Abstract
Anterior cervical discectomy and fusion (ACDF) with cage alone (ACDF-C) is associated with a significant incidence of subsidence, local kyphosis, and migration. The use of concurrent plate augmentation may decrease the incidence of these complications while improving the fusion rate. The purpose of the study is to present our results with ACDF with cage and plate augmentation (ACDF-CPA) and to compare these results to previous reports of outcomes following ACDF-C. We evaluated the radiologic and clinical parameters of 83 patients (266 fusion sites) who had an ACDF-CPA between March 2002 and May 2006. Radiologic parameters included fusion rate, fusion time, fusion type, site of pseudoarthrosis and rate and degree of subsidence. Clinical parameters included complications and overall outcomes assessed with Robinson’s criteria; 79 of 83 patients showed bony fusion (95.1%) at last follow-up postoperatively, and there was no significant difference in fusion rate between the number of fusion levels. Type I (pseudoarthrosis) was noticed in 9 patients (12 fusion sites), type II in 14 (19 fusion sites), and type III in 60 (235 fusion sites). Five type I and all type II fusions converged into type III by the last follow-up; 76 of 83 patients (91.6%) experienced good clinical outcomes. Pseudoarthrosis occurred more commonly in more proximal locations, and the subsidence rate was significantly greater in two-level fusions when compared with single-level fusions (P = 0.046). There were four metal-related complications. Plate augmentation in one- or two-level anterior cervical fusions for degenerative cervical spine disorders may improve fusion rates and reduce subsidence and complication rates, resulting in improved clinical outcomes.
Keywords: Anterior cervical discectomy and fusion, Cage, Plate construct
Introduction
Anterior cervical discectomy and fusion (ACDF) with autograft is widely employed in the surgical treatment of degenerative cervical spine diseases. Unfortunately, the use of autogenous bone is associated with a significant degree of donor-site morbidity [1–4]. To avoid this adverse outcome, various bone graft substitutes, such as allograft [5, 6], xenograft [4], DBM (demineralized bone matrix) [2], and cages [1, 6–14] have been used.
Cervical cages composed of diverse materials (metal, carbon, PEEK, etc.) are currently used in ACDF. Polyetheretherketone (PEEK) cages have been widely utilized due to their biomechanical similarities to human bone, which prevent weakening of the vertebral bodies by decreasing the stress shield effect on the fusion site and accelerate bone fusion [6, 8, 10, 12, 13]. Currently, many surgeons perform these procedures without metal plate fixation [1, 6–14], which has an overall fusion rate, stability at the fusion site, and restoration of foraminal height comparable to the use of autogenous bone [1, 6, 8, 10, 13]. However, controversy exists regarding this practice due to limited long-term results and a substantial incidence of complications, such as cage subsidence, anterior migration, and local kyphosis [2, 9, 15, 16].
In an effort to decrease the incidence of these complications, we performed ACDF with cages and metal plate augmentation (ACDF-CPA). The purpose of this study is to present our results with ACDF-CPA and to compare these results to previous reports of outcomes following ACDF-C (ACDF with cage alone).
Materials and methods
Materials
A total of 83 subjects (266 fusion sites) who had an anterior cervical discectomy and fusion with cage and plate augmentation for degenerative cervical spine diseases from March 2002 to May 2006 with a minimum 2-year follow-up were included in this study. There were 53 males and 30 females with a mean age of 54.3 ± 27.2 years (range 31–91 years). The average follow-up period was 44.1 ± 12.3 months (range 25–69 months). Fifty-two patients presented with radiculopathy and 31 with myelopathy. There were 41 single-level cases (82 fusion sites), 34 two-level cases (136 fusion sites), and 8 three-level cases (48 fusion sites). Of the most common procedures, there were 17 C5–C6 single-level fusions, 15 C4–C5, C5–C6 two-level fusions and 3 C4–C5, C5–C6, C6–C7 three-level fusions. This study was approved by our Institutional Review Board.
