates (biseg GDW) (°) (Fig. 3)
- Bisegemental scoliotic angle (SKW) (°)
- Sagittal and coronal translation, expressed in percentage of sagittal/a.p. VB diameter
- Spinal canal encroachment (CT/MRT) (%) in relation to the cross-sectional spinal canal area at the level of injury relative to canal cross section adjacent to the injured level
- Intraoperative findings with regard to compromised neural elements and intervertebral disc
Fig. 3.
Radiographic image as seen in sagittal CT reconstruction with help lines (red) for the purpose of measuring mono- and bisegmental Cobb angles (GDW)
Kyphotic deformity was defined as negative (−) and lordotic deformity positive (+).
- Details about the OP treatment
- Type of treatment
- Surgical techniques: approaches, implants, bone substitutes and fusion techniques, number of posterior or anterior instrumented and/or fused segments, decompression of the spinal canal, endoscopy, navigation, kypho-/vertebroplasty, OR-, and beam-on time of intraoperative fluoroscopy, and estimated blood loss
- Intraoperative complications: general, surgical, and neurological complications with or without operative revision, such as hemorrhage, inadvertent dural tear, iatrogenic spinal cord, nerve root injury, internal organ injury, revision of screw placement, conversion from endoscopic to open access procedure, and others.
- Discharge data
- Date and neurological status (Frankel/ASIA Score) at discharge from hospital
Follow-up course
At least one complete FU examination was required. Nevertheless, the online database allowed for the documentation of subsequent FU visits as well. Besides objective FU parameters, we collected information on subjective back function, health-related quality of life, and social- and work-related rehabilitation process:
- Follow-up data (FU)
- Date of FU visit
- Date of implant removal if applicable
- Alcohol and tobacco use
- Physical examination and course of treatment after discharge from hospital
- Fingertip-floor distance (cm), duration of inpatient rehabilitation at subsequent clinical institutions (weeks) and ambulatory, outpatient physical therapy (months), return to work (weeks), reintegration or change of work, activity level regained during recreational activities, back function, and comorbidities related to the surgical approach or donor site morbidity for autologous iliac crest bone grafts.
- Incidence and type of complications throughout the FU period such as death (injury or OP related), postoperative hemorrhage, cerebrospinal fluid leakage, neurologic deterioration, hardware failure, infections, deep venous thrombosis, embolism, loss of postoperative correction, and others.
- Radiological findings
- Monosegmental Cobb angle between VB endplates (monoseg GDW) (°).
- Bisegmental Cobb angle between VB endplates (biseg GDW) (°).
- Bisegmental Scoliosis angle (SKW) (°).
- Fusion (Bridwell’s criteria I)/non-fusion (Bridwell’s criteria II–IV) of anterior and posterior spondylodesis.
Data security was maintained using 128-bit encryption technology, controlled access, regular data base back-ups, mirrored server technology, and physical protection of machines [45]. The Study Administrator de-identified patients’ and surgeons’ names prior to any further evaluation.
Data management and statistical analysis
Data management and analysis was carried out with STATA (Version 9, STATA Inc. College Station, TX, USA) and SPSS (Version 13, SPSS Inc., Chicago, IL, USA) for Windows. Standardized statistical tests [t-, Wilcoxon’s-, Mann–Whitney test, χ2 test, multivariate data and regression analysis (ANOVA)] were used for comparison. Follow-up data were grouped according to the following time periods: <6, 6–12, 12–24, or >24 months after the date of injury.
Results
A study population of 733 patients from 8 centers was included. Contributing centers and patient numbers in alphabetical order: Berlin, Centre for Musculoskeletal Surgery, Charite (n = 101), Homburg/Saar, Saarland University Hospital, Department of Trauma, Hand and Reconstructive Surgery (n = 78), Medical University Innsbruck, Department of Trauma Surgery (n = 147), Trauma Hospital Klagenfurt (n = 47), Mainz, Johannes Gutenberg University Medical Center, Department of Trauma Surgery (n = 34), Trauma Center Murnau, Department of Spinal Surgery (n = 207), Ulm University Hospital Department of Trauma-, Hand-, and Reconstructive Surgery (n = 60), Wurzburg, University Hospital, Department of Trauma-, Hand-, and Reconstructive Surgery (n = 59).
Epidemiology
Demographic data
The study population consisted of 487 (66.4%) male and 246 (33.6%) female patients. The overall average patient age at the time of injury was 41 years (range 16–89 years). The patient distribution by age groups was 16–20 years n = 80 (10.9%), 21–40 years n = 298 (40.7%), 41–60 years n = 252 (34.4%), and more than 61 years n = 103 (14.1%).
Cause of injury
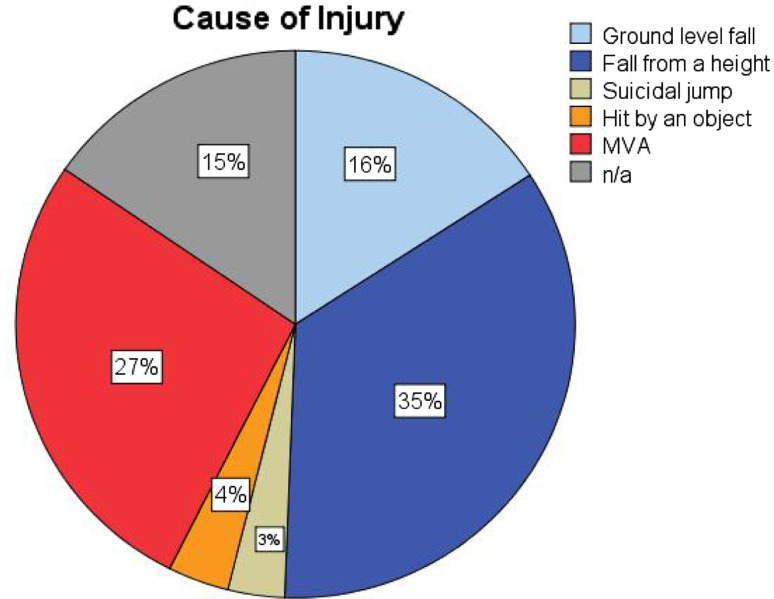
The most common causes of injury were “fall from a height” (n = 225), motor vehicle accidents (n = 173), and “simple falls” (n = 116) (Fig. 1). A significant correlation was found between patient age and cause of injury (p < 0.05): patients injured by simple falls were older with an average age of 52 years (16–89 years) when compared with those injured in an MVA with an average age of 35 years (17–79 years). There was a statistically significant correlation between the cause of injury and fracture type according to AO/Magerl classification (p < 0.001; χ2 test). The relative risk (RR) of a type A fracture from a simple fall was 1.5 times higher than for any other cause of injury (p < 0.001). At the same time, there was a 2.6 times higher RR of a type B/C injury associated with high-energy trauma, such as fall from a height or MVA, when compared with simple falls.
Fig. 1.
Causes of injury and relative frequency (n total = 620, n missing = 113): fall from a height (n = 191 blue), ground level fall (n = 255 light blue), motor vehicle accident (n = 199 red), hit by object (n = 26 orange), suicidal jump (n = 24 light brown)
Classification and level of injury
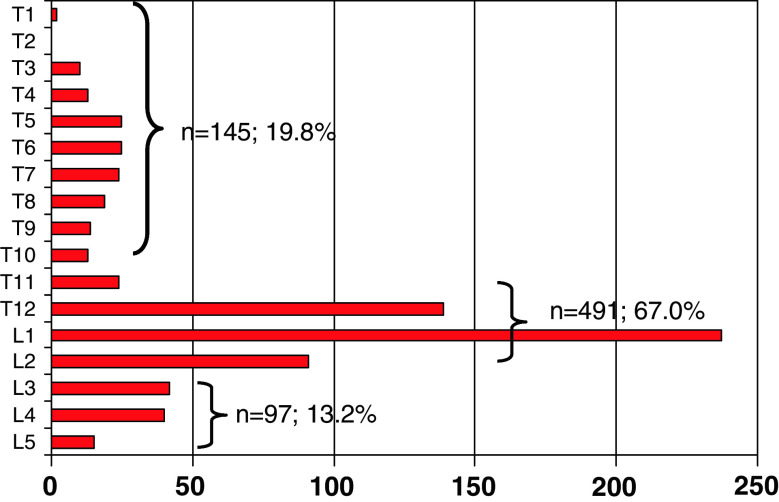
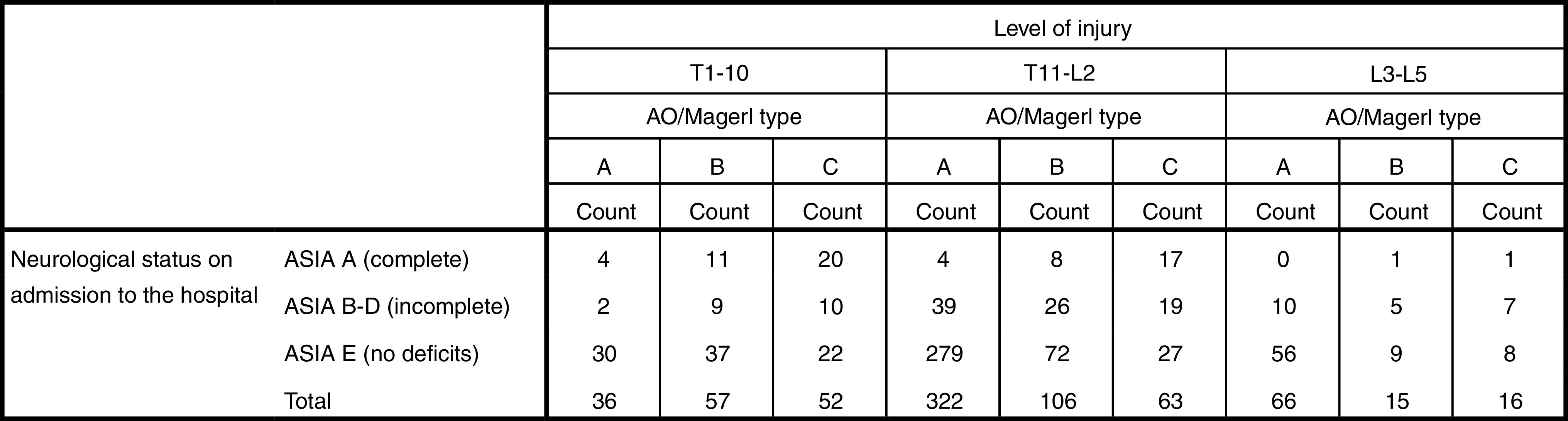
There were 424 (57.8%) type A (compression), 178 (24.3%) type B (distraction), and 131 (17.9%) type C (rotation) injuries. Within these three categories, the most common injury subtypes were burst fractures (type A3) 345 (81.4%), predominantly ligamentous disruptions (type B1) 87 (48.9%), and compression fractures with rotation (type C1) 74 (56.5%). With regard to the level of injury, we found 145 (19.8%) T-spine, 97 (13.2%) L-spine, and 491 (67.0%) TL-junction injuries (Fig. 2). The distribution of fracture types varied according to the level of injury with more B and C type injuries at the T-spine level (Table 1). Multilevel injuries were uncommon: 61 patients (8.3%) sustained injuries of more than two adjacent motion segments: and 42 (5.7%) patients had injuries of non-adjacent motion segment. The majority of 630 patients (85.9%) suffered from mono- or bisegmental spinal injuries.
Fig. 2.
Frequencies and level of the most severe injury (n total = 733)
Table 1.
