Abstract
The fate of notochord cells during disc development and aging is still a subject of debate. Cells with the typical notochordal morphology disappear from the disc within the first decade of life. However, the pure morphologic differentiation of notochordal from non-notochordal disc cells can be difficult, prompting the use of cellular markers. Previous reports on these notochordal cell markers only explored the occurrence in young age groups without considering changes during disc degeneration. The aim of this study, therefore, was to investigate presence, localization, and abundance of cells expressing notochordal cell markers in human lumbar discs during disc development and degeneration. Based on pilot studies, cytokeratins CK-8, -18 and -19 as well as Galectin-3 were chosen from a broad panel of potential notochordal cell markers and used for immunohistochemical staining of 30 human lumbar autopsy samples (0–86 years) and 38 human surgical disc samples (26–69 years). In the autopsy group, 80% of fetal to adolescent discs (0–17 years) and 100% of young adult discs (18–30 years) contained many cells with positive labeling. These cells were strongly clustered and nearly exclusively located in areas with granular changes (or other matrix defects), showing predominantly a chondrocytic morphology as well as (in a much lesser extent) a fibrocytic phenotype. In mature discs (31–60 years) and elderly discs (≥60 years) only 25 and 22–33%, respectively, contained few stained nuclear cells, mostly associated with matrix defects. In the surgical group, only 16% of samples from young adults (≤47 years) exhibited positively labeled cells whereas mature to old surgical discs (>47 years) contained no labeled cells. This is the first study describing the presence and temporo-spatial localization of cells expressing notochordal cell markers in human lumbar intervertebral discs of all ages and variable degree of disc degeneration. Our findings indicate that cells with a (immunohistochemically) notochord-like phenotype are present in a considerable fraction of adult lumbar intervertebral discs. The presence of these cells is associated with distinct features of (early) age-related disc degeneration, particularly with granular matrix changes.
Keywords: Notochordal cells, Aging, Disc degeneration, Cell differentiation
Introduction
The pathophysiology of disc degeneration and related low-back pain is still not well explored despite intensive research activities in this field. Recent investigations provide evidence that distinct alterations of the extracellular matrix of the intervertebral disc are associated with histopathologic changes [5, 30, 41]. Since the matrix is synthesized [49], modulated and thereby controlled by the disc cells, it seems essential to identify changes in both, cell function and phenotype [31].
The origin of the nucleus pulposus and the fate of notochordal cells have been subject to a continuous debate [7, 13, 23, 34, 39, 43]. Some reports suggest that the adult nucleus pulposus cells derive from cells “invading” into the disc [16, 24, 51, 55] others indicate that these cells originate from involution of the notochord [7, 17, 34, 43, 52]. The latter hypothesis is supported by an ultrastructural investigation [50] and by immunohistochemical detection of certain cytokeratin isotypes expressed by notochordal cells [11, 47]. Previous investigations on the natural notochord history failed to detect cells with the typical notochordal cell morphology over the age of 10 years [17, 35, 36]. However, these findings are mainly based on the detection of notochordal cells based on routine histology, i.e. looking for typical cell morphology. In the developing fetus, pronounced change of the notochordal phenotype can already be observed. It can be hypothesized that these modifications continue in the post-fetal period and lead to a substantial change in the morphology of the notochordal cells to a predominantly chondrocytic or much lesser extent fibrocytic appearance [12]. The identification of notochordal cells solely by routine histological criteria may therefore be incomplete.
Previous studies have reported that notochordal disc cells express distinct cell markers [12, 18, 46, 47]. Cytokeratin types CK-8, CK-18, and CK-19 have been shown to be expressed by fetal notochordal cells [11, 12, 29]. Interestingly, cells with ultrastructural characteristics of notochordal cells have also been identified in the adult nucleus pulposus [50]. Additionally, in one study on the fate of notochordal cells, the use of cytokeratins as markers suggests that those cells persist in the nucleus pulposus of adults [47]. Galectin-3, which was also shown to be a further notochordal cell marker, also seems to be expressed in adult discs [12]. Galectin-3 belongs to the group of galactoside-binding proteins, but is also capable to interact with many different peptides (among them cytokeratins) and polynucleotides. It is involved in a variety of intra- and extracellular processes, such as the regulation of RNA-splicing, the modulation of cell-adhesion, or extracellular signaling [6, 8, 14, 20, 26, 32, 37, 40, 44, 48]. Galectin-3 also appears to participate in the recognition of advanced glycation endproducts (AGEs), which have recently been shown to play a role in disc degeneration [30, 38, 45].
To the best of our knowledge, the temporo-spatial localization of notochordal cell markers during the process of both aging and disc degeneration has so far not been investigated. In using autopsy and surgical lumbar intervertebral disc specimens of all ages, we investigated presence, localization, and abundance of cells expressing notochordal cell markers by immunohistochemistry. These findings were correlated with both individual age and histological signs of disc degeneration to explore a potential role of these cells in disc degeneration.
Materials and methods
Study population and tissue preparation
Two distinct study populations were used in this investigation.
Autopsy group
This group comprised of 30 individuals from whom lumbar motion segments had been removed at autopsy. Age of the individuals ranged from fetal (26th week of gestation) to senile age (86 years). None of these individuals died of a consuming illness (e.g. tumor, infection) or had a known history of a back problem requiring treatment. Thin sagittal slices (of approx. 5 mm thickness) of the complete motion segment were fixed in 4–6% buffered formaldehyde (pH 7.4), subsequently decalcified (0.1 M EDTA, pH 7.4) and finally embedded in paraffin wax as routinely performed [5, 31].
Surgical group
This group comprised of 38 disc samples that had been obtained during surgery for painful lumbar disc degeneration and/or disc herniation (protrusion, extrusion, or sequestration). The samples were obtained from individuals (23 males, 15 females; age range 26–69 years) with known clinical symptoms, radiological features, and histological degree of disc degeneration. All samples were obtained from patients undergoing a spinal intervention (discectomy or spinal fusion) because of incapacitating back or leg pain. Painful disc degeneration was identified by positive provocative discography and/or the presence of severe Modic changes [54].
