Abstract
Pathomechanisms of injured-nerve pain have not been fully elucidated. Radicular pain and chronic constriction injury models have been established; however, producing these models is complicated. A sciatic nerve-pinch injury is easy to produce but the reliability of this model for evaluating pain behavior has not been examined. The current study evaluated pain-related behavior and change in pain markers in the dorsal root ganglion (DRG) of rats in a simple, sciatic nerve-pinch injury model. In the model, the sciatic nerve was pinched for 2 s using forceps (n = 20), but not injured in sham-operated animals (n = 20). Mechanical and thermal hyperalgesia were measured every second day for 2 weeks using von Frey filaments and a Hargreaves device. Calcitonin gene-related peptide (CGRP), activating transcription factor-3 (ATF-3), phosphorylated p38 mitogen activated protein (Map) kinase (p-p38), and nuclear factor-kappa B (NF-κB; p65) expression in L5 DRGs were examined at 4 and 7 days after surgery using immunohistochemistry. The proportion of neurons immunoreactive for these markers was compared between the two groups. Mechanical (during 8 days) and thermal hyperalgesia (during 6 days) were found in the pinch group rats, but not in the sham-operated animals (p < 0.05); however, hyperalgesia was not significant from days 10 to 14. CGRP, ATF-3, p-p38, and NF-κB expression in L5 DRGs was upregulated in the nerve-injured rats compared with the sham-operated rats (p < 0.01). Our results indicate that a simple sciatic nerve pinch produced pain-related behavior. Upregulation of the pain-marker expression in the nerve-injury model suggested it could be used as a model of pain. However, it was not considered as suitable for long-term studies.
Keywords: Rat, Pain, Nerve, Pinch
Introduction
Neuropathic pain is severe pain originating from peripheral nerve injury. Several rodent models for injured-nerve pain have been reported. For example, the models used to study neuropathic pain hypersensitivity in rodents have included chronic constriction of the sciatic nerve [4], partial nerve lesion [24], spinal segmental nerve injury [16], and disc herniation [15]. These models show prolonged mechanical and thermal hyperalgesia, and upregulation of cytokines and neuropeptides in dorsal root ganglion (DRG) neurons and in the spinal dorsal horn. However, the methods for producing these models are complicated.
Some authors have reported that rats in a sciatic nerve-pinch model showed pain-related behavior. Kato et al. [14] reported that a robust increase in pinch-induced mechanical hyperalgesia was evident at 6, 7, 9, 10, 11, and 12 days in rats. Bester et al. reported that sciatic nerve pinch resulted in inconsistent, but marked tactile allodynia manifesting first at 3 weeks and persisting in rats for up to 52 weeks [5]. George et al. reported that nerve-pinch injury induced a transient period of hypoalgesia to heat followed by the development of hyperalgesia to heat and mechanical stimulation by day 10 [8]. A sciatic nerve-pinch model is easy to produce; however, the reliability of this model for evaluating pain-related behavior and changes in neuropeptides and cell signaling in DRG neurons has not been fully examined.
To investigate pain-related behavior and changes in neuropeptide and cell signaling in DRG neurons (4 markers) in the current study, the following method was planned. For pain-related behavior, rats were evaluated for mechanical and thermal hyperalgesia using von Frey filaments and a Hargreaves device after nerve injury.
To examine changes in neuropeptide and cell signaling in DRG neurons, we have investigated the induction of activating transcription factor-3 (ATF3), a member of the activating transcription factor/cAMP-responsive element binding protein (ATF/CREB) family [9]. ATF2 is constitutively expressed by small-diameter DRG cells and is regulated by NGF [7]. ATF3, in contrast, is induced de novo in sensory and motor neurons after peripheral nerve injury and is widely used as a marker of DRG injury [22].
Calcitonin gene-related peptide (CGRP) is present in about 45% of the primary sensory neurons in the DRG [10]. DRG neuronal size is correlated with the caliber of primary afferent axons, and usually CGRP is localized to small neurons, which are usually associated with unmyelinated axons [10]. These axons terminate mainly in laminae I and II of the spinal dorsal horn and carry information from peripheral nociceptors [10]. A study of electrophysiologically-characterized neurons in lumbar dorsal root ganglia using a dye injection method revealed CGRP-like immunoreactivity in 46% of C-fiber neurons, 33% of A-δ fiber neurons, and 17% of A-α/β fiber neurons [19]. These data show that CGRP is mainly a marker of pain perception, although under some circumstances it is a marker of proprioception.