Level of injury and fracture type according to the AO/Magerl classification [54]
| AO/Magerl classification/fracture type | Total | |||
|---|---|---|---|---|
| A | B | C | ||
| Level of injury | ||||
| T1–T10 | 36 | 57 | 52 | 145 |
| T11–L2 | 322 | 106 | 63 | 491 |
| L3–L5 | 66 | 15 | 16 | 97 |
| Total | 424 | 178 | 131 | 733 |
Neurological deficits on admission to the hospital
Complete motor and sensory neurological deficits (Frankel/ASIA A) were present in 66 (9%) patients and incomplete neurological deficits (Frankel/ASIA B–D) present in 540 (74%) patients. No neurological deficits (Frankel/ASIA E) were present in 540 (74%) patients. More severe spinal injuries correlated with a higher incidence of neurologic deficits (Frankel/ASIA A–D) on admission to the hospital [type A (11.1%), type B (33.1%) and type C injuries (57.4%) p < 0.01]. Concomitantly, neurological deficits varied significantly (p < 0.01) according to the fracture type and level of injury (Table 2): 38.6% of the T-spine injuries were associated with neurological deficits (ASIA A–D) in contrast to 23% in TL junction or 25.7% of L-spine injuries (p < 0.001).
Table 2.
Neurological status according to the Frankel/ASIA score on admission to the hospital with regard to the level of injury and AO/Magerl fracture type
Concomitant injuries
The anatomical region and severity were graded according to the Abbreviated Injury Scale (AIS). At least one or more concomitant injuries were present in 376 (51.3%) of 733 patients. Apart from AIS Thoracolumbar and Lumbar spine categories, the most common concomitant injuries were located in the extremities and pelvic ring, 33.7% of the patients. Severe, life threatening or critical injuries were most commonly related to head, neck, and cervical spine injuries in 29 (3.9%) patients. Of those patients with concomitant injuries, 177 (24.1%) had 1, 94 (12.8%) 2 and 105 (14.3%) patients 3–4 additional injuries. The incidence of concomitant injuries increased with injury classification: type A (42%), B (62%), and type C (66%) injuries (p < 0.01). The average Injury Severity Score (ISS) score was 14.6 (POSTERIOR 15.6, ANTERIOR 11.4, and COMBINED 13.8). Higher ISS scores represent more severe injuries. Patients with neurological deficits had average ISS 18 versus ISS 13 in those without neurological deficits (p < 0.05). According to the AO/Magerl classification, the average ISS was 11 for type A, 17 for type B, and 21 for type C injuries.
Treatment and radiological findings
Operative procedure
The surgical treatment for these injuries was POSTERIOR 380 (51.8%), ANTERIOR 34 (4.6%), and COMBINED 319 (43.5%). Table 3 summarizes surgical technique, level of injury, fracture classification, neurological status, and other relevant parameters. POSTERIOR procedures were carried out more often in T-spine injuries 65% than TL junction 47% and L-spine injuries 57% (p = 0.002). Equal numbers of COMBINED and POSTERIOR treatments, 47% were used for TL-junction injuries.
Table 3.
Summary of characteristic baseline parameters and patient numbers according to the level of injury and surgical technique
| Parameter | T-spine (n = 145) | TL junction (n = 491) | L-spine (n = 97) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| POSTERIOR | COMBINED | ANTERIOR | POSTERIOR | COMBINED | ANTERIOR | POSTERIOR | COMBINED | ANTERIOR | |
| Patient number (n) | 94 (64.8%) | 47 (32.4%) | 4 (2.8%) | 231 (47.0%) | 232 (47.3%) | 28 (5.7%) | 55 (56.7%) | 40 (41.2%) | 2 (2.1%) |
| Ø age (years) | 41 (17–85) | 35 (17–67) | 37 (17–52) | 42 (17–89) | 41 (16–80) | 39 (22–64) | 46 (16–85) | 40 (16–71) | 58 (41–73) |
| Fx type A | 21 (22.3%) | 13 (27.7%) | 2 (50%) | 164 (71%) | 133 (57.3%) | 25 (89.3%) | 27 (67.3%) | 27 (67.5%) | 2 (100%) |
| Fx type B | 45 (47.9%) | 11 (23.4%) | 1 (25%) | 47 (20.3%) | 57 (24.6%) | 2 (7.1%) | 12 (21.8%) | 3 (7.5%) | |
| Fx type C | 28 (29.8%) | 23 (48.9%) | 1 (25%) | 20 (8.7%) | 42 (18.1%) | 1 (3.6%) | 6 (10.9%) | 10 (25.0%) | |
| Frankel/ASIA E | 58 (61.7%) | 27 (57.4%) | 4 (100%) | 187 (81%) | 164 (70.7%) | 27 (96.4%) | 45 (81.8%) | 26 (65%) | 2 (100%) |
| Frankel/ASIA B–D | 14 (14.9%) | 7 (14.9%) | 34 (14.7%) | 49 (21.1%) | 1 (3.6%) | 9 (16.4%) | 13 (32.5%) | ||
| Frankel/ASIA A | 22 (23.4%) | 13 (27.7%) | 10 (4.3%) | 19 (8.2%) | 1 (1.8%) | 1 (2.5%) | |||
| Polytrauma | 29 (30.9%) | 15 (31.9%) | 1 (25%) | 16 (6.9%) | 21 (9.1%) | 1 (3.6%) | 5 (9.1%) | 4 (10%) | |
| Median VAS Spine Score before injury | 96 (n = 67) | 95 (n = 37) | 59 (n = 2) | 95 (n = 173) | 92 (n = 181) | 86 (n = 22) | 97 (n = 40) | 85 (n = 32) | 68 (n = 2) |
Timing and hospital stay
The majority of patients were admitted on the day of injury with a median of 2 days (SD 5.1) between the injury and first surgical intervention. 32 patients received a single posteroanterior procedure (COMBINED) within 3 days of injury. The majority, 287, COMBINED patients were treated with a two-staged procedure: posterior surgery on average 2 days after injury followed by a second anterior procedure 9 days after injury. The average hospital stay for all OP patients was 19 days: POSTERIOR 14 days, ANTERIOR 18 days, and COMBINED 24 days. The delay until surgery was significantly longer for compression fractures than distraction and rotation injuries: 3 days (type A), 2 days (type B), and 1 day (type C) (p < 0.001).
Implants and instrumentations
Posterior
Angular stable posterior implants were used in 330 (87%) cases in combination with 281 (73.9%) additional transverse stabilizers in 58 patients with T-spine, 180 TL junction, and 43 L-spine injuries. For the POSTERIOR patients, 275 had one to two motion segments injured [T-spine n = 59 (63.4%), TL junction n = 30 (13.2%), L-spine n = 7 (12.8%)], and 96 more than two motion segments injured. Cancellous and cortical autologous bone graft from the posterior iliac crest was used for posterior spondylodesis at the T-spine level in 41.5%, TL junction 16.9%, and L-spine in 18.2% of the cases. A “trauma” PLIF (posterior lumbar interbody fusion) was used in 36 patients with TL junction (n = 30), or L-spine injury (n = 6).
Combined
Angular stable implants were used for the posterior instrumentation in 312 (97.8%) patients. No significant difference between COMBINED and POSTERIOR patients was seen with regard to the extent of the spinal injury assessed by the number of treated motion segments [COMBINED 258 (80.9%) 1–2 motion segments, 48 (15.1%) 3–4 motion segments, and 13 (4.1%) cases with up to a maximum of 6 motion segments)]. Posterior spondylodesis were used in 78 (24.5%) of COMBINED cases.
Anterior
A total of 353 anterior procedures were done in the ANTERIOR or COMBINED treatment groups. A less-invasive thoracoscopic approach was used in 238 (67.4%) cases, instead of conventional thoracotomies (n = 29, 8.2%), lumbotomies (n = 51, 14.4%), thoracophrenolumbotomies (n = 8, 2.3%), or other non-specified anterior approaches (n = 19, 5.4%). In 230 (65.1%) of these patients, the extent of injury was 1 (n = 75), 2 (n = 155), 3 (n = 7), or 4 (n = 11) motion segments. Treated levels were missing for 105 (29.7%) cases. Anterior angular stable plates were used in 184 patients for T-spine (n = 31), TL junction (n = 133), and L-spine (n = 1) injuries. ANTERIOR spondylodesis bridged one (n = 133, 37.7%), two (n = 135, 38.2%), three (n = 3, 0.8%), or four (n = 2; 0.6%) motion segments (missing n = 5, 1.4%). The most common donor site for autologous bone grafts was the anterior (n = 137; 38.8%) or the posterior (n = 40; 11.3%) iliac crest. Interbody support implants (cages) were used in 188 patients. Two hundred fifteen anterior interbody fusions were performed with autologous bone grafts (n = 84) or cages (n = 131) in combination with an anterior plate system. Standalone interbody fusions were done in 117 cases with bone grafts (n = 67) or cages (n = 50) alone (missing n = 21).
Operative details
The average OR time in COMBINED was significantly longer 298 min (T-spine 350 min, TL junction 286 min, L-spine 309 min) than in POSTERIOR 152 min (T-spine 179 min, TL junction 139 min, L-spine 157 min) and ANTERIOR 208 min (T-spine 214 min, TL junction 213 min, L-spine 125 min) (p < 0.001). Intraoperative fluoroscopic images in two planes were taken in 543 (74.1%) cases. The radiation exposure was significant longer in COMBINED 246 s (T-spine 311 s, TL junction 255 s, L-spine 270 s) than POSTERIOR 168 s (T-spine 199 s, TL junction 150 s, L-spine 222 s), or ANTERIOR 215 s (T-spine 305 s, TL junction 204 s, L-spine 265 s) (p < 0.001). Operative treatment of T-spine injuries was associated with more radiation exposure in all three treatment subgroups. In 95 (13%) patients, computer-assisted surgery was used for intraoperative navigation. A cellsaver® was used in 361 (49%) of the cases. The average blood loss of 958 ml (20–5,300 ml, n = 170) was highest in COMBINED.
Surgical decompression
The relative traumatic spinal canal encroachment was measured (%) with the help of CT or MRI images. Data from 508 (69.3%) of 733 patients were available with T- (n = 72), TL junction (n = 364), or L-spine injury (n = 72). The average spinal canal encroachment was 35.6% (range 5–95%) of the unaffected spinal canal diameter at the level of injury with 36.5% at the T-spine, 34.1% TL junction, and 42.3% at the L-spine. The RR to suffer from neurological deficits (ASIA A–D) was 3.5 higher in cases with spinal canal encroachment in comparison to patients without spinal canal encroachment (p < 0.001). The spinal canal encroachment (%) correlated significantly with the neurological status at the time of admission to the hospital (p < 0.001). For patient with T-spine injuries and spinal canal encroachment, 51.4% had neurologic deficits, as compared to 26.1% with TL junction, and 30.6% with L-spine injuries. Fifty-five patients with a complete neurologic deficit showed 70% (T-spine 60%, TL junction 75%) spinal canal encroachment at the time on admission to the hospital. On average, 92 patients with incomplete neurologic deficits showed 50% (T-spine 35%, TL junction 60%, and L-spine 60%) of spinal canal encroachment. In 358 patients without neurological deficits, the median spinal canal encroachment was 20% (T-spine 10%, TL junction 20%, L-spine 30%). Intraoperative myelography was used in 27 cases. Eighteen patients required surgical dural repair of a traumatic dural tear. Surgical decompression was performed from posterior (n = 171), anterior (n = 76), or both sides (n = 40). Pre- and postoperative spinal canal encroachment did not show significant differences between surgical techniques POSTERIOR (38.2 vs. 19.1%), ANTERIOR (16.8 vs. 9.6%), or COMBINED (43.1 vs. 19.9%) (p = 0.523).
Complications
Nine patients died from life threatening severe other than spinal injuries (AIS ≥ 4). Three patients were in the age group 21–40 years, two 41–60 years, and four patients greater than 60 years of age. The exact cause of death was not noted other than two patients having pulmonary emboli.
The perioperative course was uneventful for 623 (85%) out of 733 patients [T-spine n = 112 (77.2%), TL junction n = 426 (86.8%), L-spine n = 85 (87.6%)] (missing n = 5, 0.7%).
One hundred five (14.3%) patients sustained intraoperative [n total = 56 (7.7%): TS 17 (11.7%), TL junction 29 (5.9%), LS 10 (10.3%) or postoperative (n total = 69 (9.4%): TS 21 (14.5%), TL junction 42 (8.6%), LS 6 (6.2%)] complications. 39 (5.3%) patients required surgical revision for their complications [T-spine 13 (8.2%), TL junction 20 (3.7%), L-spine 6 (5.4%)] (missing n = 231) (Table 4). The incidence of intraoperative complications was significantly higher in COMBINED (10.7%; n = 34) than POSTERIOR patients (5.9%; n = 22) (p = 0.021).