Surgical procedure
Bone graft was acquired via a 1-cm skin incision on the anterior superior iliac spine. Cancellous bone sufficient to fill the cage was harvested using a trocar (7-mm diameter; AO Synthesis, Switzerland). We employed the Smith-Robinson left anterior cervical approach to gain access to the cervical spine. After removing a portion of the anterior longitudinal ligament and intervertebral disc, we used the Caspar dilator to expand the interval between adjacent vertebral bodies by approximately 2–3 mm. The subchondral bone was exposed by removing any osteophyte, remaining intervertebral disc, and the cartilaginous endplates of the upper and lower vertebral bodies. For subjects requiring spinal decompression, a portion of the posterior longitudinal ligament was also removed. Using lateral plain radiographs, we determined the cage size, the insertion angle of screws, and the angle of lordosis of the metal plates. For the segments to be fused, cervical lordosis was maintained by placing a PEEK cage (Solis cage, Stryker Biotech, Hopkinton, MA) filled with cancellous bone in the area 1–2 mm posterior from the anterior cortex of the vertebral body. For plate augmentation, we used a CSLP Plate (Cervical Spine Locking Plate, AO Synthes). Postoperatively, a Philadelphia brace was worn for 4 weeks. Subjects were instructed to wear a soft collar brace for an additional 2 weeks to provide additional stability while addressing concerns of comfort.
Methods
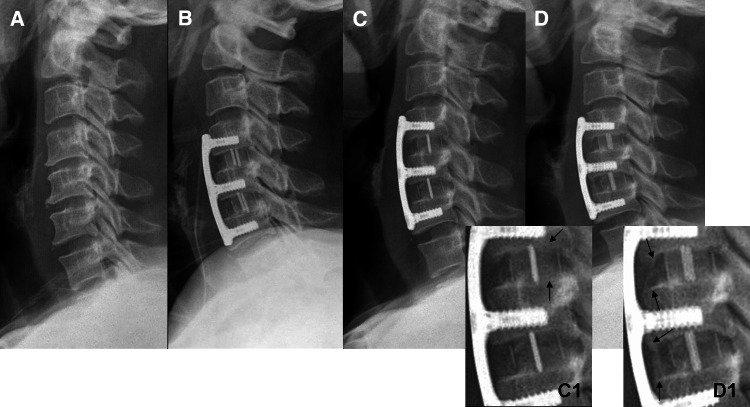
For the radiologic evaluation of fusion status, we examined AP and lateral plain radiographs at 3, 6, 9, 12, and 24 months postoperatively (Fig. 1a–d). All radiographs were performed with the subjects at supine position against the cartridge with the X-ray beam 1 m away, in an effort to eliminate any variability in magnification. According to the modified method proposed by Kim et al. [17], fusion status was divided into three types: (1) type I (pseudoarthrosis) defined as an absence of bridging bone between grafted bone and vertebral bodies, presenting as a radiolucent defect or a Halo sign; (2) type II (borderline) defined as cases that did not meet the criteria of types I or III; (3) type III (fusion) defined as bone bridging beyond the endplates of adjacent vertebra in the interior of the cage (Fig. 1c1) or bone bridging formed up to the anterior or posterior portion of the cage (Fig. 1d1). Fusion status was determined using the above-mentioned criteria at each time point. The pseudoarthrosis rate and fusion type were evaluated and compared for each fusion level.
Fig. 1.
A 61-year-old man who presented with cervical myelopathy and underwent two-level ACDF with cage and plate augmentation at C4–C5 and C5–C6. Plain lateral radiographs at (a) preoperative (b) immediately postoperative (c C1) postoperative 3 months, and (d D1) postoperative 2 years visits. c and (C1) show bone bridging (arrows type III) formed between grafted bone in the cage and vertebral endplates. d and (D1) show new bone formation (arrows type III) in the anterior portion of cage
Cage subsidence was determined based on the difference in distance between the central point of the lower margin of the upper vertebra and the central point of the upper margin of the lower vertebra in the fusion sites on lateral radiographs immediately postoperatively and at the last follow-up. All radiologic images were obtained and viewed on our institution’s PACS (Picture Achieving and Communication System) and cage subsidence measurements were made using PACS. The incidence and degree of cage subsidence was examined and compared at each fusion level. Metal-related complications, such as screw backout, as well as the presence or absence of neurologic complications, infection, and other complications were also examined.
We applied Robinson’s classification system for the evaluation of clinical outcomes [18]. Subjective symptoms, presence of pain, necessity of analgesics, and limitations of active daily living were recorded. Outcomes were rated as excellent, good, moderate, and poor. Good or excellent results were considered to be “good” outcomes. Clinical outcomes were compared for different fusion levels. Dysphagia was evaluated using the dysphagia score proposed by Bazaz [19]. We considered the occasional sensation of dysphagia with solid foods as “moderate” and frequent episodes as “severe”. Donor-site morbidity was defined as infection or pain at the donor site that persisted greater than 1 month postoperatively.