It has been reported that the nuclear factor-kappa B (NF-κB) plays a crucial role in regulating proinflammatory-cytokine gene expression and the transfer of nociceptive information [2, 3]. NF-κB is activated in DRG after partial sciatic nerve injury and is crucial for hyperalgesia [6, 18]. On the other hand, p38 mitogen activated protein (Map) kinase (p38) is phosphorylated by stress signals such as inflammatory cytokines, heat shock, ultraviolet light, and ischemia, and it plays a pivotal role in the mediation of cellular responses to a variety of signaling molecules [29]. p38 has also been reported to be activated in rat DRG following peripheral tissue inflammation and nerve injury distal to DRG [13, 17, 21]. Intrathecal administration of a p38 inhibitor has been shown to reduce hyperalgesia, suggesting that activated p38 may play an important role in inflammatory and neuropathic pain [21, 26].
The purpose of the current study was to evaluate pain-related behavior and changes in putative pain markers (CGRP, ATF-3, phosphorylated p38, and NF-κB) in the DRG in a simple, sciatic nerve-pinch model.
Materials and methods
All protocols for animal procedures were approved by the Ethics Committees of our institution following National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996 revision).
Rat nerve-injury model
Forty 6-week-old male Sprague–Dawley rats (200–250 g) were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneally (i.p.). Their left sciatic nerves were exposed. We pinched the sciatic nerves once for 2 s with 2-mm-wide smooth forceps (pinch group; n = 20). The sciatic nerve was pinched gradually, and pinching was stopped if the rat showed cramps in its hind paw. In sham-operated rats, the left sciatic nerves were exposed; however, the sciatic nerves were not pinched in animals from this group (sham-operated group, n = 20). In the 40 rats used in these studies, we examined only pain behavior in 20 rats and only immunohistochemistry in a further 20 rats separately. We did not examine pain behavior in the 20 rats used for immunohistochemistry.
Evaluation of mechanical hyperalgesia
Twenty rats were evaluated for tactile hyperalgesia (sham-operated group, n = 10; pinch group, n = 10). There were no animal dropouts on any day. Mechanical thresholds of the left hind paw were assessed using von Frey filaments with bending forces of 1.20 g (noxious stimulation) before surgery and at 2, 4, 6, 8, 10, 12, and 14 days after surgery. The von Frey filaments were applied to left hind paws for five trials at approximately 5 min intervals. The responses to these stimuli were ranked as follows: 0, no response; 1, withdrawal from the von Frey filament; and 2, immediate flinching or licking of the hind paw. Scores of five trials in each animal were added (Total score form 0 to 10 in each animal). The average nociceptive score was calculated [27].
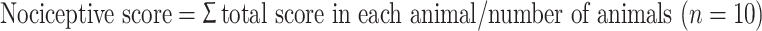 |
Evaluation of thermal hyperalgesia
Thermal nociceptive thresholds in rat hind paws were evaluated using a Hargreaves device (Ugo Basile, Varese, Italy). The animals were placed in a Plexiglas testing chamber (17 cm × 22 cm) with the floor maintained at 28°C. Rats were deposited into individual transparent acrylic boxes. A heat stimulus (150 mcal/s per cm2) was delivered using a 0.5-cm diameter radiant heat source positioned under the plantar surface of the paw to be tested. A cutoff time of 20 s was used after it was ascertained that no tissue damage was produced within this time. Three trials were performed at each time point, and the latencies of paw withdrawal for all trials were averaged.