Table 4.
Numbers (n) and incidence (%) of documented intra- and postoperative complications
| Complication | Numbers (n) | |
|---|---|---|
| Intraoperative (n total = 56, 7.7%) | Hemorrhage | 35 |
| Misplaced screws | 18 | |
| Conversion endoscopy—open surgery | 8 | |
| Miscellaneous | 8 | |
| Postoperative (n total = 69, 9.4%) | Death | 6 |
| Infection | 10 | |
| Wound healing problems | 14 | |
| Thrombosis/embolism | 7 | |
| Implant misplacement/loss of correction | 8 | |
| Miscellaneous | 24 |
Intraoperative complications
Hemorrhage was the most common complication in COMBINED (n = 25) and POSTERIOR (n = 10) subgroups (p = 0.002). Malpositioned pedicle screws had to be revised in 18 cases with T-spine (n = 7), TL junction (n = 7), or L-spine (n = 4) injuries. Endoscopic approaches were converted to open surgery in six cases and eight patients suffered from miscellaneous complications.
Postoperative complications
Documented within the database were 10 postoperative infections, 14 (1.9%) wound healing problems, 7 (1%) thrombosis/emboli, a total of 14 (2%) malpositioned implants of those 5 were located at the T-spine, 7 at the TL junction, and 2 at the L-spine. In 8 (1.1%) cases, a loss of correction was noticed and 24 (3.3%) were miscellaneous complications.
Complications and surgical revision
Revision surgery for postoperative complications was necessary in 39 (5.3%) patients. Details were reported for 25 cases: 4 infections, 5 wound healing problems, 1 thrombosis/embolus, 6 malpositioned implants, 1 loss of correction/implant failure, 1 incompletely decompressed spinal canal, 1 postoperative hemorrhage, 4 pleural effusions, 1 neurological deterioration, and 1 postoperative seroma.
Neurological course during hospitalization
On average, patients with complete neurological deficits (Frankel/ASIA A) were hospitalized longer, 28 days, than those with incomplete (Frankel/ASIA B–D), 22 days, or without neurological deficits, 16 days (p < 0.001). Initially, 592 (80.8%) patients had no neurological deficits, 92 (12.6%) incomplete, and 49 (6.7%) patients complete neurological deficits. There was improvement of at least 1 Frankel grade or more in 75 patients: 78.9% (n = 15) in Frankel/ASIA B patients, 42.7% (n = 17) in Frankel/ASIA C, and 53.8% (n = 43) in Frankel/ASIA D patients. One patient deteriorated and had a worse outcome on the Frankel/ASIA Scale than on admission. The potential for improvement of the initial neurological deficit was greater in incomplete lesions at the TL junction than complete neurological deficits at the T-spine.
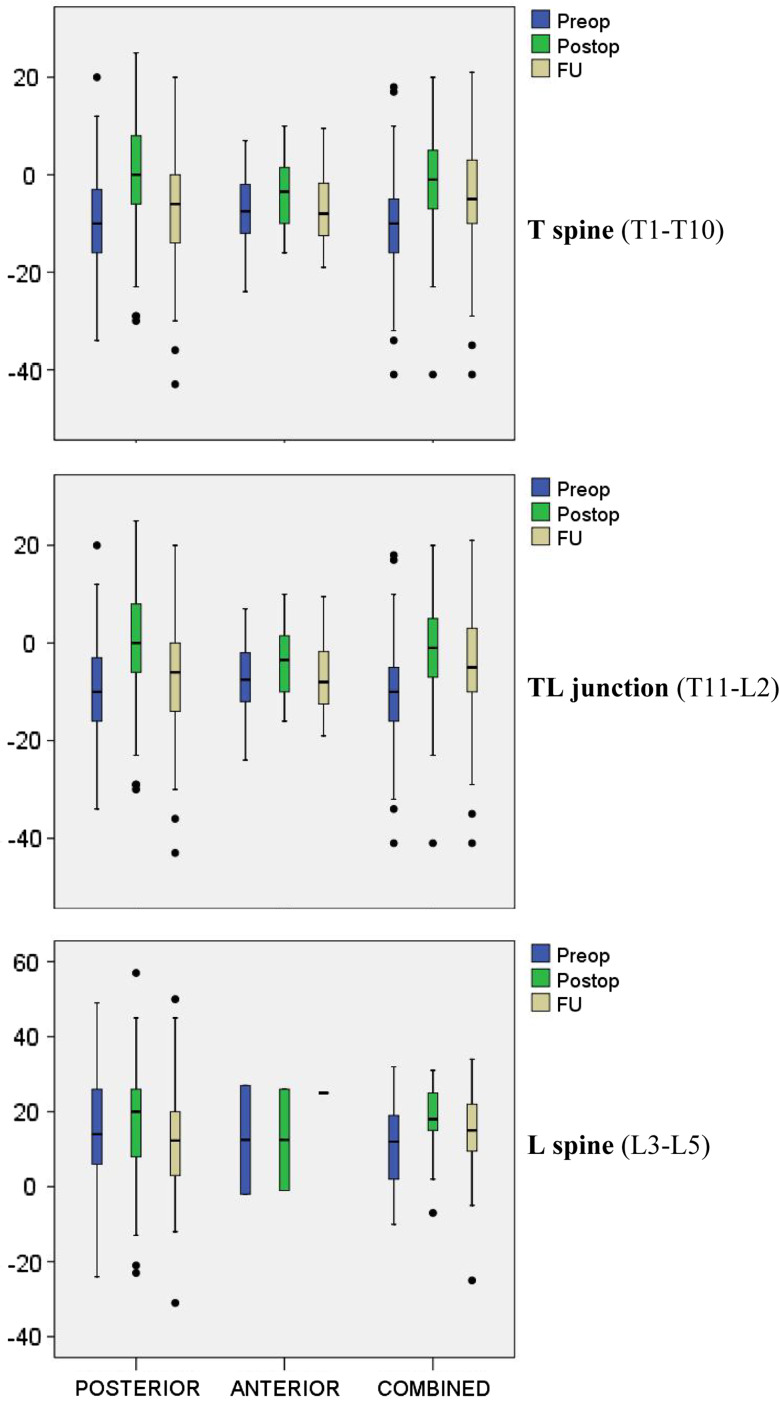
Radiological findings and treatment groups during hospitalization
The pre- and postoperative sagittal spinal alignments were measured as the mono and bisegmental Cobb angles (Fig. 3). Pre- and postoperative kyphotic deformity was more pronounced at the T-spine [preop −19°; n = 138/postop −14° (n = 127)], than TL junction (preop −10°; n = 484/postop 0°; n = 474), or L-spine (preop 13°; n = 94/postop 19° n = 90). On average, the posttraumatic deformity was corrected by 5.7° at the T-spine (n = 123), 9.3° TL junction (n = 472), and 5.1° L-spine (n = 90) level. Postoperative alignment did not depend on the type of injury [Magerl/AO A–C (p = 0.151) or different surgical techniques (POSTERIOR vs. COMBINED (p = 0.34)].
Follow-up (FU)
A total of 558 (76%) patients with T-spine (n = 112), TL junction (n = 366), and L-spine injuries (n = 60) were assessed for FU during a 30-month period from 1 January 2004 until 31 May 2006. Patient FU according to surgical technique was POSTERIOR, n = 280, ANTERIOR, n = 29 and COMBINED, n = 249. In some patients the results of several, consecutive FU visits were documented and have been analyzed accordingly.
Timing and FU visits
Overall, the average time to FU was 15 months (6–45 months). The average hospital stay of patients available for FU was 19 days (T-spine 25 days, TL junction 17 days, L-spine 22 days). The mean hospital stay was 14 days after POSTERIOR, 18 days ANTERIOR and 24 days COMBINED surgery. During the FU period, 382 (72.2%) [POSTERIOR (n = 205) or COMBINED (n = 177)] of the patients had an implant removal in T-spine n = 73 (67%), TL junction n = 259 (75.2%), or L-spine n = 50 (64.1%) injuries. The median time to implant removal was 12 months (3–48 months).
Rehabilitation
On average 4 weeks after initial surgery (0–50 weeks), patients were treated on an inpatient basis at rehabilitation facilities. No significant differences between surgical techniques (3 weeks POSTERIOR, 4 weeks ANTERIOR and COMBINED each) were found. The duration of an inpatient rehabilitation program was significantly longer in patients with (1) neurological deficits (Frankel/ASIA A–D Ø 10.9 weeks) as compared to patients without neurological deficits (Frankel/AISA E Ø 4.2 weeks), (2) polytraumatized patients (Ø 8.6 weeks vs. isolated spinal trauma Ø 6.4 weeks), and (3) patients with more than one concomitant injury [Ø 8 vs. Ø 7.0 weeks up to 1 relevant concomitant injury (p < 0.05)]. On average an ambulatory rehabilitation program was continued for 4 months (0–32 months) lasting 3 months after POSTERIOR, 4 months after ANTERIOR, and 4.5 months after COMBINED (p > 0.05) surgery.
Complications during the FU period
Complications were reported in 52 (9.1%) patients during the FU period after POSTERIOR (n = 39), ANTERIOR (n = 4), or COMBINED (n = 9) surgery. Twenty-three patients had complications within 1–2 years after the initial treatment. Eighteen patients had revision surgery for 11 infections, 1 pleural effusion, 1 seroma, 1 infection with the implant removal, 2 loss of correction/implant failure, 1 hernia, and 1 implant cut out. Thirty-four patients with complications were managed non-operatively for: 2 infections, 10 losses of correction/implant failure, severe pain, persistent drop-foot, cerebrospinal fluid fistula, decubitus after rehabilitation, hematoma after implant removal, or adjacent fracture. Other implant associated complications reported were misplaced screw, implant loosening, four broken pedicle screws, and two dislocations. Complications following the implant removal were nerve/spinal cord injury, dural tear, and three broken pedicle screws left in situ.
Neurological course during FU
The neurological status of 557 (99.8%) patients was assessed at least once or subsequently several times throughout the 2.5-year FU period (missing n = 1). In cases with more than one FU visit, the most recent outcome was considered for further analysis. Neurological FU data were obtained during the period between 1 and 2 years after the injury in 58% of cases. Table 5 compares the initial and FU Frankel/ASIA scores with regard to the level of injury:
Table 5.
Neurological status according to the Frankel/ASIA Score on admission to the hospital and outcome at follow-up with regard to the level of injury
| Frankel/ASIA on admission (n) | Frankel/ASIA at follow-up (n) | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | n total (%) | |
| T-spine (T1–T10) | ||||||
| A | 19 | 0 | 0 | 1 | 1 | 21 (23.5) |
| B | 0 | 1 | 0 | 3 | 0 | 4 (3.6) |
| C | 0 | 0 | 2 | 0 | 2 | 4 (3.4) |
| D | 0 | 0 | 0 | 0 | 7 | 7 (6.3) |
| E | 0 | 0 | 0 | 1 | 75 | 76 (67.9) |
| n total (%) | 19 (17.0%) | 1 (0.9%) | 2 (1.8%) | 5 (4.5%) | 85 (75.9%) | 112 (100) |
| TL junction (T11–L2) | ||||||
| A | 7 | 1 | 3 | 3 | 3 | 17 (4.6%) |
| B | 0 | 2 | 3 | 2 | 0 | 7 (1.9%) |
| C | 0 | 0 | 2 | 5 | 4 | 11 (3.0%) |
| D | 1 | 0 | 0 | 13 | 25 | 39 (10.6%) |
| E | 0 | 0 | 0 | 3 | 289 | 292 (79.8%) |
| n total (%) | 8 (2.2%) | 3 (0.8%) | 8 (2.2%) | 26 (7.1%) | 321 (87.7%) | 366 (100%) |
| L-spine (L3–L5) | ||||||
| A | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 0 | 0 | 1 | 1 | 0 | 2 (2.5%) |
| C | 0 | 0 | 0 | 1 | 2 | 3 (3.8%) |
| D | 0 | 0 | 0 | 5 | 12 | 17 (21.5%) |
| E | 0 | 0 | 0 | 3 | 54 | 57 (72.2%) |
| n total (%): | 0 | 0 | 1 (1.3%) | 10 (12.7%) | 68 (86.1%) | 79 (100%) |
80 (60.6%) out of 132 patients improved at least one Frankel/ASIA grade after surgery; 469 (84.1%) out of 557 patients remained unchanged; and 8 (1.4%) patients had a worse outcome than initially documented. Seven of the patients with a neurological deterioration were initially examined as neurologically intact (Frankel/ASIA E) and Frankel/ASIA D at FU. One patient was reported to be Frankel/ASIA A without additional comments or possible reasons for deterioration reported. The rate of neurological improvement was 59.6% (34 out of 57 patients) after POSTERIOR surgery and 61.3% (46 out of 75 patients) after COMBINED surgery. Binary logistic regression analysis did not show statistically significant differences in neurological improvement and surgical technique taking patient age and fracture level into consideration.