Determination of the degree of histological degeneration
In all samples, the histological degree of disc tissue degeneration was determined as previously described in detail [5]. Briefly, the parameters cellularity, occurrence, and extent of cleft and tear formation, granular and/or mucoid matrix degeneration and cell necrosis were ranked, forming the “histodegeneration score” (HDS).
Identification of suitable notochordal markers
In pilot studies, antibodies against various cytokeratins (CK-5, -6, -7, -8, -10, -13, -15, -18, -19, -20, and pan CK, clone AE1/AE3) and against Galectin-3 were tested to obtain qualitative information about their expression in the intervertebral disc. Two small histomorphological representative sample series were used: five fetal autopsy discs (with well recognizable notochordal cells) and five adult discs (without morphologically evident notochordal cells or cell residues). Histologically, notochordal cells in the fetal period were identified based on their characteristic appearance, i.e. polyhedral or round cells with usually round nucleus, dispersed fine chromatin and eosinophilic highly vacuolated cytoplasm (physaliferous). The cytoplasm usually contains large amounts of glycogen. With age, the initial tightly packed cells (cluster) became more and more separated by large volumes of mucoid matrix. In addition, double labeling experiments were performed using Galectin-3 and CK -8, -18 or -19 in fetal and adult samples. Based on the results of the pilot studies (see below), antibodies against pan CK AE1/AE3 (which includes reactivity against CK-8 and -19 among other CKs, but not CK-18), CK-18 and Galectin-3 were used for immunohistochemical staining of 30 autopsy samples (autopsy group) and 38 surgical disc samples (surgical group).
Immunohistochemical staining protocol
For the staining of cytokeratins, we used antibodies that are routinely used in diagnostic surgical pathology examinations in our laboratory. For detailed information about sample pre-treatment and antibody concentration, see Table 1. The specificity of the immunohistochemical reactions had been tested extensively in routine samples. The appropriate slides were deparaffinized and pretreated according to established protocols [4, 30, 53], either by heating in citrate buffer or by enzymatic pretreatment (0.25% pepsin). The pretreated samples were incubated with the following primary monoclonal antibodies or antibody-mixtures: CK AE1/AE3 (Zytomed, Berlin, Germany), CK-20 (BioLogo, Kronshagen, Germany), CK-19 (Dako, Hamburg, Germany), CK-18 (Dako, Hamburg, Germany), CK-15 (Acris, Herford, Germany), CK-10/13 (Zytomed, Berlin, Germany), CK-8 (Zytomed, Berlin, Germany), CK-7 (Zytomed, Berlin, Germany), CK-5 (Zytomed, Berlin, Germany), CK-6 (BioLogo, Kronshagen, Germany). In addition, a polyclonal antibody was used for the detection of Galectin-3 (Santa Cruz Biotech., Santa Cruz, USA). The specificity of this antibody had been tested extensively prior to the investigation on colonic and intestinal epithelium. Visualization was performed using the Super Sensitive Detection System (Biogenex, San Ramon, USA) with alkaline-phosphatase conjugated streptavidin and Fast Red as chromogen. For double staining experiments, the Histostain plus Kit (Zytomed, Berlin, Germany) was used with peroxidase conjugated streptavidin and aminoethylcarbazol (AEC) as chromogen. In each batch, positive controls (human fetal intervertebral discs) as well as negative controls (by omission of the primary antibody and use of non-immunized normal serum) were included.
Table 1.
Immunohistochemical staining protocol
| Antibody | Source | Concentration | Pre-treatment |
|---|---|---|---|
| CK-5 | Zytomed Systems, Berlin, GE | 1:75 | Boiling |
| CK-6 | BioLogo, Kronshagen, GE | 1:10 | Enzymatic |
| CK-7 | Zytomed Systems, Berlin, GE | 1:100 | Enzymatic |
| CK-8 | Zytomed Systems, Berlin, GE | 1:50 | Enzymatic |
| CK-10 | Zytomed Systems, Berlin, GE | 1:100 | None |
| CK-13 | Zytomed Systems, Berlin, GE | 1:30 | Boiling |
| CK-15 | Acris, Herford, GE | 1:50 | Enzymatic |
| CK-18 | Dako, Hamburg, GE | 1:100 | Enzymatic |
| CK-19 | Dako, Hamburg, GE | 1:50 | Enzymatic |
| CK-20 | BioLogo, Kronshagen, GE | 1:200 | Boiling |
| AE1/AE3 | Zytomed Systems, Berlin, GE | 1:140 | Boiling |
| Galectin-3 | Santa Cruz Biotech., Santa Cruz, USA | 1:50 | Enzymatic |
Boiling procedure 10 min. in citrate buffer, pH 6.0, in a pressure cooker
Enzymatic 0.1% pronase E (Merck, Darmstadt, GE, K38823533845) 10 min., 37°C
Data evaluation
In the pilot studies, the staining pattern was evaluated qualitatively, i.e. with respect to presence or absence of the respective parameter (when compared to positive controls).
For the autopsy and surgical samples, a quantitative morphometric analysis was performed (as previously described [31]) by randomly selecting 20 fields of each anatomic subset (i.e. of nucleus pulposus, inner and outer anulus fibrosus) and by counting the positively stained cells as a fraction of all cells in the area.
The amount of stained nuclear cells (anular cells showed almost no reaction) was assigned to one of four groups: – (no cells labeled), + few cells (<10% cells labeled), ++ intermediate amount of cells (10–50% cells labeled) and +++ abundant positive cells (>50% cells labeled).
A Spearman rank correlation test was performed to test for statistical association between immunohistochemical data and histological disc degeneration (HDS). A P value of 0.05 was set as the level of statistical significance (two tailed).
Results
Identification of suitable notochordal markers
In all five fetal disc samples, typical notochordal cells were identified by their clustered arrangement and their physaliferous morphology embedded in a loose, mucoid matrix (see “Materials and methods”). These cells were exclusively present in the nucleus pulposus and stained distinctly positive for pan-cytokeratin (AE1/AE3), the specific cytokeratins CK-8, -18, -19 and for Galectin-3 with an exclusively cytoplasmic pattern (Figs. 1a, 2a, 3a). Annular cells and cells in the endplates showed no staining. Not all remaining cytokeratins tested revealed any specific staining.