Immunohistochemical detection of CGRP, ATF-3, phosphorylated-p38 (p-p38), and NF-κB (p65) in L5 DRG
Twenty rats were evaluated for CGRP, ATF-3, p-p38, and NF-κB expression (sham-operated group, n = 10; pinch group, n = 10). At 4 (each group, n = 5) and 7 (each group, n = 5) days after surgery, rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and perfused transcardially with 250 ml 4% paraformaldehyde (PFA) in phosphate buffer (0.1 M, pH 7.4). The left L5 DRGs were resected and cut into 10-μm sections on a cryostat. Sections were mounted on poly-l-lysine-coated slides. They were incubated in a blocking solution containing 0.3% Triton X-100 and 5% skim milk in 0.01 M phosphate buffered saline (PBS) for 60 min at room temperature. Sections were processed for immunohistochemical detection of CGRP by incubation with rabbit antibody to CGRP (1:1,000; Chemicon, Temecula, CA), of ATF-3 by incubation with a rabbit antibody to ATF3 (1:800; Santa Cruz Biotechnology, Santa Cruz, CA), of p-p38 by incubation with rabbit antibody to p-p38 (1:1,000; Cell Signaling, Beverly, MA), and of NF-κB by incubation with a rabbit antibody to NF-κB (p65, 1:800; Santa Cruz Biotechnology, Santa Cruz, CA), and for 20 h at 4°C, followed by incubation with goat anti-rabbit Alexa 488-fluorescein-conjugated antibody (1:400; Molecular Probes, Eugene, OR). The sections were examined using a fluorescence microscope (Nikon, Japan). We counted cells in 10 sections of each DRG. Immunoreactive cells were counted at ×400 magnification using a counting grid. The ratio of CGRP-, ATF-3-, p-p38-, and NF-κB-immunoreactive cells to total DRG neurons per 0.0225 mm2 was counted in DRG sections and averaged for each animal in a blinded manner.
Statistical analysis
The paw withdrawal latencies of hind paws were compared using a one-way analysis of variance (ANOVA) for repeated measurements. For multiple comparisons, we used Dunnett’s post hoc test. Comparison of the ratio of CGRP-, ATF-3-, p-p38-, and NF-κB-immunoreactive neurons between groups was made using a one-way ANOVA followed by Dunnett’s post hoc test. p < 0.05 was considered statistically significant and error bars indicate SEM.
Results
Behavior
Mechanical and thermal hyperalgesia caused by nerve pinch
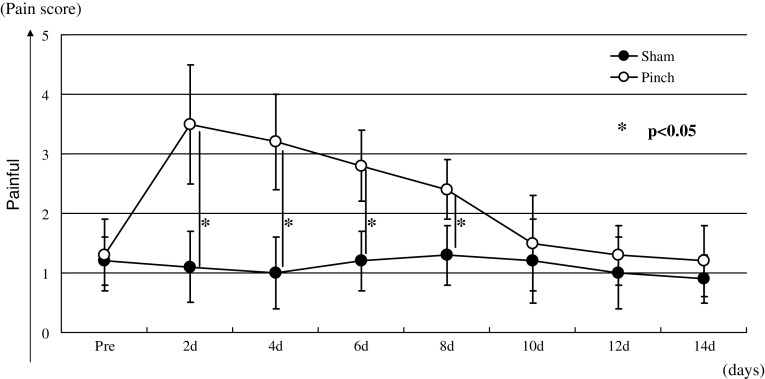
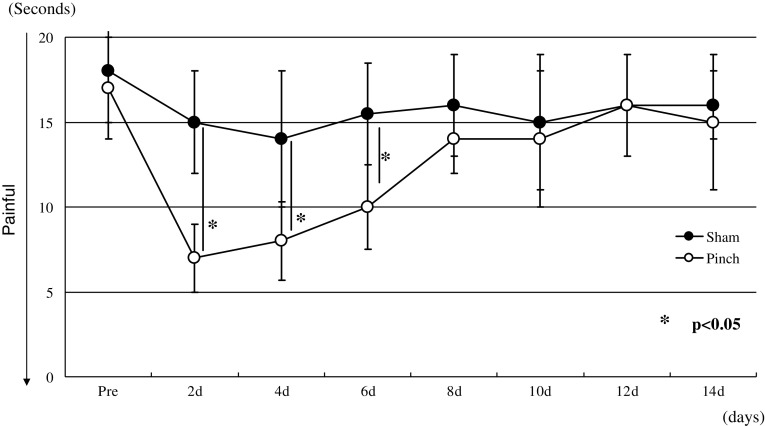
Animals in the pinch group displayed significant mechanical hyperalgesia that continued through day 8 compared with animals in the sham-operated group (p < 0.05) (Fig. 1). However, the mechanical hyperalgesia was not seen from days 10 to 14 (Fig. 1). The pinch group displayed significant thermal hyperalgesia that continued through day 6 compared with animals in the sham-operated group (p < 0.05) (Fig. 2). However, the thermal hyperalgesia was not seen from days 8 to 14 (Fig. 2).
Fig. 1.
Comparison of mechanical hyperalgesia between the pinch and sham-operated groups. Data are shown as mean ± SEM
Fig. 2.