Two independent spine surgeons evaluated the fusion status of each fusion level twice, 2 months apart. The inter-(κ1) and intraobserver (κ2) reliability of these readings were evaluated using κ statistics. Both the inter- and intraobserver reliability were excellent (κ1 = 0.88 and κ2 = 0.82). Fisher’s exact test and Student’s t test were used in the comparison of different fusion level groups. All statistical analyses were performed using SPSS (version 15, SPSS, Chicago, IL), and statistical significance was defined as P < 0.05.
Results
Fusion time and fusion rate according to the number of fusion levels
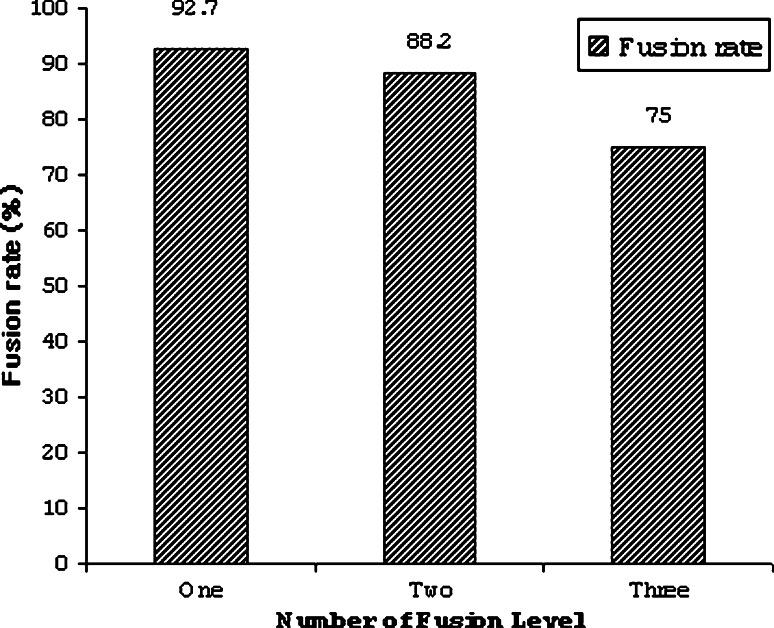
Mean fusion time was 4.2 ± 2.4 months (range 3–12 months) after surgery. Type III fusion occurred in 60 cases (72.3%), 69 cases (83.1%), 76 cases (91.6%), 79 cases (95.1%), and 79 cases (95.1%) at postoperative 3, 6, 9, 12 and 24 months, respectively (Table 1). The fusion rate according to the number of fusion levels at 3 months postoperatively was 92.7% (38/41 cases) of one-level fusions, 88.2% (30/34 cases) of two-level fusions and 75.0% (6/8 cases) of three-level fusions. Fusion rate tended to decrease as the number of fusion levels increased; however, there was no statistically significant difference between the groups (P = 0.317, Fig. 2).
Table 1.
Distribution of different fusion types at different time points
| 3 months (%) | 6 months (%) | 9 months (%) | 12 months (%) | 24 months (%) | |
|---|---|---|---|---|---|
| Type I | 9 (10.8) | 7 (8.4) | 5 (6.1) | 4 (4.8) | 4 (4.8) |
| Type II | 14 (16.9) | 7 (8.4) | 2 (2.4) | 0 | 0 |
| Type III | 60 (72.3) | 69 (83.1) | 76 (91.6) | 79 (95.1) | 79 (95.1) |
Fig. 2.
Fusion rate shows a tendency to decrease as the number of fusion levels increase; however, there is no statistically significant difference between groups (P = 0.317)
Classification of fusion type according to modified Kim’s criteria
According to the modified Kim’s criteria, at 3 months, type I fusion was noted in nine cases/12 fusion sites (3 cases/4 fusion sites in one-level fusions, 4 cases/6 fusion sites in two-level fusions, 2 cases/2 fusion sites in three-level fusions). Type II fusion was noted in 14 cases/19 fusion sites (2 cases/2 fusion sites in one-level fusions, 10 cases/15 fusion sites in two-level fusions, 2 cases/2 fusion sites in three-level fusions). Type III was seen in 60 cases/235 fusion sites.