Fig. 1.
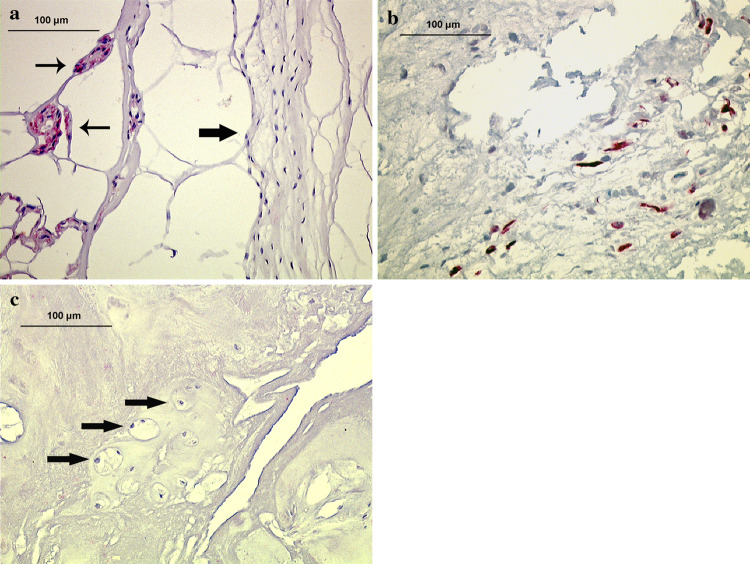
Immunolocalization of pan CK AE1/AE3 (staining cytokeratin-8 and -19 among others) in disc tissue of various age groups (autopsy group). a Fetal nucleus pulposus and pre-anulus region (arrows), showing typical labeling of notochordal cells (28th week, disc level L3/4). b Young adult nucleus pulposus tissue (21 years, disc level L2/3), showing numerous positively stained cells that are exclusively labeled in granular matrix areas (arrows) and adjacent to matrix defects (×200)
Fig. 2.
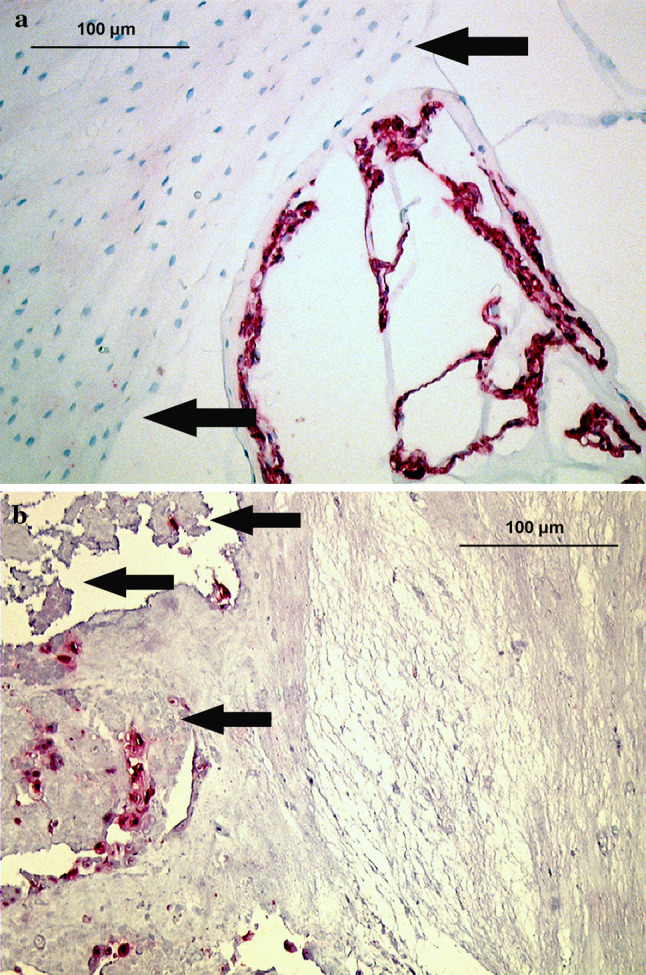
Immunolocalization of cytokeratin-18 (CK-18) in disc tissue of various age groups (autopsy group). a Fetal nucleus pulposus tissue (small arrows) and notochord sheath and pre-anulus region (big arrow), showing typical positive labeling of notochordal cells (28th week, disc level L3/4). b Young adult nucleus pulposus tissue (25 years, disc level L3/4), showing numerous positively labeled cells. c Old adult nucleus pulposus tissue (77 years, disc level L3/4), showing no positively stained disc cells (arrows) (×200)
Fig. 3.