Clinical FU data
Alcohol use was noted in 4.5% of patients, and nicotine use in 18.8% of the patients. The RR for wound healing problems was 2.9 times higher in smokers than non-smokers. The range of spinal motion was assessed by flexion tests, which measured the distance (cm) from fingertips to the floor distance (FBA) in 413 patients. The average FBA in patients younger than 40 years was 10.8 cm versus 16.3 cm of those older than 40 years. Female patients showed better (11.1 cm) flexion test results than male (14.8 cm) patients. No significant correlations were found between the radiological findings (GDW) and flexion test results (FBA). The subjective back function was documented at least once in 361 cases, and repeatedly measured in 196 patients. The subjective back function improved over time such that 17.7% of the patients reported full back function (100%) after 6–12 months, 19.4% after 1–2 years, and 32.7% after >2 years after the initial injury. The back functioning data were obtained at FU 1–2 years after the injury for 58.1% (n = 324) of reported cases. At this time, 21.1% of the patients reported full back function (100%), 51.3% had minor difficulties every now and then (75%), 20.6% had frequent difficulties with minor limitations (50%), 5.2% had frequent severe difficulties or pronounced limitations (25%), and 1.6% reported permanent, major, and disabling back problems (0%). Full back function was regained in 24% of the patients after POSTERIOR, 14% ANTERIOR, and 17% COMBINED (p = 0.005) surgery. With regard to the level of injury, full back function (100%) was reported in 17% after T-spine, 23% TL junction, and 14% L-spine injuries (p > 0.05). The comorbidity at the donor site of autologous bone grafts at the iliac crest was evaluated by an ordinal scale of 1–5: 175 (56%) of 311 patients reported no difficulties at the donor site (100%), 56 (18%) reported minor difficulties every now and then (75%), 25 (8%) had frequent difficulties with minor limitations (50%), and 1 patient complained of frequent, severe difficulties or pronounced limitations (25%). No pain or morbidity by the posterior access to the spine (100%) was noted by 300 (56.7%) of the patients, 33.8% (n = 179) had minor difficulties every now and then (75%), 6% (n = 40) had frequent difficulties with minor limitations (50%), 1.5% (n = 8) had frequent, severe difficulties or pronounced limitations (25%), and 1 patient (0.2%) complained of disabling limitations (0%) (missing n = 1). According to the level of injury, there were 49.5% of patients with T-spine, 60.2% TL junction, and 51.3% L-injuries without complaints of comorbidities by the posterior access (p > 0.05). Odds ratio for patients with 1–2 level injury without complaints from the posterior approach (59%) was higher than in patients with >2 level injuries and a larger surgical approach (45.1%) (p = 0.007). With an anterior approach, 61.2% (n = 170) of the patients had no complaints (100%), 30.2% (n = 84) had minor difficulties every now and then (75%), 6.5% (n = 18) had frequent difficulties with minor limitations (50%), 1.8% (n = 5) had frequent, severe difficulties or pronounced limitations (25%), and 1 patient (0.4%) complained of disabling limitations (0%) (missing n = 1). In addition 57.1% of patients with T-spine, 62.6% TL junction, and 57.9% L-spine injury had no complaints about any comorbidity from the anterior approach at FU (p > 0.05). The relative frequency of patients without complaints (100%) in conjunction with a minimally invasive endoscopic anterior approach (n = 104, 63.8%) was higher than open anterior surgery (n = 10, 55.6%).
VAS spine score
The VAS spine score (0–100 points) is a self-reporting tool to measure subjective back functioning [44]. A higher VAS score represents less back problems. Younger patients scored higher than older patients before injury: 16–40 years 85 points, 41–60 years 77 points, and 70 points in patients more than 60 years (p < 0.001). The mean VAS spine scores before injury was 80 with significant differences between subgroups POSTERIOR 86 points, ANTERIOR 81 points and COMBINED 74 points (p < 0.001). VAS spine scores according to the level of injury were 80 points in T-spine, 82 in TL junction, and 73 points in L-spine injuries. The subjective outcome at FU was reassessed in 468 (83.9%) patients. Influencing factors, such as patient age, gender, fracture type and the time of the FU examination were considered for the following analysis (ANOVA). The average VAS spine scores at FU was 58.4 points with statistically significant difference between POSTERIOR (64.9 points) than COMBINED (47.8 points) (p = 0.004) subgroups in T-spine injuries only. The baseline VAS spine score before injury (p < 0.001) and neurological deficits (Frankel/ASIA A–D: 53.4 points vs. Frankel/ASIA E: 61.3 points) (p < 0.017) had a significant impact on the outcome at FU as well. Table 6 shows the summary of the VAS spine score results and score loss throughout the course of treatment and level of injury. A significant correlation was observed between a greater fingertip-floor distance (cm) and less points in the VAS spine scores at FU (r = 0.079, p < 0.001). No significant correlations were found between radiological measurements (mono- and bisegmental GDW) and VAS spine scores.
Table 6.
Patient numbers and average visual analog scale (VAS) spine score prior to the injury (preoperative) and follow-up
| Level of injury | Surgical technique | VAS spine score | |||||
|---|---|---|---|---|---|---|---|
| Preoperative | Follow-up | Score difference | |||||
| Number (%) | Mean | Number (%) | Mean | Number (%) | Mean | ||
| n/a | 439 (78.7) | 80 | 468 (83.9) | 58.4 | 389 (69.7) | −15.76 | |
| T-spine (T1–T10) | Total | 85 (75.9) | 80.4 | 87 (77.7) | 59.1 | 72 (64.3) | −17.6 |
| POSTERIOR | 52 (74.3) | 84.9 | 57 (81.4) | 64.9 | 44 (62.9) | −15.3 | |
| ANTERIOR | 1 (33.3) | ||||||
| COMBINED | 32 (82.1) | 72.6 | 30 (76.9) | 47.8 | 28 (71.8) | −21 | |
| TL junction (T11–L2) | Total | 289 (79.0) | 81.5 | 315 (86.1) | 59.5 | 261 (71.3) | −15.2 |
| POSTERIOR | 129 (76.8) | 86.8 | 149 (88.7) | 62.9 | 117 (69.6) | −13.9 | |
| ANTERIOR | 20 (83.3) | 81.3 | 19 (79.2) | 63.9 | 16 (66.7) | −11.9 | |
| COMBINED | 140 (80.5) | 76.6 | 147 (84.5) | 55.3 | 128 (73.6) | −16.8 | |
| L-spine (L3–L5) | Total | 65 (81.3) | 73.4 | 66 (82.5) | 52.4 | 56 (70) | −16.1 |
| POSTERIOR | 32 (76.2) | 83.1 | 31 (73.8) | 65.5 | 26 (61.9) | −12.5 | |
| ANTERIOR | 2 (100) | 67.5 | 2 (100) | 41 | 2 (100) | −26.5 | |
| COMBINED | 31 (86.1) | 63.9 | 33 (91.7) | 40.7 | 28 (77.8) | −18.8 | |
An average score difference of 389 patients was calculated according to the level of injury and surgical technique
Rehabilitation: occupation and recreational activities
Occupation before the time of injury was reported by 543 (97.3%) patients returning for FU. The relative frequency of patients involved in jobs with physical activity was 30.5%, 16.7% were unable to work or unemployed, and 21% had a sedentary job or did light physical labor (29.2%). The relative frequency of COMBINED patients engaged in jobs with physical activities (33%) was higher than in POSTERIOR patients (30%). At the same time, COMBINED patients (12.4%) were less likely to be retired or unable to work than POSTERIOR patients (22.2%) (p = 0.001). The relative frequency of polytraumatized patients available for FU within different surgical subgroups was POSTERIOR 12.9%, ANTERIOR 3.1%, and COMBINED 10.8%.
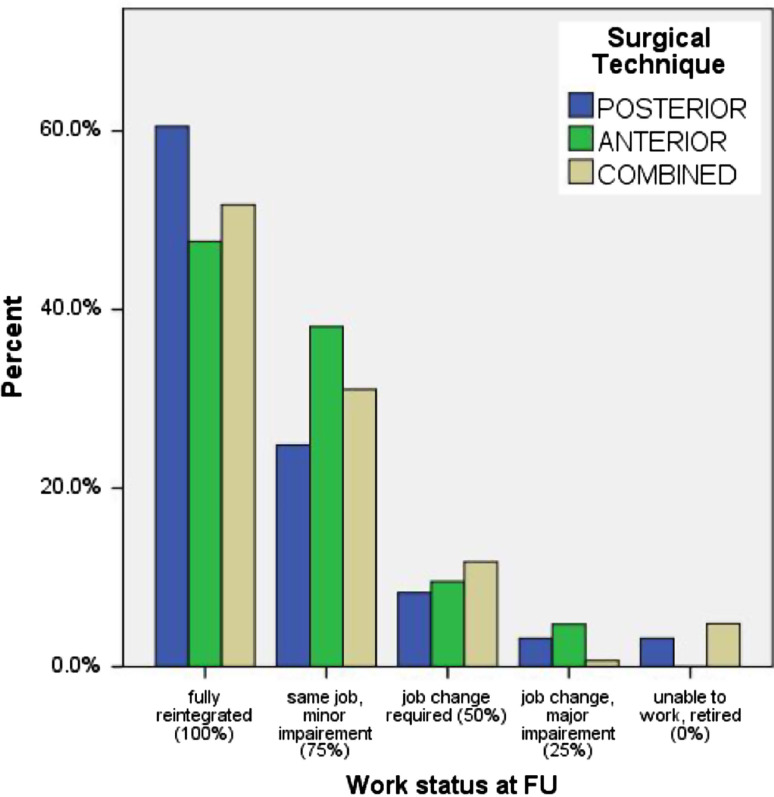
The median period of disability from work of 323 (57.9%) patients was 4 months. The level of injury did not have a significant influence on the disability time from work, but patients with neurological deficits were absent from work 9 months as compared to 5 months of those without neurological deficits (p < 0.001). Patient’s status with regard to the rehabilitation into work life (0, 25, 50, 75, or 100%) was assessed: 180 (32.3%) patients were fully rehabilitated and had returned to their previous occupation (100%), 92 (16.5%) returned to their same occupation with minor impairment (75%), 32 patients (5.7%) had to change jobs due to the injury (50%), 7 (1.3%) patients had significant impairments despite a job chance (25%), and 12 (2.2%) patients remained unfit to work or filed for early retirement (0%). 71.1% of patients with a sedentary occupation were fully rehabilitated (100%) into their previous jobs as compared to 38.9% of those involved in physical labor. Fully rehabilitated patients into work life occurred in 49.2% of T-spine, 58.7%, TL junction, and 48.6% L-spine injury. Figure 4 displays the work status after POSTERIOR and COMBINED treatment at FU.
Fig. 4.