Of the nine subjects with radiographic evidence of pseudoarthrosis (type I fusion) at 3 months follow-up, five subsequently developed into type III fusions (2 one-level cases at 6 months, 1 one-level case and 1 two-level case at 9 months, and 1 three-level case at 12 months). One of the four subjects with persistent radiographic evidence of pseudoarthrosis remained symptomatic and required a revision surgery. This was a two-level fusion which likely failed due to the patient’s poor compliance with postoperative care, resulting in cage subsidence and loosening of the hardware. Following subsequent posterior fixation, this patient developed a fusion mass 6 months after reoperation (Table 2). The other three patients do not have any significant symptoms and are being observed clinically. These developments suggest that full fusion may be achieved over time without the need for revision surgery, especially in cases in which hardware failure or neurologic symptoms are absent.
Table 2.
Summary of cases resulting in unsatisfactory clinical outcomes
| Case | Age/sex | Fusion level | Diagnosis | Symptom onset | Clinical outcome | Note |
|---|---|---|---|---|---|---|
| 1 | 35/M | C5–C6 | Myelopathy | 6 months | Fair | Junctional stenosis |
| 2 | 40/F | C5–C6 | Radiculopathy | 3 months | Fair | Degenerative change |
| 3 | 83/M | C3–C4 | Myelopathy | 1 year | Poor | Degenerative change |
| 4 | 69/M | C4–C5–C6 | Myelopathy | 3 years | Fair | Metal failure → revision |
| 5 | 61/M | C4–C5–C6 | Myelopathy | 2 months | Fair | Sustained myelopathy |
| 6 | 47/M | C5–C6–C7 | Myelopathy | 6 months | Poor | Junctional stenosis |
| 7 | 60/M | C4–C5–C6–C7 | Myelopathy | 10 years | Poor | Degenerative change |
Cases that transformed from type II fusion into type III fusion over the follow-up period were composed of two-one level cases and five-two level cases at 6 months, four-two-level cases and one-three-level case at 9 months, and one-two level case and one-three-level case at 12 months. Overall fusion rate at 2 years postoperatively was 95.1% (79 of 83 cases).
Location of pseudoarthrosis formation in the fusion site
Pseudoarthrosis in type I, one-level fusions (3 cases/4 fusion sites) developed at the lower vertebra of the fusion site in one case, at the upper vertebra in one case, and at both upper and lower vertebrae in one case. All pseudoarthroses in the two- (4 cases/6 fusion sites) and three-level fusions (2 cases/2 fusion sites) occurred at the upper vertebra of the fusion site. Overall, pseudoarthrosis developed more frequently at the upper than lower vertebra, occurring in the upper vertebra at ten fusion sites and in the lower vertebra at two fusion sites.
Incidence and degree of cage subsidence according to the number of fusion levels
Cage subsidence occurred in two cases (4.9%) of one-level fusion, five cases (14.7%) of two-level fusion, and one case (12.5%) of three-level fusion, with a significantly increased incidence rate of cage subsidence in multilevel fusions (P = 0.046). When comparing the postsurgical radiographs with the most recent follow-up radiographs, 2.55 mm (2.2–2.9 mm) subsidence was observed in one-level fusion and 2.97 mm (2.3–3.8 mm) subsidence was observed in multilevel fusion, with an average of 2.8 mm (2.2–3.8 mm) subsidence observed overall. The degree of cage subsidence was significantly greater in multilevel fusion cases (P = 0.039).
Clinical outcomes and complications
Excellent or good clinical outcomes were achieved in 76 of 83 (91.6%) cases, with desirable clinical outcomes achieved in 38 of 41 (92.7%) one-level fusions, 31 of 34 (91.2%) two-level fusions, and 7 of 8 (87.5%) three-level fusions. Clinical outcomes did not differ significantly between multilevel fusions and single-level fusions (P = 0.693).
Four subjects (4.8%) developed dysphagia that persisted for greater than 6 weeks. All cases spontaneously resolved within 3 months. There were no cases of donor-site infection and five cases of pain persisting greater than 1 month. All donor-site pain resolved within 3 months.