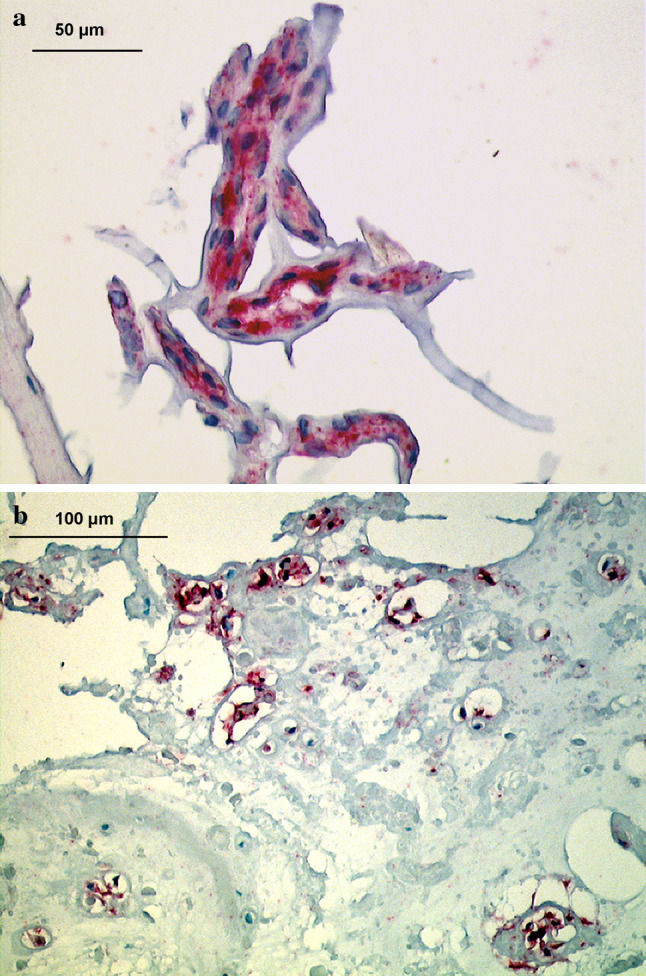
Immunolocalization of Galectin-3 in disc tissue of various age groups (autopsy group). a Fetal nucleus pulposus, showing typical positive labeling of notochordal cells (28th week, disc level L3/4) (×400). b Young adult nucleus pulposus tissue (25 years, disc level L3/4), showing numerous positive cells comparable to the cytokeratin pattern (×200)
In three of the five adult samples, labeled cells were seen for pan-cytokeratin (AE1/AE3), CK-8, -18, -19 and Galectin-3 (Figs. 1b, 2b, 3b). The cell morphology of the labeled cells was not different from that of non-labeled cells, i.e. the positive cells presented as typical fibrochondrocytic cells of the nucleus pulposus. The staining for CK-18 was slightly more inconsistent and revealed positive labeling in fewer samples. Like in the fetal samples, all positive cells exclusively showed cytoplasmic staining and were only found in or at the border of granular tissue changes. The remaining cytokeratins showed no specific staining in the adult discs. Double-labeling experiments demonstrated co-localization of Galectin-3 with CK-8, CK-18 or CK-19 (Fig. 4). Based on these pilot studies, antibodies against CK AE1/AE3 (detecting CK-8 and CK-19 among others), CK-18 and Galectin-3 were used for immunohistochemical staining in the autopsy and surgical biopsy group.
Fig. 4.
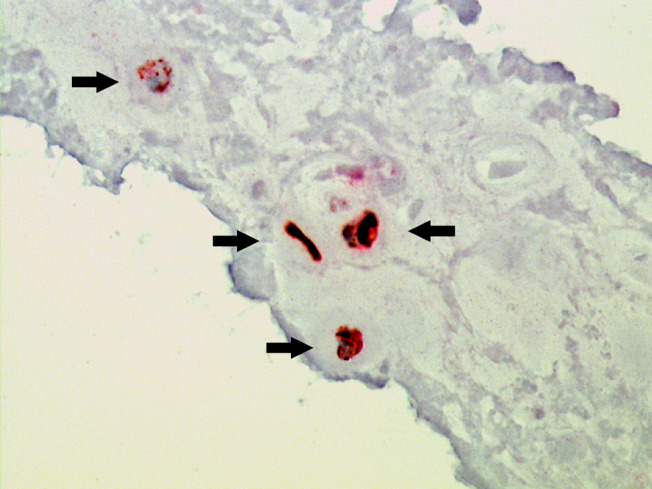
Immunohistochemical double labeling experiments. The simultaneous staining of CK-19 (red color) and Galectin-3 (brown color) shows exact cellular co-localization of both parameters (arrows) (×400)
Notochordal phenotype pattern in the autopsy group
Fetal to adolescent discs
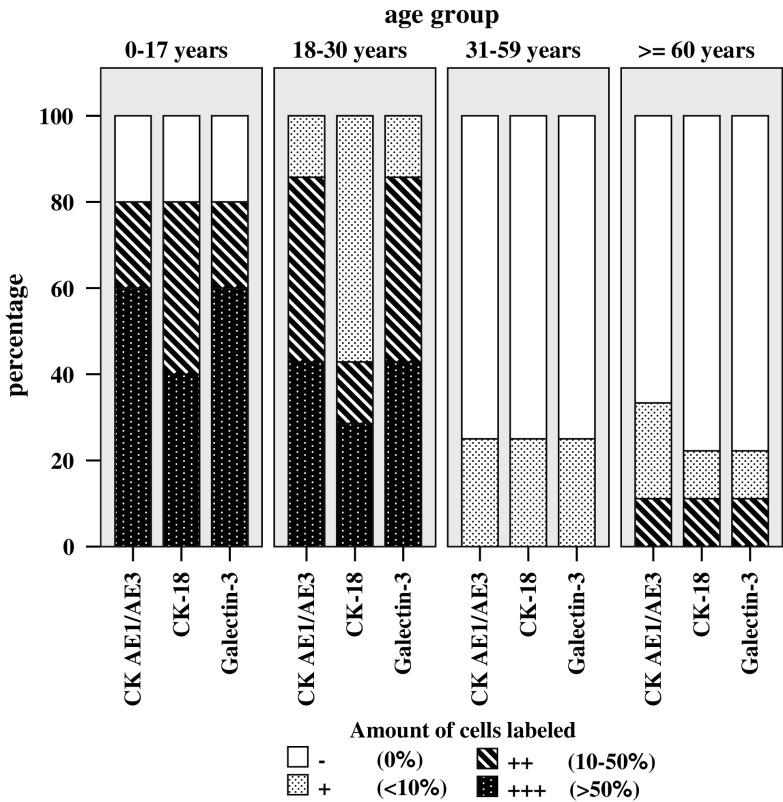
In the fetal, juvenile and adolescent age group (0–17 years, HDS 0–4), cells with typical notochordal appearance were intensively labeled for CK AE1/AE3 (i.e. CK-8 and -19), CK-18, and Galectin-3 (Table 2, Figs. 1a, 2a, 3a). In samples without morphologically typical notochordal cells, no positively stained cells were present. Positively labeled cells were exclusively located in the nucleus pulposus. In 80% of all cases, numerous CK-positive cells were seen (60% of cases in Grade +++) and further 20% of cases were ranked as Grade ++ (Fig. 5). In the two adolescents discs (13 and 16 years), no positive cells were seen.
Table 2.