Relative frequency of patients and work status at follow-up according to five categories with regard to surgical technique (blue POSTERIOR, green ANTERIOR, light brown COMBINED): (100%) fully reintegrated, same job before the time of injury; (75%) same job, but minor limitation on the job; (50%) change in jobs necessary; (25%) change in job with limitations; (0%) unable to work or retired
A total of 557 patients reported on their activity level during leisure time at FU, most data being obtained (n = 324) during the time period 1–2 years after the injury: 141 (25.3%) patients were able to return to the same recreational activities before the time of injury (100%); 237 (42.5%) participated in the same activities with minor limitations (75%); 139 (24.9%) had major limitations and changed activities during leisure time (50%); 22 (3.9%) were only capable of every day activities without additional activities during their leisure time (25%); and 18 (3.2%) were dependent on someone’s help or were in the need for care (0%). Repetitive measures of 196 patients showed improvement over time with regard to relative frequency of patients enjoying an unrestricted activity level during their leisure time (100%) from 16.8% after 6–12 months, 22.1% after 1–2 years and 38.1% after more than 2 years after the time of injury. Overall, 25.3% (n = 141) of the patients regained a (100%) activity level at their last FU. POSTERIOR (n = 87 (31.2%) surgery rendered more patients fully functional during their leisure time than COMBINED [n = 50 (20.1%)] surgery (p = 0.001). Patients without neurologic deficits were more likely to regain 100% of their activity level than patients with neurologic deficits [OR 5.7 (p < 0.001)].
Radiological measurements
A detailed listing of the radiological measurements of the bisegmental Cobb angles (GDW) is presented with regard to surgical technique and level of injury at FU (Table 7). The postoperative correction of radiological malalignment and loss of correction at FU was calculated as the difference between FU and postoperative measurements. No significant differences or changes in the scoliotic deformity (SW) were observed over time or between treatment subgroups.
Table 7.
Summary radiological results and measurements (bisegmental Cobb angle) throughout the course of treatment with regard to the level of injury and surgical technique
| Level of injury | OR technique | Radiological results Cobb angle biseg (°) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Correction | Follow-up | Loss of correction | |||||||
| Number (%) | Mean | Number (%) | Mean | Number (%) | Mean | Number (%) | Mean | Number (%) | Mean | ||
| OP (n = 558) | 550 (98.5) | −8.8 | 539 (96.6) | −0.7 | 534 (95.7) | 8.1 | 536 (96.1) | −5.4 | 59 (93) | −4.5 | |
| T-spine | Total | 107 (95.5) | −19.6 | 103 (92) | −13.9 | 99 (88.4) | 6.1 | 108 (96.4) | −18.1 | 100 (89.3) | −4.6 |
| T1–T10 | POSTERIOR | 66 (94.3) | −19.4 | 61 (87.1) | −14.3 | 58 (82.9) | 5.9 | 69 (98.6) | −18.7 | 61 (87.1) | −5.3 |
| ANTERIOR | 3 (100) | −7.7 | 3 (100) | −6.3 | 3 (100) | 1.3 | 2 (66.7) | −13 | 2 (66.7) | −4.5 | |
| COMBINED | 38 (97.4) | −20.8 | 39 (100) | −13.9 | 38 (97.4) | 6.7 | 37 (94.9) | −17.4 | 37 (94.9) | −3.6 | |
| TL junction | Total | 364 (99.5) | −10.3 | 358 (97.8) | −0.9 | 357 (97.5) | 9.3 | 351 (95.9) | −5.8 | 344 (94) | −4.8 |
| T11–L2 | POSTERIOR | 166 (98.8) | −10.1 | 161 (95.8) | −0.1 | 160 (95.2) | 9.9 | 163 (97) | −6.6 | 157 (93.5) | −6.25 |
| ANTERIOR | 24 (100) | −7.5 | 24 (100) | −3 | 24 (100) | 4.6 | 23 (95.8) | −6.3 | 23 (95.8) | −3.3 | |
| COMBINED | 173 (100) | −10.9 | 173 (99.4 | −1.4 | 173 (99.4) | 9.5 | 165 (94.8 | −4.9 | 164 (94.3) | −3.6 | |
| L−spine | Total | 79 (98.8) | 12.7 | 78 (97.5) | 17.7 | 78 (97.5) | 4.7 | 77 (96.3 ) | 13.9 | 75 (93.8) | −3.1 |
| L2–L5 | POSTERIOR | 42 (100) | 14.1 | 41 (97.6) | 17.3 | 41 (97.6) | 2.8 | 40 (95.2) | 12.8 | 39 (92.9) | −3.7 |
| ANTERIOR | 2 (100) | 12.5 | 2 (100) | 12.5 | 2 (200) | 0 | 1 (50) | 1 (50) | |||
| COMBINED | 35 (97.2) | 11.2 | 35 (97.2) | 18.5 | 35 (97.2) | 7.3 | 36 (100) | 14.8 | 35 (97.2) | −3.1 | |
The average amount of correction of the traumatic deformity (pre vs. postop) and loss of correction until FU (post vs. FU) were calculated accordingly
Sagittal profile
The traumatic kyphotic deformity was corrected in all three OP subgroups (Fig. 5). At the time of injury, the kyphotic deformity varied according to the level of injury with an average of −20° at the T-spine, −10° TL junction, and 12° at the L-spine. Postoperative measurements were −14° T-spine, −1° TL junction, and 18° L-spine resulting in a correction of 6° at the T-spine, 9° TL junction, and 5° at the L-spine. At FU, the average biseg GDW was −19° T-spine, −6° TL junction, and 14° L-spine correspond to a loss of correction of −5° T-spine, −5° TL junction, and −3° L-spine. COMBINED treatment showed the best radiological results at all levels of injury with less loss of correction (T-spine −3.6°, TL junction −3.6°, L spine −3.1°). The FU results after POSTERIOR treatment (T-spine −18.7°, TL junction −6.6, L spine 12.8°) showed more residual kyphotic deformity than COMBINED treatment (T-spine −17.4°, TL junction −4.9°, L spine 14.8°). Multivariate analysis (ANOVA) was carried out with consideration of different distributions of fracture type, patient age, level of injury, and deformity at the time of admission to the hospital between different surgical subgroups. Radiological FU data were obtained during the 1–2 years for 54.7% (n = 305) of the cases [>2 years 18.3% (n = 102), and 6–12 months 17.9% (n = 100)] after the time of injury. The ANOVA of the monoseg. [COMBINED (−2.8°) vs. POSTERIOR (−7.4°) (p < 0.001)] and biseg GDW [COMBINED (−3.8°) vs. POSTERIOR (−6.1°) (p = 0.005)] showed significantly less loss of correction after COMBINED surgery. The level of injury, radiological deformity before surgery, and patient age had a significant effect on the radiological results at FU (p < 0.001). The monoseg GDW of patients with (−8.3°) or without (−8.6°) a posterior spondylodesis and POSTERIOR treatment did not differ significantly at FU, but significant less loss of correction when measuring the biseg GDW in patients with a posterior spondylodesis (−5.8°) than without (−8.2°) (p = 0.049). After COMBINED treatment, no significant differences between patients with or without a bony posterior spondylodesis were noted. The implant removal did not have a significant influence on radiological FU results in POSTERIOR patients. Instead patients with an implant removal (biseg GDW −4.4°) in the COMBINED treatment subgroup showed a significantly greater loss of correction at FU than in COMBINED patients without implant removal (biseg GDW −0.9°) (p < 0.05). Different combinations and surgical techniques for anterior column support and reconstruction are possible: anterior strut graft, VB replacement implants (cages), with or without additional anterior plates. The use of an additional anterior plates did not result in better radiological FU results in either one of the combinations with strut grafts or cages (p = 0.34). Cages showed less loss of correction at FU (biseg GDW of 0.3° vs. −3.7°) than bony strut graft.
Fig. 5.
Boxplots diagrams of the preoperative-, postoperative- and biseg Cobb angles (°) at FU with regard to surgical technique, level of injury
Matched-pair analysis of ANTERIOR vs. COMBINED surgery
A matched-pair analyses was done for the comparison of compression (type A) fractures after isolated ANTERIOR treatment with a bone graft or cage and anterior plate (n total = 31) versus COMBINED posteroanterior treatment with a bone graft or cage without anterior plate (n total = 122). A frequency match with the following criteria resulted in two identical groups with a 1–2 ratio (ANTERIOR n = 19 to COMBINED n = 37):
compression injuries (Magerl/AO type A);
no neurologic deficits (Frankel/ASIA E);
TL-junction injuries;
patient age 21–60 years;
FU >6 months.
Significant differences between subgroups included the intraoperative average blood loss of 400 ml in ANTERIOR surgery versus 675 ml COMBINED and superior postoperative radiological correction of the traumatic deformity with COMBINED (GDW biseg 3.5°) than ANTERIOR (GDW biseg −5°) surgery (p < 0.05).
Discussion
During the last decade, treatment options for thoracolumbar spinal injuries have changed considerably. Less-invasive approaches and new implant designs have been developed and broadened the spine surgeon’s therapeutic options. As a consequence, the Spine Study Group of the German Association of Trauma Surgery initiated the second, prospective multicenter study (MCSII). The aim of this study was to address recent changes in spinal surgery to supplement the 1994–1996 MCSI results [41–43], and to redefine the “state-of-the-art” of TL spinal injury treatment in German-speaking countries. Data collection was accomplished with a new, internet-based application [45]; 733 consecutive patients receiving operative treatment from eight trauma centers were included during a 24-month period. Up to now, there are only two large prospective multicenter studies published on treatment of thoracolumbar spinal fractures [31, 41–43]. A multicenter study is a logistic challenge and requires detailed planning, close monitoring, and a motivated study group. In compliance with these requirements, multicenter studies allow for the recruitment of larger, thus more representative study populations than individual case series or retrospective literature reviews [23, 83]. A newly developed database system was evaluated and used to collect all study parameters [45]. Nevertheless, the authors believe that the system can be improved and can become more efficient, when enhanced by routines that will facilitate the data input and allow for a better data backflow to individual participating centers and the clinical study administration. Hu et al. [37] detected 944 patients with spinal injuries in an insurance database (MHSIP) of patients admitted to hospital over 36-month time period. The gender ratio was ♀60: ♂40 of rather young male (2nd and 3rd life decade) and older female patients (6–7th life decade). According to this and the literature, the typical traumatic spinal injury in male patient is 60–70% of the cases and 30–40 years of age [31, 41, 83]. Because of the characteristic distribution of several typical key parameters, e.g. patient age, gender distribution, and level of injury, this study population is representative for patients with acute traumatic spinal injuries and can be as compared to those of similar studies [31, 76, 78]. Over time a slight increase in the average patient age (MCSI 39 vs. MCSII 41 years) can be detected and is in accordance with other literature [39] and databases [80] resulting in an average age for patients with traumatic spinal injuries of 28.6 years between 1973 and 1977, and 33.5 years between 1982 and 1989 [16]. During the years of 1997 until 2001 and in a Canadian population, Pickett et al. [64] calculated an average patient age of 42 years. In the pertinent literature high-energy trauma “fall from a height” (40–60%) and “motor vehicle accidents” (25–40%) are described as the main causes of injury [10, 31, 39, 41, 63]. A high-energy trauma usually correlates with a higher incidence of more severe type B and type C spinal injuries [54]. More recently, the so-called “simple” falls at ground level has become the second leading cause of injury with increasing numbers of elderly patients sustaining traumatic spinal injuries. In the 1970s, 16.5% of traumatic spinal injuries with neurologic deficits were caused by simple falls and have increased up to 23.8% in the early 2000s [39]. Appropriate precautionary measures to avoid simple falls in geriatric patients are essential [53] and should be addressed specifically to prevent this source of spinal injuries in the near future [64]. The most exposed levels of traumatic spinal injuries are the thoracolumbar junction and mid-thoracic spine (T5–T7) [31, 41, 54].
Until the 1990s, the focus of interest was mainly on posterior stabilization techniques [83], whereas anterior [21, 38, 40] and combined procedures [6, 22, 74] were spotlighted during the past two decades. This trend was reflected by the numbers from 1994 to 1996 MCSI and this study showing a continuous increase in combined surgery for TL-junction injuries from 34% (MCSI) up to 47% (MCSII). At the same time, less-invasive procedures, e.g. endoscopic procedures and intraoperative navigation, have further broadened the therapeutic spectrum.