Comparison of thermal hyperalgesia between the pinch and sham-operated groups. Data are shown as mean ± SEM
Immunohistochemistry
CGRP, ATF-3, p-p38, and NF-κB expression in DRG
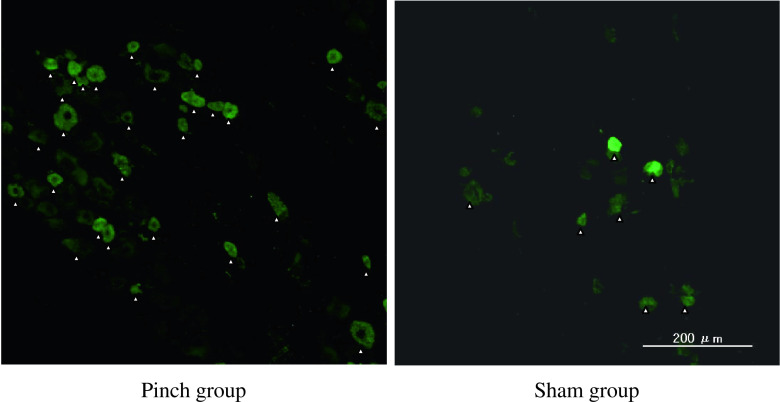
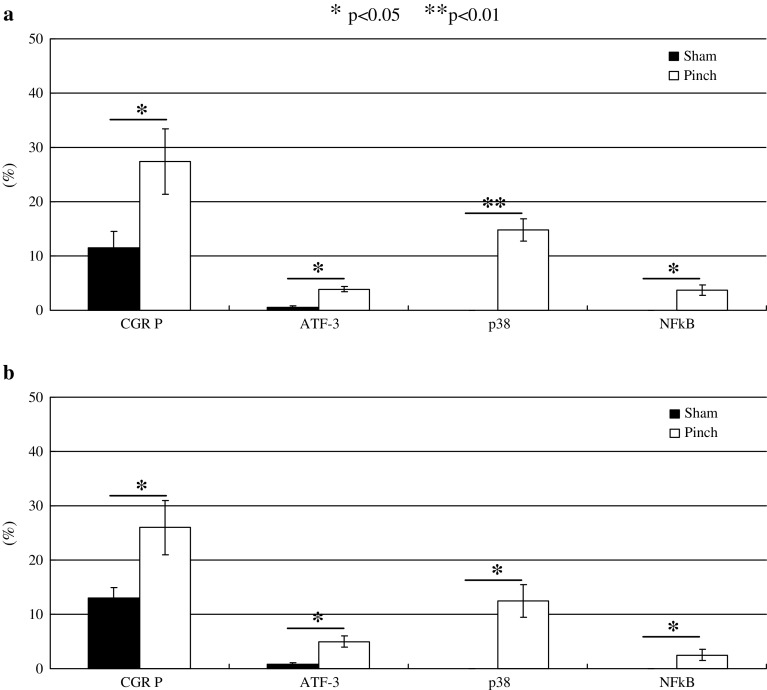
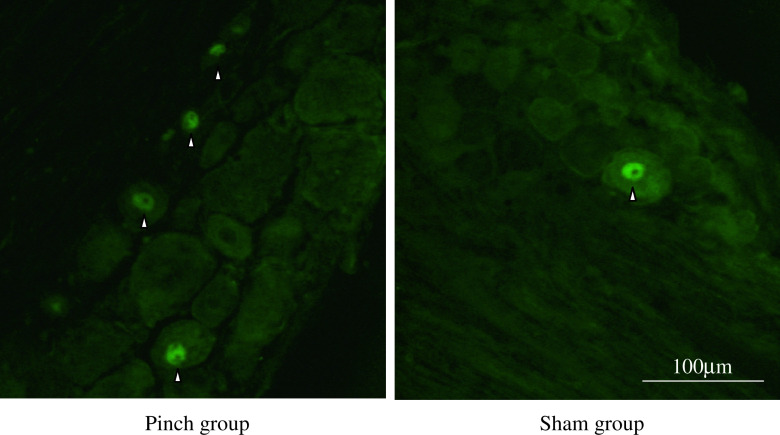
The ratio of CGRP-immunoreactive DRG neurons in the pinch group rats was significantly higher than that in the sham-operated rats on days 4 and 7 (p < 0.05) (Figs. 3 and 7).
Fig. 3.
CGRP immunoreactivity (arrowheads) in the cytoplasm of DRG neurons in the pinch group rats and in sham-operated animals on day 4
Fig. 7.