Abundance of cells with typical notochordal morphology and cells expressing notochordal cell markers in the nucleus pulposus (autopsy group)
| Specimen number | Age | Sex | HDS NP | Cells exhibiting typical notochordal morphology | Cells exhibiting notochordal cell markers | ||
|---|---|---|---|---|---|---|---|
| CK AE1/AE3 | CK-18 | Galectin-3 | |||||
| 1 | 28th week | M | 0 | +++ | +++ | +++ | +++ |
| 2 | 32nd week | F | 1 | +++ | +++ | +++ | +++ |
| 3 | 1 day | M | 2 | +++ | +++ | ++ | +++ |
| 4 | 2 days | F | 3 | +++ | +++ | +++ | +++ |
| 5 | 1 month | M | 2 | +++ | +++ | ++ | +++ |
| 6 | 2 months | F | 3 | +++ | +++ | +++ | +++ |
| 7 | 2 years | M | 2 | ++ | ++ | ++ | ++ |
| 8 | 8 years | M | 2 | ++ | ++ | ++ | ++ |
| 9 | 13 years | M | 4 | − | − | − | − |
| 10 | 16 years | F | 3 | − | − | − | − |
| 11 | 18 years | M | 8 | − | + | + | + |
| 12 | 20 years | M | 11 | − | ++ | + | ++ |
| 13 | 21 years | M | 13 | − | ++ | + | ++ |
| 14 | 22 years | M | 10 | − | +++ | +++ | +++ |
| 15 | 24 years | M | 9 | − | ++ | + | ++ |
| 16 | 25 years | M | 10 | − | +++ | ++ | +++ |
| 17 | 25 years | M | 13 | − | +++ | +++ | +++ |
| 18 | 31 years | M | 12 | − | − | − | − |
| 19 | 47 years | F | 15 | − | + | + | + |
| 20 | 47 years | F | 16 | − | − | − | − |
| 21 | 58 years | F | 16 | − | − | − | − |
| 22 | 66 years | M | 17 | − | − | − | − |
| 23 | 77 years | F | 16 | − | ++ | ++ | ++ |
| 24 | 77 years | F | 15 | − | − | − | − |
| 25 | 77 years | M | 14 | − | − | − | − |
| 26 | 79 years | F | 15 | − | − | − | − |
| 27 | 81 years | M | 16 | − | + | − | − |
| 28 | 81 years | F | 17 | − | + | + | + |
| 29 | 85 years | F | 14 | − | − | − | − |
| 30 | 86 years | F | 15 | − | − | − | − |
− absent, + <10% cells labeled, ++ intermediate amount of cells (10–50% cells labeled), +++ >50% cells labeled
Fig. 5.
Morphometric evaluation of immunohistochemical reaction results for pan-CK, CK-18 and Galectin-3 in the autopsy series. The histogram shows the distribution of negative/positive cells and the extent of positive staining in the four age groups, juvenile/adolescent (0–17 years), young adult (18–30 years), mature adult (31–60 years) and elderly age group (≥60 years). Categorization: − no reaction, + few labeled cells, i.e. <10% of cells stained, ++ intermediate amount of stained cells with 10–50% of cells stained, +++ numerous labeled cells, i.e. >50% of cells stained
Young adults
In the young adult age group (18–30 years, HDS 8–13), a 100% of cells showed cytoplasmic reaction for the chosen notochordal markers (Table 2; Figs. 1b, 2b, 3b). Labeled cells were frequently found in clusters and nearly exclusively located in or adjacent to areas with granular changes, tissue clefts and/or tears. Those cells showed predominantly a chondrocytic morphology or to a minor extent a fibrocytic phenotype and were mainly located in the nucleus pulposus and only very rarely in the inner anulus fibrosus. Not every matrix defect and granular degeneration contained labeled cells. The evaluation of the amount of labeled notochordal cells in defect versus non-defect areas was difficult, since there was a clear predominance of notochordal cells in the defect areas. However, when counting the labeled cells in those areas adjacent to matrix defects a major increase in the proportion of stained cells is seen which comprises a rise of between 155 and 200% (i.e. doubling) of non-defect areas. In 43% of discs those were ranked Grade +++ (abundant stained cells), in 43% of discs those were ranked Grade ++ (intermediate) and in 14% of discs those were ranked Grade + (few cells) (Fig. 5), showing comparable results to the juvenile age group.
Mature individuals
In the mature age group (31–60 years, HDS 12–16), stained cells—if observed—were also mostly located in areas with matrix defects in the nucleus pulposus. The evaluation of the amount of labeled notochordal cells in defect versus non-defect areas was difficult, since there was a clear predominance of notochordal cells in the defect areas. In 75% of discs no labeled cells were seen, the remaining 25% of discs were ranked grade + (few positive cells). Interestingly, the amorphous granular material itself sometimes showed a specific staining at the rim of the granular tissue fragments (Fig. 6).
Fig. 6.

Immunohistochemical labeling of amorphous granular matrix material. The immunostaining for pan-cytokeratin (AE1/AE3) (mature adult autopsy group, 31 years, disc level L4/5) reveals a specific positive labeling of part of the granular material (arrows) (×400)
Elderly individuals
The elderly age group (≥60 years, HDS 14–17) showed a comparable staining result to the mature age group. In 67–77% of discs, no labeled cells were seen (Table 2; Fig. 5), the remaining discs were ranked in 11% grade ++ (intermediate) and in 11–22% grade + (few positive cells).
Statistical analysis
The Spearman rank correlation test revealed a highly significant correlation for all cases between cytokeratin and Galectin markers (P < 0.001). In the young adults (18–30 years), mature individuals (31–60 years) and elderly individuals (≥60 years), there was a significant correlation (P < 0.05) for all antibodies between the notochordal phenotype and granular changes, but no correlation was seen with age or the other characteristics of histological degeneration.
Notochordal phenotype pattern in the surgical group
In the surgical specimens (26–69 years), positive labeling was identified for the selected notochordal cell markers (Fig. 7a, b). Again, labeled cells were seen only in nuclear disc areas. In the age group up to 47 years, we identified an intermediate amount of positive cells in 13% and very few positive cells in further 3% of samples. Extensive labeling was not observed in this set of samples. In the age group older than 47 years, none of the samples exhibited a positive staining. The statistical analysis revealed a significant correlation between the amount of cells expressing notochordal cell markers and granular changes (P < 0.05), but not with any other parameters.