In this study, angular stable implants were used in 87% of the posterior and 55% of anterior surgical procedures. This trend can be explained as a result of the superior biomechanical properties of angular stable plate or rod systems when compared with conventional implants [34, 52]. In this context, the relevance of newly developed screw designs and their configuration within the VB have to be considered as well [33, 72, 75]. New expandable anterior devices can be implanted via thoracoscopic approaches; thus, reducing the perioperative morbidity and pain with better lung function as compared to conventional thoracotomies [8, 15, 50, 65]. Evidence-based guidelines for thoracolumbar fracture treatment are desirable, but scarce in the pertinent literature [82]. Instead, the treatment recommendations for burst fractures (AO/Magerl type A3) will vary and cover the complete therapeutical spectrum from non-operative to rather sophisticated combined posteroanterior surgical reconstruction techniques [1, 56, 57, 82]. Danisa et al. [21] compared unstable burst fractures at the TL junction without neurological deficits after anterior, posterior and combined instrumentations. They found significantly less OR time, less intraoperative blood loss and superior cost-effectiveness in favor of the posterior instrumentations with similar results of the remaining postoperative radiological deformity, pain, and rehabilitation time. POSTERIOR and COMBINED patients of this study did not show significant differences with regard to age and gender distribution, although higher numbers of the more severe injuries (AO/Magerl type B/C) with neurological deficits were located in the COMBINED subgroup.
Overall, neurologic deficits in TL spinal injuries occur in 22–45% of the cases [23, 31, 35, 54] and have a strong impact on the overall prognosis and patient outcome. In the literature, there are no specific recommendations on how and when to address spinal canal encroachment from retropulsed bony fragments and some authors do not directly relate posttraumatic neurologic deficits to spinal canal encroachment [4, 20, 62, 66]. In a meta-analysis of 275 publications, Boerger et al. found that surgical decompression of the spinal canal solely to attempt to improve neurological deficits would not be justified [11]. Nevertheless, patients of this study with a pronounced spinal canal encroachment did have a significantly higher risk of neurological deficits and confirmed by a majority of relevant literature [25, 55, 61, 81]. Experimental [17, 24, 77] and clinical studies [19, 27, 49, 68] substantiated that a rapid surgical decompression and spinal canal clearance from bony fragments in patients with incomplete neurological deficits renders beneficial results with a better outcome [12, 40, 55].
Mono- and bisegmental Cobb angles are reliable parameters for the assessment of the radiological or sagittal spinal alignment throughout the course of treatment [48]. This study showed better correction of the posttraumatic deformity and favorable radiological FU results after COMBINED surgical treatment to the POSTERIOR or ANTERIOR surgical subgroups.
The spectrum of observed intra- and postoperative complications in spinal surgery has been reported before [70]. Overall, 83% of the cases remained uneventful and without complications until FU. Both MCS revealed a higher complication rate after COMBINED [15.1% (MCSII) vs. 13.7% (MCSI)] than POSTERIOR [11.7% (MCSII) vs. 14.1% (MCSI)] surgery. A realistic estimate of perioperative complication in spinal surgery is thus 15% and supported by findings of other pro- [67] and retrospective studies [70, 83].
558 (76%) of 733 patients could be recruited for at least one or more than one consecutive FU examinations during the 30-month period of time.
The average time of inpatient rehabilitation was 3–4 weeks followed by 4 months of outpatient rehabilitation. One decade ago, rehabilitation periods were significantly longer with 6 weeks inpatient rehabilitation and 11 months outpatient rehabilitation [43]. Possible explanations for the shortening of the registered time period for rehabilitation programs could have either medical reasons, e.g. an improved treatment, or could be attributed to non-medical factors as well, e.g. a more restrictive granting of funds by governmental medical sponsors and/or private insurance companies. It is possible that patients with neurological deficits or polytraumatized patients require longer rehabilitations to recover from their injuries [35, 36]. During hospitalization, the numbers of patients with neurologic deficits decreased from 21 to 14%. In the pertinent literature, recovery rates range from 17 to 100% after incomplete and 0–100% after complete neurologic deficits [23, 28, 43, 83]. In this study, 73% (69 out of 95 patients) recovered from incomplete neurologic deficits, respectively 44% (12 out of 27 patients) from complete spinal cord injury (Frankel/ASIA A). Recovery from neurologic deficits was slightly higher after COMBINED (61.3%; 46 out of 75 patients) as compared to POSTERIOR treatment (59.6%; 34 out of 57 patients). When looking at neurological recovery, the severity of the initial neurologic injury [83], as well as the time interval between the injury and first decompression should be considered [18, 69]. A traumatic spinal injury with neurologic deficits is a complex, multifactorial event usually adhering to multiple biological and pathomechanical determinates, making it difficult to determine the true impact of a specific surgical technique with regard to neurologic recovery. Some authors report a better neurologic outcome after anterior decompression [14, 32, 47, 55], while others did not find statistically significant differences [21, 26, 83]. Up till now, the authors are not aware of strong support in favor of a particular surgical technique with superior results in terms of better neurologic recovery.
Different psychometric scores have been developed to measure the clinical success of treatment methods besides established objective clinical parameters to allow for better, indepth subjective assessment from the patient’s point of view [13]. In this study, the VAS spine score was chosen to measure the subjective functioning after spinal injuries. Statistically significant differences between COMBINED and POSTERIOR subgroups at FU were found after injury at the T-spine level only. 1–2 years after the initial injury on average 19% of the patients (24% POSTERIOR and 17% COMBINED) regained their full and unrestricted back function, 25% (31% POSTERIOR and 20% COMBINED) were able to participate at the same activity level during leisure time, and 32% had resumed the same job before the time of injury without limitations. McLain et al. reported 70% of patients after significant traumatic TL spinal injury and posterior surgery being able to maintain full time employment 5 years after the injury; of those patients 54% remained on the same job and 16% had to change jobs [58]. This study showed that a persistent neurologic impairment is the most important predictor of the overall functional outcome after acute traumatic thoracolumbar spinal injury. In accordance with our findings, the magnitude of the neurologic injury correlated significantly with the reintegration to work as well (p < 0.00005) [58]. In a prospective randomized study Korovessis et al. compared COMBINED with isolated POSTERIOR surgery for type A3 burst fractures of the middle lumbar spine (L2–L4). Their findings indicated better functional outcome in the SF36 role physical scale SF36 (p = 0.05) and bodily pain scale (p = 0.06), shorter OR time, less blood loss and less perioperative complications after posterior surgery. Nevertheless, Korovessis et al. [46] recommended COMBINED surgery for A3 burst fractures because POSTERIOR treatment alone was not able to maintain the surgically achieved correction. Dickman et al. in a meta-analysis of 58 publications did not find differences in the functional outcome and pain after combined or posterior treatment for TL spinal injuries [23], nor did Danisa et al. [21] in their study in terms of pain and reintegration into work life. The comparison of ANTERIOR vs. COMBINED surgery for type A compression fractures in this study did not demonstrate significant differences of the subjective or functional outcome, but favorable radiological results with a better realignment of the sagittal profile following COMBINED surgery. Meta-analyses and literature reviews of the radiological outcomes after surgery for TL injury are limited [23], or showed similar findings of approximately 10° loss of correction after long-, short-posterior or combined surgery as well [83]. A higher primary and overall construct biomechanical stability of a combined posteroanterior stabilization might imply the significant better radiological results after COMBINED surgery [9, 73]. Esses et al. [26] looked at ANTERIOR vs. POSTERIOR instrumentations, and found that posterior instrumentations (11.3°) were superior to anterior instrumentation (9.3°) for the reconstruction of the sagittal profile with less remaining kyphotic deformity at a 12 months (12–20 months) FU, but without statistical significance. Been et al. [6] compared long-term results after 6 years of posterior vs. combined instrumentations and found no clinical-functional differences between both the groups, but a higher rate of implant failure and more loss of correction after posterior surgery alone. Several studies have highlighted the importance of a sufficient reconstruction of the anterior load-bearing column as a precaution to prevent a secondary loss of correction and implant failure [51, 59, 60, 79]. For these reasons and based on this study’s finding, the concept of combined posterior–anterior (COMBINED) surgery for the treatment of Magerl type B, C and type A3 compression injuries that are perceived to be insufficient to resist a physiological axial load has become increasingly established within members of the German Spine Study Group during the past 10 years. This trend is reflected by an increasing number for COMBINED surgery when comparing MCSI and MCSII. There is a controversy about the use of artificial VB replacement implants (cages) or autologous strut grafts. In this study, less loss of correction was observed when using cages as compared to bone grafts. Despite some technical advances in recent expandable cages [71], it remains the surgeon’s preference and individual decision in favor of cages or bone graft when in need for anterior vertebral column support due to a lack of long-term results for any of the latest titanium cages. The relevant comorbidity and complications at the donor site of autologous bone grafts should be considered [29]. In accordance with the findings of Wang et al. [84], we did not see significant influence of POSTERIOR spondylodesis on the radiological FU results. Wang et al. [84] found that in cases with short-posterior instrumentations, a shorter OR time, less blood loss and no additional comorbidity due to the harvest of autologous bone graft would be beneficial. This study showed that short angular stable posterior pedicle screw instrumentation systems have replaced longer hock and rod constructs or plate systems. Short instrumentations in 1–2 motion segment injuries rendered less patient complaint at the surgical access site than in patients with multi-segment instrumentations (>2 motion segment injuries).
In summary, our study showed (1) better radiological FU results after combined posterior–anterior surgery (COMBINED) but (2) better clinical, functional outcome after posterior surgery (POSTERIOR) alone. Possible explanations for these findings could include the higher patient number of more severe type B and type C injuries with a higher incidence of concomitant neurological injuries, or the higher morbidity of a combined surgical approach. It remains questionable if better radiological results, e.g. a better reconstruction of the physiological spinal alignment will eventually become significant with regard to the subjective outcome on a long-term basis over years to come. The MCSII findings do not allow for distinct or evidence-based treatment recommendations, but represent a comprehensive update of today’s common clinical practice in eight large trauma centers involved with the operative care for acute thoracolumbar spinal injuries. Randomised multicenter study designs with access to similar large patient numbers will be needed to find the answers and solutions to remaining questions for an improved care of patients with in the future.
Summary and conclusions
The second, internet-based multicenter study (MCSII) of the Spine Study Group of the German Association of Trauma Surgery (Deutsche Gesellschaft für Unfallchirurgie) is a representative patient collection of acute traumatic thoracolumbar (T1–L5) injuries. The MCSII results are an update of those obtained with the first multicenter study (MCSI) more than a decade ago. The aim of the study was to assess and bring into focus: (1) epidemiologic data, (2) surgical and radiological outcome, and (3) 2-year FU results of these injuries.
According to the Magerl/AO classification, there were 424 (57.8%) compression fractures (A type), 178 (24.3%) distractions injuries (B type), and 131 (17.9%) rotational injuries (C type). B and C type injuries carried a higher risk for neurological deficits, concomitant injuries, and multiple vertebral fractures. The level of injury was located at the thoracolumbar junction (T11–L2) in 67.0% of the case.
380 (51.8%) patients were operated on by posterior stabilization and instrumentation alone (POSTERIOR), 34 (4.6%) had an anterior procedure (ANTERIOR), and 319 (43.5%) patients were treated with combined posteroanterior surgery (COMBINED). 65% of patients with thoracic (T1–T10) and 57% with lumbar spinal (L3–L5) injuries were treated with a single posterior approach (POSTERIOR). 47% of the patients with thoracolumbar junction (T11–L2) injuries were either operated from posterior or with a combined posterior–anterior surgery (COMBINED) each. Short angular stable implant systems have replaced conventional non-angular stable instrumentation systems to a large extent. The posttraumatic deformity was restored best with COMBINED surgery. T-spine injuries were accompanied by a higher number and more severe neurologic deficits than TL junction or L-spine injuries. At the same time, T-spine injuries showed less potential for neurologic recovery especially in paraplegic (Frankel/AISA A) patients. 5% of all patients required revision surgery for perioperative complications.