Discussion
Bony fusion was achieved at an average of 4.2 months when utilizing ACDF-CPA. This is longer than the average of 6–10 weeks required with ACDF with autogenous bone graft in single-level fusions, but <3–6 months required with ACDF-C [10, 20]. In addition, we had a higher fusion rate (72.3%, 60 of 83 cases) at 3 months postoperatively than that previously reported of PEEK cages alone (16.7%) [10, 20]. One possible contributor to the delayed fusion time and low fusion rate seen in ACDF-C is the diverse bone substitutes that fill the cages, such as allograft, DBM, and hydroxyapatite, all of which have a lower osteoinductive capacity than autograft. Another significant factor that likely contributes to this difference is the continuous micro motion at the fusion site that occurs with natural neck motion. This suggests that metal plate fixation could significantly enhance the stability at the fusion site and produce more desirable fusion rates and times.
A number of studies have evaluated the rate of cage subsidence following ACDF-C. Barsa et al. [15] observed a cage subsidence rate of 18% (18 of 100 cases), with all subsidence occurring between the anterior portion of the cage and the anterior cortical bone of the patient. Among the 20 fusion sites where subsidence occurred, 17 occurred in the lower vertebra, 1 occurred in the upper vertebra, and 1 occurred in both the upper and lower vertebrae. Lee et al. [11] found that cage subsidence of more than 2 mm occurred in 17 of their 36 cases (44.7%), with an average of 3.09 mm and maximum of 8 mm. Schmieder et al. [16] also reported a high subsidence rate of 44.8% (30 of 67 cases). However, both Lee and Schmieder reported that none of their cases required revision surgery, despite their high subsidence rates because cervical lordosis was radiologically present throughout follow-up. These reports illustrate a possible discrepancy between radiologic apparent cage subsidence and the clinical relevance of these findings. In contrast, Gercek et al. [9] experienced more severe consequences of cage subsidence in their study, with subsidence developing in five of their nine cases (55.6%). One of these cases developed radiculopathy from cage subsidence, consequent intervertebral foramen collapse, and breakage of the cage at 15 months postoperatively.
In our study, ACDF-CPA was associated with a cage subsidence rate of 9.6% (8 cases total, 2 one-level fusions, 5 two-level fusions, and 1 three-level fusions) with an average subsidence of 2.8 mm (2.2–3.8 mm). Among these cases, one developed pseudoarthrosis that required posterior fixation. Thus, when compared with the subsidence rates seen after ACDF-C, ACDF-CPA is associated with a noticeable reduction in the rate and degree of subsidence, which is likely due to the increase in stability and maintenance of cervical lordosis with the use of metal plate fixation. The metal plates also likely reduced the stress on the host bone that is encountered in procedures with cage alone.
Although the use of plate augmentation in ACDF is not universally employed, there are several reports illustrating its efficacy in supporting fusion formation. Song et al. [21] found that ACDF-CPA achieved a high fusion rate in the treatment of single-level degenerative cervical disease, with proper maintenance of the intervertebral disc space and lordosis. Emery et al. [22] and Wang et al. [23] studied the development of pseudoarthrosis after multilevel fusion without plate augmentation, and both reported high pseudoarthrosis rates of 44 and 37%, respectively. However, Papadopoulos et al. [24] implemented plate augmentation in multilevel fusions and found that only 2 of their 46 cases (4%) developed pseudoarthrosis. This drastic difference in pseudoarthrosis rates following multilevel fusions suggests the necessity of plate augmentation. In our study, there were no cases of pseudoarthrosis in the single-level fusions and only 9.5% of multilevel cases developed pseudoarthrosis. This further illustrates the role that plate augmentation may have in reducing the incidence of pseudoarthrosis.
A high rate of good clinical outcomes has been achieved following ACDF with plate augmentation. Wang et al. [23] reported achieving good clinical outcomes in 88% of their 30 cases when they utilized metal plate fixation in three-level ACDF with autograft. Papadopoulos et al. [24] also reported good clinical results in 38 of their 46 cases (83%). In comparison, studies that only utilized a cage in single-level fusions reported a range of good clinical outcomes from 74 to 100% [6, 7]. In our study, we obtained good clinical results in 76 of our 83 cases (91.6%). Of our seven cases with unsatisfactory results, six had myelopathy prior to surgery and three developed adjacent level degeneration. Revision surgery was performed in only one case due to pseudoarthrosis and hardware failure (Table 2).