Fig. 7.
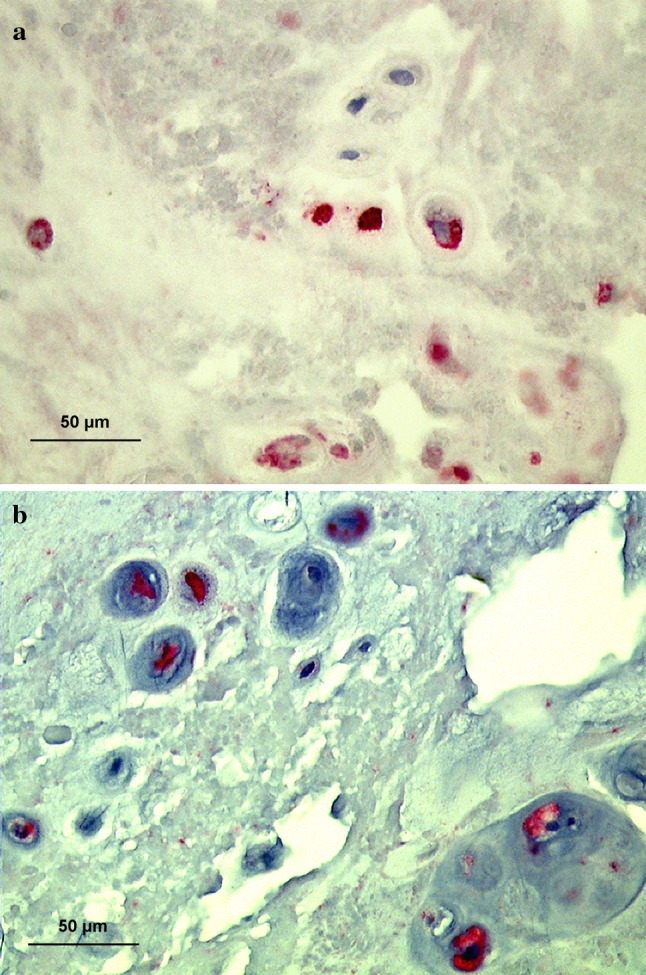
Immunolocalization of notochordal markers in the surgical material a Pan-cytokeratin (AE1/AE3) positive cells are present in a mature adult surgical disc sample (36 years, disc level L4/5) with clusters of positively labeled cells. b Galectin-3 positive cells are seen in a mature adult surgical disc sample (36 years, disc level L4/5) with clusters of positively labeled cells (×400)
Discussion
This is the first study analyzing the presence of cells with notochordal phenotype in relation to age-related changes of adult human lumbar intervertebral discs. Most interestingly, our immunohistochemical study identifies cells exhibiting notochordal cell markers in the young and middle age group without showing the typical notochordal cell morphology. In addition, we provide evidence that the occurence of cells with notochordal immunohistochemical phenotype significantly correlates with granular matrix changes and that there is an obvious association with cleft formation in the nucleus pulposus, which was evident in the young adult and the mature adult age groups, but not seen in the elderly age group. The latter effect may be because elderly individuals often do not show cell reaction (proliferation/apoptosis, etc.) adjacent to the clefts and tears.
The loss of cells with typical notochordal phenotype (physaliferous) and the coincident onset with signs of disc degeneration has lead to speculations about their role in the preservation of disc function. Although interspecies comparison—premature loss of notochordal cells from chondrodystrophic breeds with higher incidence of intervertebral disc degeneration—gave some support for this idea, the conclusive evidence for this hypothesis is still missing.
Our results support previous studies showing that cells in the fetal and juvenile nucleus pulposus with the typical morphology of the notochord (physaliferous) express the markers cytokeratin CK-8, -18, -19 and Galectin-3 [11, 12, 29, 47, 56]. Double labeling experiments show a clear co-localization of specific cytokeratins (CK-8, -18, -19) and Galectin-3. There is an ongoing debate on the presence or absence of CK-18 in notochordal cells [11, 29]. Our autopsy series reveals an overall similar proportion of CK-18 positive discs as for the other markers, while the CK-18 positive cases were somewhat less frequent in the surgical material than the other markers. The reason may be because a clear distinction of nuclear and anular region is sometimes difficult to make in surgical material, particularly in disc herniation specimens. Oguz et al. [33] reported on a ubiquitous expression of Galectin-3 in rat intervertebral discs and stressed that this protein is inappropriate as a marker for notochordal cells. In our study on human intervertebral discs, we observed the expression of Galectin-3 in the nucleus pulposus and few positive cells in the inner anulus fibrosus. However, a general and widespread labeling of cells in the endplate or outer anulus fibrosus was not seen. These somehow conflicting results are probably related to differences in the investigated species (rat/human). The natural course of the notochord in different species shows a substantial variation concerning the loss of notochordal cells with age [1, 15, 18, 19]. The coexpression of Galectin-3 with cytokeratin markers strengthens our observation of a distinct cell population in the intervertebral disc with notochordal phenotype. We found that in many adult discs, a significant fraction of cells expressed notochordal markers without exhibiting the typical notochordal cell morphology. These cells are almost exclusively present in the nucleus pulposus and are rarely seen in the adjacent inner anulus. The occurrence of cells with notochordal markers in the inner anulus fibrosus may result from some difficulties to clearly distinguish between the inner anulus and nucleus pulposus compartments in the presence of degenerative tissue disarrangements. At present, our data do not allow to determine whether these cells are (direct) transdifferentiated descendents of the original notochordal cells or “de”-differentiated local chondrocyte-like cells de novo expressing notochordal cell markers. The different morphologies of the stained adult cells (chondrocytic and fibrocytic, dendritic [21]) may indicate a differentiation versatility of cells with notochordal markers and emphasizes the difficulty to identify cell populations by morphology alone.