Follow-up data of 558 (76.1%) patients were available and collected during a 30-month period from 1 January 2004 until 31 May 2006. On average, a posterior implant removal was carried out in a total of 382 COMBINED and POSTERIOR patients 12 months after the initial surgery. On average, the rehabilitation process required 3–4 weeks of inpatient treatment, followed by another 4 months of outpatient therapy and was significantly shorter when compared with MCSI in the mid-1990s. From the time of injury until FU 80 (60.6%) of 132 patients with initial neurological deficits improved at least one grade on the Frankel/ASIA scale; 8 (1.3%) patients deteriorated. A higher recovery rate was observed for incomplete neurological injuries (73%) than complete neurological injuries (44%). Different surgical approaches did not have a significant influence on the neurologic recovery until FU. Nevertheless, neurological deficits are the most important factors for the functional outcome and prognosis of TL spinal injuries.
POSTERIOR patients had a better functional and subjective outcome at FU than COMBINED patients. However, the posttraumatic radiological deformity was best corrected in COMBINED patients and showed significantly less residual kyphotic deformity (biseg GDW: −3.8° COMBINED versus −6.1° POSTERIOR) at FU (p = 0.005). The sagittal spinal alignment was better maintained when using VB replacement implants (cages) in comparison to iliac strut grafts. Additional anterior plate systems did not have a significant influence on the radiological FU results.
In conclusion, comprehensive data of a large patient population with acute thoracolumbar spinal injuries has been obtained and analyzed with this prospective internet-based multicenter study. Thus, updated results and the clinical outcome of the current operative treatment strategies in participating German and Austrian trauma centers have been presented. Nevertheless, it was not possible to answers all remaining questions to contradictory findings of the subjective, clinical outcome and corresponding radiological findings between different surgical subgroups. Randomized-controlled long-term investigations seem mandatory and the next step in future clinical research of Spine Study Group of the German Trauma Society.
Acknowledgments
The authors like to thank all clinical collaborators of the Spine Study Group of the German Association of Trauma Surgery Spine for their exceptional commitment to this project throughout the past years. Special thanks go to PD. Dr. L. Audigé for his expert advice and Mark Kanodi, MSc for help with the manuscript preparation.
Conflict of interest statement
This study was supported by the German Association of Trauma Surgery.
Footnotes
Comorbidities were graded according to an anatomical injury scale (Abbreviate Injury Scale = AIS) [2] from 0, meaning “no injury” up to 5, meaning “critical injury, survival unlikely”.
References
- 1.Agus H, Kayali C, Arslantas M. Nonoperative treatment of burst-type thoracolumbar vertebra fractures: clinical and radiological results of 29 patients. Eur Spine J. 2005;14(6):536–540. doi: 10.1007/s00586-004-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Association for Automotive Medicine (1980) Die abgekürzte Verletzungsskala (AIS). Revidierte Fassung ed. Morton Grove, IL 60053, USA
- 3.American Spinal Injury Association (1992) ASIA classification: Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injuries Association, Chicago [DOI] [PubMed]
- 4.Atlas SW, Regenbogen V, Rogers LF, Kim KS. The radiographic characterization of burst fractures of the spine. AJR Am J Roentgenol. 1986;147(3):575–582. doi: 10.2214/ajr.147.3.575. [DOI] [PubMed] [Google Scholar]
- 5.Baker SP, O’Neill B, Haddon WJ, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. doi: 10.1097/00005373-197403000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Been HD, Bouma GJ. Comparison of two types of surgery for thoraco-lumbar burst fractures: combined anterior and posterior stabilisation vs. posterior instrumentation only. Acta Neurochir (Wien) 1999;141(4):349–357. doi: 10.1007/s007010050310. [DOI] [PubMed] [Google Scholar]
- 7.Beisse R, Muckley T, Schmidt MH, Hauschild M, Buhren V. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2(2):128–136. doi: 10.3171/spi.2005.2.2.0128. [DOI] [PubMed] [Google Scholar]
- 8.Beisse R, Potulski M, Bühren V. Thorakoskopisch gesteuerte ventrale Plattenspondylodese bei Frakturen der Brust- und Lendenwirbelsäule. Operat Orthop Traumatol. 1999;11(1):54–69. doi: 10.1007/s00064-006-0083-8. [DOI] [PubMed] [Google Scholar]
- 9.Bence T, Schreiber U, Grupp T, Steinhauser E, Mittelmeier W. Two column lesions in the thoracolumbar junction: anterior, posterior or combined approach? A comparative biomechanical in vitro investigation. Eur Spine J. 2007;16(6):813–820. doi: 10.1007/s00586-006-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensch FV, Koivikko MP, Kiuru MJ, Koskinen SK. The incidence and distribution of burst fractures. Emerg Radiol. 2006;12(3):124–129. doi: 10.1007/s0010140-005-0457-5. [DOI] [PubMed] [Google Scholar]
- 11.Boerger TO, Limb D, Dickson RA. Does ‘canal clearance’ affect neurological outcome after thoracolumbar burst fractures? J Bone Joint Surg Br. 2000;82(5):629–635. doi: 10.1302/0301-620X.82B5.11321. [DOI] [PubMed] [Google Scholar]
- 12.Bohlman HH, Kirkpatrick JS, Delamarter RB, Leventhal M (1994) Anterior decompression for late pain and paralysis after fractures of the thoracolumbar spine. Clin Orthop Relat Res (300):24–29 [PubMed]
- 13.Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders: summary and general recommendations. Spine. 2000;25(24):3100–3103. doi: 10.1097/00007632-200012150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Bradford DS, McBride GG (1987) Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin Orthop (218):201–216 [PubMed]
- 15.Buhren V, Beisse R, Potulski M. Minimally invasive ventral spondylodesis in injuries to the thoracic and lumbar spine. Chirurg. 1997;68(11):1076–1084. doi: 10.1007/s001040050326. [DOI] [PubMed] [Google Scholar]
- 16.Burney RE, Maio RF, Maynard F, Karunas R. Incidence, characteristics, and outcome of spinal cord injury at trauma centers in North America. Arch Surg. 1993;128(5):596–599. doi: 10.1001/archsurg.1993.01420170132021. [DOI] [PubMed] [Google Scholar]
- 17.Carlson GD, Gorden CD, Oliff HS, Pillai JJ, LaManna JC. Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am. 2003;85-A(1):86–94. [PubMed] [Google Scholar]
- 18.Clohisy JC, Akbarnia BA, Bucholz RD, Burkus JK, Backer RJ. Neurologic recovery associated with anterior decompression of spine fractures at the thoracolumbar junction (T12–L1) Spine. 1992;17(8 Suppl):S325–S330. doi: 10.1097/00007632-199208001-00019. [DOI] [PubMed] [Google Scholar]
- 19.Croce MA, Bee TK, Pritchard E, Miller PR, Fabian TC. Does optimal timing for spine fracture fixation exist? Ann Surg. 2001;233(6):851–858. doi: 10.1097/00000658-200106000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai LY, Wang XY, Jiang LS (2007) Neurologic recovery from thoracolumbar burst fractures: is it predicted by the amount of initial canal encroachment and kyphotic deformity? Surg Neurol 67(3):232–237 [DOI] [PubMed]
- 21.Danisa OA, Shaffrey CI, Jane JA, Whitehill R, et al. Surgical approaches for the correction of unstable thoracolumbar burst fractures: a retrospective analysis of treatment outcomes. J Neurosurg. 1995;83(6):977–983. doi: 10.3171/jns.1995.83.6.0977. [DOI] [PubMed] [Google Scholar]
- 22.Defino HL, Rodriguez-Fuentes AE. Treatment of fractures of the thoracolumbar spine by combined anteroposterior fixation using the Harms method. Eur Spine J. 1998;7(3):187–194. doi: 10.1007/s005860050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickman CA, Yahiro MA, Lu HT, Melkerson MN. Surgical treatment alternatives for fixation of unstable fractures of the thoracic and lumbar spine. A meta-analysis. Spine. 1994;19(20 Suppl):2266S–2273S. doi: 10.1097/00007632-199410151-00003. [DOI] [PubMed] [Google Scholar]
- 24.Dimar JR, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine. 1999;24(16):1623–1633. doi: 10.1097/00007632-199908150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Dunn HK (1984) Anterior stabilization of thoracolumbar injuries. Clin Orthop (189):116–124 [PubMed]
- 26.Esses SI, Botsford DJ, Kostuik JP. Evaluation of surgical treatment for burst fractures. Spine. 1990;15(7):667–673. doi: 10.1097/00007632-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13–B26. doi: 10.1016/j.injury.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Frankel HL, Hancock DO, Hyslop G, Melzak J, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia I. Paraplegia. 1969;7(3):179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 29.Frymoyer JW, Howe J, Kuhlmann D (1978) The long-term effects of spinal fusion on the sacroiliac joints and ileum. Clin Orthop Relat Res (134):196–201 [PubMed]
- 30.Gebhard F, Weidner A, Liener UC, Stockle U, Arand M. Navigation at the spine. Injury. 2004;35(Suppl 1):S–S45. doi: 10.1016/j.injury.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Gertzbein SD. Scoliosis Research Society. Multicenter spine fracture study. Spine. 1992;17(5):528–540. doi: 10.1097/00007632-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Ghanayem AJ, Zdeblick TA (1997) Anterior instrumentation in the management of thoracolumbar burst fractures. Clin Orthop (335):89–100 [PubMed]
- 33.Goldhahn J, Reinhold M, Stauber M, Knop C, et al. Improved anchorage in osteoporotic vertebrae with new implant designs. J Orthop Res. 2006;24(5):917–925. doi: 10.1002/jor.20133. [DOI] [PubMed] [Google Scholar]
- 34.Haug RH, Street CC, Goltz M. Does plate adaptation affect stability? A biomechanical comparison of locking and nonlocking plates. J Oral Maxillofac Surg. 2002;60(11):1319–1326. doi: 10.1053/joms.2002.35732. [DOI] [PubMed] [Google Scholar]
- 35.Hebert JS, Burnham RS. The effect of polytrauma in persons with traumatic spine injury. A prospective database of spine fractures. Spine. 2000;25(1):55–60. doi: 10.1097/00007632-200001010-00011. [DOI] [PubMed] [Google Scholar]
- 36.Heyde CE, Ertel W, Kayser R. Management of spine injuries in polytraumatized patients. Orthopade. 2005;34(9):889–905. doi: 10.1007/s00132-005-0847-0. [DOI] [PubMed] [Google Scholar]
- 37.Hu R, Mustard CA, Burns C. Epidemiology of incident spinal fracture in a complete population. Spine. 1996;21(4):492–499. doi: 10.1097/00007632-199602150-00016. [DOI] [PubMed] [Google Scholar]
- 38.Huang TJ, Chen JY, Shih HN, Chen YJ, Hsu RW. Surgical indications in low lumbar burst fractures: experiences with anterior locking plate system and the reduction-fixation system. J Trauma. 1995;39(5):910–914. doi: 10.1097/00005373-199511000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Jackson AB, Dijkers M, Devivo MJ, Poczatek RB. A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch Phys Med Rehabil. 2004;85(11):1740–1748. doi: 10.1016/j.apmr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 40.Kaneda K, Abumi K, Fujiya M. Burst fractures with neurologic deficits of the thoracolumbar–lumbar spine. Results of anterior decompression and stabilization with anterior instrumentation. Spine. 1984;9(8):788–795. doi: 10.1097/00007632-198411000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Knop C, Blauth M, Bühren V, Hax PM, et al. Operative Behandlung von Verletzungen des thorakolumbalen Übergangs: Teil 1 “Epidemiologie”. Unfallchirurg. 1999;102(12):924–935. doi: 10.1007/s001130050507. [DOI] [PubMed] [Google Scholar]