Previous studies have reported donor-site morbidity in anywhere from 9.4 to 49% of subjects along with a spectrum of other complications (infection, fracture, seroma and neurologic complication, etc.) [25–27]. Pitzen et al. [28] have suggested filling the cage with local autogenous bone, as an effort to eliminate any complications at the iliac crest. Although this technique seems reasonable, we did not employ this practice due to the inferior quality of local bone when compared with iliac crest cancellous bone. The heat that develops during drilling of the endplates and the presence of bone fragments from osteophytes can result in decreased viability of cells (osteocytes, osteoblasts, and mesenchymal precursor cells), ultimately adversely affecting the fusion rate and time. Even with these limitations, Pitzen et al. achieved 91.3% fusion rate, warranting future studies evaluating the use of local bone versus iliac crest bone graft in these cervical procedures. Nonetheless, in the present study, donor-site pain following harvesting of autogenous cancellous bone using a 7-mm bone punch biopsy was minimal and resolved in all except five cases within the first week postoperatively.
Complications secondary to the use of plate augmentation may be anticipated based on the prior reports. In our study, four subjects developed dysphagia that persisted for greater than 6 weeks; however, all cases resolved within 3 months. This suggests that the additional metal plate augmentation did not contribute to significant esophageal irritation or dysphagia.
One potential drawback associated with the use of plate augmentation is the additional cost of the plates and screws. This increased cost translates to approximately 16.7–50% more than the cage alone procedure. Future studies are warranted to evaluate the cost effectiveness of ACDF-CPA versus ACDF-C.
Limitations of our study include its retrospective nature and its failure to include a cage-only group. Future studies will need to prospectively compare the results of ACDF-C and ACDF-CPA groups.
Conclusion
Plate augmentation in one- or two-level anterior cervical fusions for degenerative cervical spine disorders may improve fusion rates and reduce subsidence and complication rates, resulting in improved clinical outcomes.
Footnotes
This study was performed by the approval of the Institutional Review Board of Jeju National University Research Council (IRB-2008-29).
References
- 1.Celik SE, Kara A, Celik S. A comparison of changes over time in cervical foraminal height after tricortical iliac graft or polyetheretherketone cage placement following anterior discectomy. J Neurosurg Spine. 2007;6:10–16. doi: 10.3171/spi.2007.6.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Demircan MN, Kutlay AM, Colak A, Kaya S, Tekin T, Kibici K, Ungoren K. Multilevel cervical fusion without plates, screws or autogenous iliac crest bone graft. J Clin Neurosci. 2007;14:723–728. doi: 10.1016/j.jocn.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Shad A, Leach JC, Teddy PJ, Cadoux-Hudson TA. Use of the Solis cage and local autologous bone graft for anterior cervical discectomy and fusion: early technical experience. J Neurosurg Spine. 2005;2:116–122. doi: 10.3171/spi.2005.2.2.0116. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y, Chopin D, Hardouin P, Lu J. Clinical, radiological and histological study of the failure of cervical interbody fusions with bone substitutes. Eur Spine J. 2006;15:1196–1203. doi: 10.1007/s00586-005-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426–435. doi: 10.1016/j.spinee.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Liao JC, Niu CC, Chen WJ, Chen LH. Polyetheretherketone (PEEK) cage filled with cancellous allograft in anterior cervical discectomy and fusion. Int Orthop. 2008;32:643–648. doi: 10.1007/s00264-007-0378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho DY, Lee WY, Sheu PC. Treatment of multilevel cervical fusion with cages. Surg Neurol. 2004;62:378–385. doi: 10.1016/j.surneu.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Cho DY, Liau WR, Lee WY, Liu JT, Chiu CL, Sheu PC. Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery. 2002;51:1343–1349. doi: 10.1097/00006123-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gercek E, Arlet V, Delisie J, Marchesi D. Subsidence of stand-alone cervical cages in anterior interbody fusion: warning. Eur Spine J. 2003;12:513–516. doi: 10.1007/s00586-003-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni AG, Hee HT, Wong HK. Solis cage (PEEK) for anterior cervical fusion: preliminary radiological results with emphasis on fusion and subsidence. Spine J. 2007;7:205–209. doi: 10.1016/j.spinee.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Seok KS, Lee JH, et al. Outcome analysis of single level anterior cervical fusion using interbody PEEK cage with autologous bone graft. J Korean Orthop Assoc. 2009;44:93–101. doi: 10.4055/jkoa.2009.44.1.93. [DOI] [Google Scholar]