We provide some evidence that cells with an immunohistochemical notochordal phenotype are frequently (albeit not exclusively) associated with granular matrix changes and early cleft formation, especially in young adult discs. Most interestingly, these cells are rarely found outside areas with tissue destruction. Therefore, one might speculate that there is a potential involvement of these cells in extracellular matrix derangement, especially in the onset of this process. Although our data—at present—do not allow to draw absolute conclusions we feel encouraged to hypothesize that the destructive potential of cells may—at least in part—be due to expression of Galectin-3, which has been shown to have many intra- and extracellular signaling functions [32], and which is actively involved in the regulation of the cell-binding of various cell types to the extracellular matrix [27, 32]. Galectin-3 influences cell differentiation [28] acts as an inflammation mediator [2] and is expressed by phagocytic cells (mainly neutrophils) where it increases the production of free reactive oxygen species (so-called ROS) [22, 57]. Degenerating adult discs contain a specific cell population that exhibits phagocytic properties which seems to be substantially involved in tissue degradation [31], e.g. by production, liberation, and activation of major matrix metalloproteinases (MMPs) [42, 53]. In this context, it has to be mentioned that Galectin-3 is a substrate of gelatinases (MMP-2 and -9) which have also recently been identified in the degenerating disc [3, 25]. Our data may therefore indicate a (indirect) destructive potential of notochordal cells, despite the fact that notochordal cells increase the proteoglycan synthesis in vitro [9, 10]. Even though we cannot exclude with certainty the possibility that the expression of Galectin-3 could equally well be a response to damage as a cause of it, one would expect higher staining intensities in more degenerated discs, but this was not the case (no significant correlation to the amount of histodegenerative changes).
Interestingly, not only cells stained for notochordal markers, but also a considerable fraction of amorphous granular material, which may be a precursor of cleft formation in the disc, showed a specific fragmented staining pattern especially for pan-CK (AE1/AE3). This may—at least in part—indicate a cellular origin of these structures, suggesting that these are remnants of “dead or apoptotic” disc cells, possibly “dead” cells with positive reaction with notochordal cell markers. However, the authors acknowledge that the role of this cell type in disc degeneration so far remains speculative and further studies are required before more conclusive statements can be made.
In conclusion, this is the first paper to describe the occurrence and temporo-spatial distribution of cells with an immunohistochemical notochordal phenotype without exhibiting the typical morphologic notochord cell morphology in adult human intervertebral discs and indicates a close correlation of these cells with early degenerative changes.
Acknowledgments
This study was made possible by grants from the Hartmann-Müller Foundation, Switzerland (No 1047) and the AOSpine (SRN 02/103 and AOSBRC-07-03). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of AOSpine.
References
- 1.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconj J. 2004;19:575–581. doi: 10.1023/B:GLYC.0000014088.21242.e0. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, Tuan RS, Albert TJ. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27:1291–1296. doi: 10.1097/00007632-200206150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Boos N, Nerlich AG, Wiest I, von der MK, Aebi M (1997) Immunolocalization of type X collagen in human lumbar intervertebral discs during ageing and degeneration. Histochem Cell Biol 108:471–480 [DOI] [PubMed]
- 5.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Liu FT, Yang RY. Roles of galectin-3 in immune responses. Arch Immunol Ther Exp (Warsz) 2005;53:497–504. [PubMed] [Google Scholar]
- 7.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Erwin WM, Ashman K, O’donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and Up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- 10.Erwin WM, Inman RD. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine. 2006;31:1094–1099. doi: 10.1097/01.brs.0000216593.97157.dd. [DOI] [PubMed] [Google Scholar]
- 11.Goetz W, Kasper M, Fischer G, Herken R. Intermediate filament typing of the human embryonic and fetal notochord. Cell Tissue Res. 1995;280:455–462. doi: 10.1007/BF00307819. [DOI] [PubMed] [Google Scholar]
- 12.Goetz W, Kasper M, Miosge N, Hughes RC. Detection and distribution of the carbohydrate binding protein galectin-3 in human notochord, intervertebral disc and chordoma. Differentiation. 1997;62:149–157. doi: 10.1046/j.1432-0436.1997.6230149.x. [DOI] [PubMed] [Google Scholar]
- 13.Guehring T, Wilde G, Sumner M, Grunhagen T, Karney GB, Tirlapur UK, Urban JP. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60:1026–1034. doi: 10.1002/art.24407. [DOI] [PubMed] [Google Scholar]
- 14.Guevremont M, Martel-Pelletier J, Boileau C, Liu FT, Richard M, Fernandes JC, Pelletier JP, Reboul P. Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann Rheum Dis. 2004;63:636–643. doi: 10.1136/ard.2003.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen HJ. A pathologic-anatomical interpretation of disc degeneration in dogs. Acta Orthop Scand. 1951;20:280–293. doi: 10.3109/17453675108991175. [DOI] [PubMed] [Google Scholar]
- 16.Heiberg J (1880) Über die Zwischenwirbelgelenke und Knochenkerne der Wirbelsäule bei den Neugeborenen und ihre Verhältnisse zur Chorda. Mitteilungen aus der Embryologie, Institut der Universität Wien, pp 119–129
- 17.Horwitz T (ed) (1977) The Human Notochord: a study of its development and regression, variations, and pathologic derivative, chordoma. Indianapolis
- 18.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 19.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacobini C, Menini S, Oddi G, Ricci C, Amadio L, Pricci F, Olivieri A, Sorcini M, Di Mario U, Pesce C, Pugliese G. Galectin-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury: evidence for a protective role of galectin-3 as an AGE receptor. FASEB J. 2004;18:1773–1775. doi: 10.1096/fj.04-2031fje. [DOI] [PubMed] [Google Scholar]