- 42.Knop C, Blauth M, Bühren V, Hax PM, et al. Operative Behandlung von Verletzungen des thorakolumbalen Übergangs: Teil 2 “Operation und Radiologie”. Unfallchirurg. 2000;103:1032–1047. doi: 10.1007/s001130050667. [DOI] [PubMed] [Google Scholar]
- 43.Knop C, Blauth M, Buhren V, Arand M, et al. Operative Behandlung von Verletzungen des thorakolumbalen Überganges—Teil 3: Nachuntersuchung. Unfallchirurg. 2001;104(7):583–600. doi: 10.1007/s001130170089. [DOI] [PubMed] [Google Scholar]
- 44.Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. Entwicklung und Validierung des VAS-Wirbelsäulenscores [Development and validation of the Visual Analogue Scale (VAS) Spine Score] Unfallchirurg. 2001;104(6):488–497. doi: 10.1007/s001130170111. [DOI] [PubMed] [Google Scholar]
- 45.Knop C, Reinhold M, Roeder C, Staub L, et al. Internet based multicenter study for thoracolumbar injuries: a new concept and preliminary results. Eur Spine J. 2006;15(11):1687–1694. doi: 10.1007/s00586-006-0135-7. [DOI] [PubMed] [Google Scholar]
- 46.Korovessis P, Baikousis A, Zacharatos S, Petsinis G, Koureas G, Iliopoulos P. Combined anterior plus posterior stabilization versus posterior short-segment instrumentation and fusion for mid-lumbar (L2–L4) burst fractures. Spine. 2006;31(8):859–868. doi: 10.1097/01.brs.0000209251.65417.16. [DOI] [PubMed] [Google Scholar]
- 47.Kostuik JP. Anterior fixation for burst fractures of the thoracic and lumbar spine with or without neurological involvement. Spine. 1988;13(3):286–293. doi: 10.1097/00007632-198803000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Kuklo TR, Potter BK, Ludwig SC, Anderson PA, Lindsey RW, Vaccaro AR. Radiographic measurement techniques for sacral fractures consensus statement of the Spine Trauma Study Group. Spine. 2006;31(9):1047–1055. doi: 10.1097/01.brs.0000214940.11096.c8. [DOI] [PubMed] [Google Scholar]
- 49.La Rosa G, Conti A, Cardali S, Cacciola F, Tomasello F. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42(9):503–512. doi: 10.1038/sj.sc.3101627. [DOI] [PubMed] [Google Scholar]
- 50.Landreneau RJ, Hazelrigg SR, Mack MJ, Dowling RD, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56(6):1285–1289. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 51.Lim TH, An HS, Hong JH, Ahn JY, et al. Biomechanical evaluation of anterior and posterior fixations in an unstable calf spine model. Spine. 1997;22(3):261–266. doi: 10.1097/00007632-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 52.Linke B, Butscher A, Schneider R, Wahl D, Gasser B (2003) Holding strength of conventional and locking head screws. Folia Traumatol Lovaniensia 68–79
- 53.Lohmann R, Haid K, Stockle U, Raschke M. Epidemiology and perspectives in traumatology of the elderly. Unfallchirurg. 2007;110(6):553–562. doi: 10.1007/s00113-007-1286-7. [DOI] [PubMed] [Google Scholar]
- 54.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3(4):184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 55.McAfee PC, Bohlman HH, Yuan HA. Anterior decompression of traumatic thoracolumbar fractures with incomplete neurological deficit using a retroperitoneal approach. J Bone Joint Surg Am. 1985;67(1):89–104. [PubMed] [Google Scholar]
- 56.McDonough PW, Davis R, Tribus C, Zdeblick TA. The management of acute thoracolumbar burst fractures with anterior corpectomy and Z-plate fixation. Spine. 2004;29(17):1901–1908. doi: 10.1097/01.brs.0000137059.03557.1d. [DOI] [PubMed] [Google Scholar]
- 57.McEvoy RD, Bradford DS. The management of burst fractures of the thoracic and lumbar spine. Experience in 53 patients. Spine. 1985;10(7):631–637. doi: 10.1097/00007632-198509000-00007. [DOI] [PubMed] [Google Scholar]
- 58.McLain RF. Functional outcomes after surgery for spinal fractures: return to work and activity. Spine. 2004;29(4):470–477. doi: 10.1097/01.BRS.0000092373.57039.FC. [DOI] [PubMed] [Google Scholar]
- 59.McLain RF. The biomechanics of long versus short fixation for thoracolumbar spine fractures. Spine. 2006;31(11 Suppl):S70–S79. doi: 10.1097/01.brs.0000218221.47230.dd. [DOI] [PubMed] [Google Scholar]
- 60.McLain RF, Sparling E, Benson DR. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures A preliminary report. J Bone Joint Surg Am. 1993;75(2):162–167. doi: 10.2106/00004623-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Meves R, Avanzi O. Correlation among canal compromise, neurologic deficit, and injury severity in thoracolumbar burst fractures. Spine. 2006;31(18):2137–2141. doi: 10.1097/01.brs.0000231730.34754.9e. [DOI] [PubMed] [Google Scholar]
- 62.Mohanty SP, Venkatram N. Does neurological recovery in thoracolumbar and lumbar burst fractures depend on the extent of canal compromise? Spinal Cord. 2002;40(6):295–299. doi: 10.1038/sj.sc.3101283. [DOI] [PubMed] [Google Scholar]
- 63.Otte D, Sandor L, Zwipp H. Bedeutung und Mechanismen von Brust- und Lendenwirbelsaulenverletzungen bei Verkehrsunfallen [Significance and mechanism of thoracic and lumbar spine injuries in traffic accidents] Bedeutung und Mechanismen von Brust- und Lendenwirbelsaulenverletzungen bei Verkehrsunfallen. Unfallchirurg. 1990;93(9):418–425. [PubMed] [Google Scholar]
- 64.Pickett GE, Campos-Benitez M, Keller JL, Duggal N. Epidemiology of traumatic spinal cord injury in Canada. Spine. 2006;31(7):799–805. doi: 10.1097/01.brs.0000207258.80129.03. [DOI] [PubMed] [Google Scholar]
- 65.Potulski M, Beisse R, Buhren V. Thoracoscopy-guided management of the “anterior column”. Methods and results. Orthopade. 1999;28(8):723–730. doi: 10.1007/s001320050402. [DOI] [PubMed] [Google Scholar]
- 66.Rahimi-Movaghar V, Vaccaro AR, Mohammadi M. Efficacy of surgical decompression in regard to motor recovery in the setting of conus medullaris injury. J Spinal Cord Med. 2006;29(1):32–38. doi: 10.1080/10790268.2006.11753854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rampersaud YR, Moro ER, Neary MA, White K, et al. Intraoperative adverse events and related postoperative complications in spine surgery: implications for enhancing patient safety founded on evidence-based protocols. Spine. 2006;31(13):1503–1510. doi: 10.1097/01.brs.0000220652.39970.c2. [DOI] [PubMed] [Google Scholar]
- 68.Rath SA, Kahamba JF, Kretschmer T, Neff U, Richter HP, Antoniadis G. Neurological recovery and its influencing factors in thoracic and lumbar spine fractures after surgical decompression and stabilization. Neurosurg Rev. 2005;28(1):44–52. doi: 10.1007/s10143-004-0356-3. [DOI] [PubMed] [Google Scholar]
- 69.Reinhold M, Knop C, Lange U, Rosenberger R, Schmid R, Blauth M. Reposition von Verrenkungen und Verrenkungsbrüchen der unteren Halswirbelsäule [Reduction of traumatic dislocations and facet fracture-dislocations in the lower cervical spine] Unfallchirurg. 2006;109(12):1064–1072. doi: 10.1007/s00113-006-1188-0. [DOI] [PubMed] [Google Scholar]
- 70.Reinhold M, Schmid R, Knop C, Blauth M. Komplikationsspektrum operativ versorgter Wirbelsäulenverletzungen Eine Analyse der Multicenterstudien I und II der AG Wirbelsäule. Trauma Berufskrankh. 2004;7(Supp 2):281–291. [Google Scholar]
- 71.Reinhold M, Schmolz W, Canto F, Krappinger D, Blauth M, Knop C. An improved vertebral body replacement for the thoracolumbar spine A biomechanical in vitro test on human lumbar vertebral bodies. Unfallchirurg. 2007;110(4):327–333. doi: 10.1007/s00113-006-1221-3. [DOI] [PubMed] [Google Scholar]
- 72.Reinhold M, Schwieger K, Goldhahn J, Linke B, Knop C, Blauth M. Influence of screw positioning in a new anterior spine fixator on implant loosening in osteoporotic vertebrae. Spine. 2006;31(4):406–413. doi: 10.1097/01.brs.0000199894.63450.70. [DOI] [PubMed] [Google Scholar]
- 73.Sasso RC, Renkens K, Hanson D, Reilly T, McGuire RA, Jr, Best NM. Unstable thoracolumbar burst fractures: anterior-only versus short-segment posterior fixation. J Spinal Disord Tech. 2006;19(4):242–248. doi: 10.1097/01.bsd.0000211298.59884.24. [DOI] [PubMed] [Google Scholar]
- 74.Schnee CL, Ansell LV. Selection criteria and outcome of operative approaches for thoracolumbar burst fractures with and without neurological deficit. J Neurosurg. 1997;86(1):48–55. doi: 10.3171/jns.1997.86.1.0048. [DOI] [PubMed] [Google Scholar]
- 75.Seller K, Wahl D, Wild A, Krauspe R, Schneider E, Linke B. Pullout strength of anterior spinal instrumentation: a product comparison of seven screws in calf vertebral bodies. Eur Spine J. 2007;16(7):1047–1054. doi: 10.1007/s00586-007-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seybold EA, Sweeney CA, Fredrickson BE, Warhold LG, Bernini PM. Functional outcome of low lumbar burst fractures A multicenter review of operative and nonoperative treatment of L3–L5. Spine. 1999;24(20):2154–2161. doi: 10.1097/00007632-199910150-00016. [DOI] [PubMed] [Google Scholar]
- 77.Shields CB, Zhang YP, Shields LB, Han Y, Burke DA, Mayer NW. The therapeutic window for spinal cord decompression in a rat spinal cord injury model. J Neurosurg Spine. 2005;3(4):302–307. doi: 10.3171/spi.2005.3.4.0302. [DOI] [PubMed] [Google Scholar]
- 78.Siebenga J, Leferink VJ, Segers MJ, Elzinga MJ, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31(25):2881–2890. doi: 10.1097/01.brs.0000247804.91869.1e. [DOI] [PubMed] [Google Scholar]
- 79.Speth MJ, Oner FC, Kadic MA, de-Klerk LW, Verbout AJ. Recurrent kyphosis after posterior stabilization of thoracolumbar fractures 24 cases treated with a Dick internal fixator followed for 15–4 years. Acta Orthop Scand. 1995;66(5):406–410. doi: 10.3109/17453679508995575. [DOI] [PubMed] [Google Scholar]
- 80.Spinalcord Injury Information Network (2007) The National SCI Database. http://www.spinalcord.uab.edu
- 81.Trafton PG, Boyd CA., Jr Computed tomography of thoracic and lumbar spine injuries. J Trauma. 1984;24(6):506–515. doi: 10.1097/00005373-198406000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Vaccaro AR, Lim MR, Hurlbert RJ, Lehman RA, Jr, et al. Surgical decision making for unstable thoracolumbar spine injuries: results of a consensus panel review by the Spine Trauma Study Group. J Spinal Disord Tech. 2006;19(1):1–10. doi: 10.1097/01.bsd.0000180080.59559.45. [DOI] [PubMed] [Google Scholar]
- 83.Verlaan JJ, Diekerhof CH, Buskens E, van der Tweel I, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine. 2004;29(7):803–814. doi: 10.1097/01.BRS.0000116990.31984.A9. [DOI] [PubMed] [Google Scholar]
- 84.Wang ST, Ma HL, Liu CL, Yu WK, Chang MC, Chen TH. Is fusion necessary for surgically treated burst fractures of the thoracolumbar and lumbar spine?: a prospective, randomized study. Spine. 2006;31(23):2646–2652. doi: 10.1097/01.brs.0000244555.28310.40. [DOI] [PubMed] [Google Scholar]