- 12.Valvruch, Rune H, Davood J et al (2002) A prospective randomized comparison between the Cloward procedure and a carbon fiber cage in the cervical spine. Spine 27:1694–1701 [DOI] [PubMed]
- 13.Mastrolnardi L, Ducati A, Ferrante L. Anterior cervical fusion with polyetheretherketone (PEEK) cages in the treatment of degenerative disc disease. Preliminary observation in 36 consecutive cases with a minimum 12-month follow-up. Acta Neurochir (Wien) 2006;148:307–312. doi: 10.1007/s00701-005-0657-5. [DOI] [PubMed] [Google Scholar]
- 14.Meier U, Kemmesies D. Experiences with six different intervertebral disc spacers for spondylodesis of the cervical spine. Orthopade. 2004;33:1290–1299. doi: 10.1007/s00132-004-0707-3. [DOI] [PubMed] [Google Scholar]
- 15.Barsa P, Suchomel P. Factors affecting sagittal malalignment due to cage subsidence in standalone cage assisted anterior cervical fusion. Eur Spine J. 2007;16:1395–1400. doi: 10.1007/s00586-006-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmieder K, Wolzik-Grossmann M, Pechlivanis I, Engelhardt M, Scholz M, Harders A. Subsidence of the wing titanium cage after anterior cervial interbody fusion: 2-year follow-up study. J Neurosurg Spine. 2006;4:447–453. doi: 10.3171/spi.2006.4.6.447. [DOI] [PubMed] [Google Scholar]
- 17.Kim KS, Yang TK, Lee JC. The Radiologic changes in the bone fusion site after posterior lumbar interbody fusion using carbon cages impacted with laminar bone chips: follow-up study over more than 4 years. Spine. 2005;30:655–660. doi: 10.1097/01.brs.0000155421.07796.7f. [DOI] [PubMed] [Google Scholar]
- 18.Robinson RA, Smith GW. Anterolateral cervical disk removal and interbody fusion for cervical disk syndrome. Bull Johns Hopkins Hosp. 1955;96:223–224. [Google Scholar]
- 19.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery. Spine. 2002;27:2453–2458. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 20.Van Jonbergen HP, Spruit M, Anderson PG, Pavlov PW. Anterior cervical interbody fusion with a titanium box cage: early radiological assessment of fusion and subsidence. Spine J. 2005;5:645–649. doi: 10.1016/j.spinee.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Song KJ, Lee KB. A preliminary study of the use of cage and plating for single-segment fusion in degenerative cervical spine disease. J Clin Neurosci. 2006;13:181–187. doi: 10.1016/j.jocn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Emery SE, Bolesta MJ, Banks MA, Jones PK. Robinson anterior cervical fusion comparison of the standard and modified techniques. Spine. 1994;19:660–663. doi: 10.1097/00007632-199403001-00004. [DOI] [PubMed] [Google Scholar]
- 23.Wang JC, McDonough PW, Kanim LE, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for 3-level anterior cervical discectomy and fusion. Spine. 2001;26:643–647. doi: 10.1097/00007632-200103150-00015. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos EC, Huang RC, Girardi FP, Synnott K, Cammisa FP. Three-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Spine. 2006;8:897–902. doi: 10.1097/01.brs.0000209348.17377.be. [DOI] [PubMed] [Google Scholar]
- 25.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Keller EE, Triplett WW. Iliac bone graft: review of 60 consecutive cases. J Oral Maxillofac Surg. 1987;45:11–14. doi: 10.1016/0278-2391(87)90079-6. [DOI] [PubMed] [Google Scholar]
- 27.Summers BN, Eisenstein SM. Donor site pain from the ilium: a complication of lumbar spine fusion. J Bone Joint Surg (Br) 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 28.Pitzen T, Kiefer R, Munchen D, Barbier D, Reith W, Steudel WI. Filling a cervical spine cage with local autograft: change of bone density and assessment of bony fusion. Zentralbl Neurochir. 2006;67:8–13. doi: 10.1055/s-2006-921404. [DOI] [PubMed] [Google Scholar]