- 21.Johnson WE, Roberts S. Human intervertebral disc cell morphology and cytoskeletal composition: a preliminary study of regional variations in health and disease. J Anat. 2003;203:605–612. doi: 10.1046/j.1469-7580.2003.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- 23.Kim KW, Kim YS, Ha KY, Woo YK, Park JB, Park WS, An HS. An autocrine or paracrine Fas-mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine. 2005;30:1247–1251. doi: 10.1097/01.brs.0000164256.72241.75. [DOI] [PubMed] [Google Scholar]
- 24.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982–990. doi: 10.1097/00007632-200305150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kozaci LD, Guner A, Oktay G, Guner G. Alterations in biochemical components of extracellular matrix in intervertebral disc herniation: role of MMP-2 and TIMP-2 in type II collagen loss. Cell Biochem Funct. 2006;24:431–436. doi: 10.1002/cbf.1250. [DOI] [PubMed] [Google Scholar]
- 26.Krzeslak A, Lipinska A. Galectin-3 as a multifunctional protein. Cell Mol Biol Lett. 2004;9:305–328. [PubMed] [Google Scholar]
- 27.Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- 28.Liu FT. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- 29.Naka T, Iwamoto Y, Shinohara N, Chuman H, Fukui M, Tsuneyoshi M. Cytokeratin subtyping in chordomas and the fetal notochord: an immunohistochemical analysis of aberrant expression. Mod Pathol. 1997;10:545–551. [PubMed] [Google Scholar]
- 30.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine. 1997;22:2781–2795. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Nerlich AG, Weiler C, Zipperer J, Narozny M, Boos N. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine. 2002;27:2484–2490. doi: 10.1097/00007632-200211150-00012. [DOI] [PubMed] [Google Scholar]
- 32.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 33.Oguz E, Tsai TT, Di Martino A, Guttapalli A, Albert TJ, Shapiro IM, Risbud MV. Galectin-3 expression in the intervertebral disc: a useful marker of the notochord phenotype? Spine. 2007;32:9–16. doi: 10.1097/01.brs.0000250302.74574.98. [DOI] [PubMed] [Google Scholar]
- 34.Pazzaglia UE, Salisbury JR, Byers PD. Development and involution of the notochord in the human spine. J R Soc Med. 1989;82:413–415. doi: 10.1177/014107688908200714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peacock A. Observations on the prenatal development of the intervertebral disc in man. J Anat. 1951;85:260–274. [PMC free article] [PubMed] [Google Scholar]
- 36.Peacock A. Observations on the postnatal structure of the intervertebral disc in man. J Anat. 1952;86:162–179. [PMC free article] [PubMed] [Google Scholar]
- 37.Pieters RJ. Inhibition and detection of galectins. Chembiochem. 2006;7:721–728. doi: 10.1002/cbic.200600011. [DOI] [PubMed] [Google Scholar]
- 38.Pokharna HK, Phillips FM. Collagen crosslinks in human lumbar intervertebral disc aging. Spine. 1998;23:1645–1648. doi: 10.1097/00007632-199808010-00005. [DOI] [PubMed] [Google Scholar]
- 39.Rastogi A, Thakore P, Leung A, Benavides M, Machado M, Morschauser MA, Hsieh AH. Environmental regulation of notochordal gene expression in nucleus pulposus cells. J Cell Physiol. 2009;220:698–705. doi: 10.1002/jcp.21816. [DOI] [PubMed] [Google Scholar]
- 40.Reboul P, Martel-Pelletier J, Pelletier JP. Galectin-3 in osteoarthritis: when the fountain of youth doesn’t deliver its promises. Curr Opin Rheumatol. 2004;16:595–598. doi: 10.1097/01.bor.0000129663.76107.d6. [DOI] [PubMed] [Google Scholar]
- 41.Roberts S, Caterson B, Evans H, Eisenstein SM. Proteoglycan components of the intervertebral disc and cartilage endplate: an immunolocalization study of animal and human tissues. Histochem J. 1994;26:402–411. doi: 10.1007/BF00160052. [DOI] [PubMed] [Google Scholar]
- 42.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 43.Salisbury JR. The pathology of the human notochord. J Pathol. 1993;171:253–255. doi: 10.1002/path.1711710404. [DOI] [PubMed] [Google Scholar]
- 44.Sato S, Nieminen J. Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj J. 2004;19:583–591. doi: 10.1023/B:GLYC.0000014089.17121.cc. [DOI] [PubMed] [Google Scholar]
- 45.Sivan SS, Tsitron E, Wachtel E, Roughley P, Sakkee N, van der HF, Degroot J, Maroudas A (2006) Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J 399:29–35 [DOI] [PMC free article] [PubMed]
- 46.Stevens JW, Kurriger GL, Carter AS, Maynard JA. CD44 expression in the developing and growing rat intervertebral disc. Dev Dyn. 2000;219:381–390. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1060>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 47.Stosiek P, Kasper M, Karsten U. Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation. 1988;39:78–81. doi: 10.1111/j.1432-0436.1988.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 48.Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy -le-grand) 1998;44:1013–1023. [PubMed] [Google Scholar]
- 49.Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204:307–314. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- 50.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 51.Virchow R. Untersuchungen über die Entwicklungen des Schädelgrundes im gesunden und krankhaften Zustande und über den Einfluss derselben auf Schädelform, Gesichtsbildung und Gehirnbahn. Berlin: G. Reimers; 1857. [Google Scholar]
- 52.Walmsley R. The development and growth of the intervertebral disc. Edinburgh Med J. 1953;60:341–364. [PMC free article] [PubMed] [Google Scholar]
- 53.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weishaupt D, Zanetti M, Hodler J, Min K, Fuchs B, Pfirrmann CW, Boos N. Painful lumbar disk derangement: relevance of endplate abnormalities at MR imaging. Radiology. 2001;218:420–427. doi: 10.1148/radiology.218.2.r01fe15420. [DOI] [PubMed] [Google Scholar]
- 55.Weiss A. Die Entwicklung der Wirbelsäule der weissen Ratte, besonders der vordersten Halswirbel. Zeitsch Wissenschaft Zool. 1901;69:492–533. [Google Scholar]
- 56.Yamaguchi T, Suzuki S, Ishiiwa H, Shimizu K, Ueda Y. Benign notochordal cell tumors: a comparative histological study of benign notochordal cell tumors, classic chordomas, and notochordal vestiges of fetal intervertebral discs. Am J Surg Pathol. 2004;28:756–761. doi: 10.1097/01.pas.0000126058.18669.5d. [DOI] [PubMed] [Google Scholar]
- 57.Yamaoka A, Kuwabara I, Frigeri LG, Liu FT. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J Immunol. 1995;154:3479–3487. [PubMed] [Google Scholar]