The average proportions of CGRP, ATF-3, p-p38, and NF-κB-immunoreactive DRG neurons (a day 4, b day 7) in sham-operated rats and pinch group animals. Data are shown as mean ± SEM
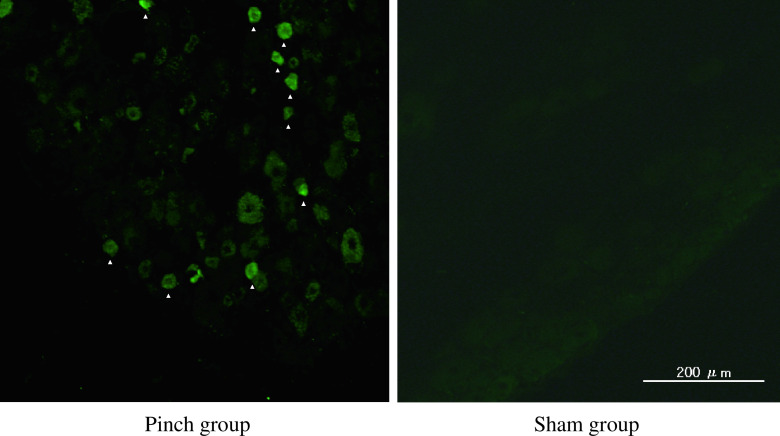
Scant ATF-3 immunoreactivity in DRG neurons was observed in the sham-operated rats (Fig. 4). Of total DRG neurons, the ratio of ATF-3-immunoreactive nuclei in cells in the DRG in the pinch group rats was significantly higher than in the sham-operated rats on days 4 and 7 (p < 0.05) (Figs. 4 and 7).
Fig. 4.
ATF-3 immunoreactivity (arrowheads) in the nuclei of DRG neurons in sham-operated rats and the pinch group animals on day 4
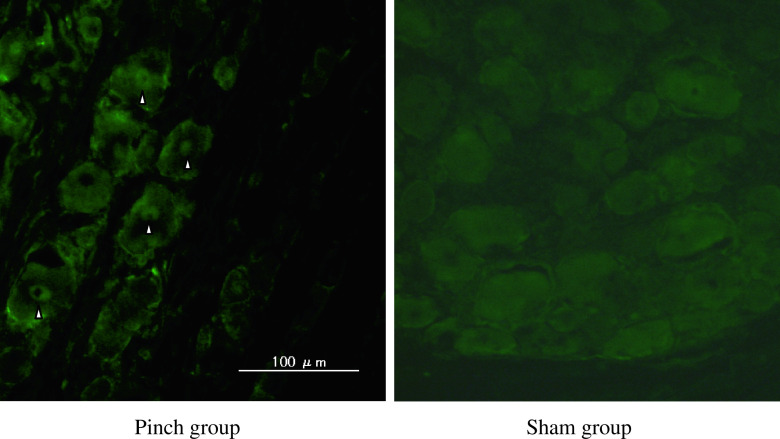
p-p38 immunoreactivity in cytoplasm of DRG neurons was not observed in sham-operated rats (Fig. 4); however, emergence of p-p38-immunoreactivity in the cytoplasm of DRG neurons in the pinch group rats was significant on days 4 and 7 (p < 0.05) (Figs. 5 and 7).
Fig. 5.
p-p38 immunoreactivity was not observed in sham-operated rats; however, it emerged in the cytoplasm of DRG neurons in the pinch group animals on day 4 (arrowheads)
Similarly to the expression of p-p38, NF-κB immunoreactivity in nuclei of DRG neurons was not observed in the sham-operated animals; however, emergence of NF-κB immunoreactivity in the nuclei of cells in the DRG in the pinch group rats was significant on days 4 and 7 (p < 0.01) (Figs. 6 and 7).
Fig. 6.
NF-κB immunoreactivity was not observed in sham-operated rats; however, it emerged in the nuclei of DRG neurons in the pinch group rats on day 4 (arrowheads)
Discussion
In the current study, a simple, sciatic nerve-pinch model produced pain-related behavior. CGRP, ATF-3, p-p38, and NF-κB expression were upregulated in the nerve-injury model rats; therefore, we concluded that this simple, sciatic nerve-pinch model can be used as a model of pain. However, this model only produced pain-related behavior for up to 6 days (thermal hyperalgesia) and 8 days (mechanical hyperalgesia).
ATF-3 is used as a marker for peripheral nerve injury. In some models of inflammatory and injured-nerve pain, inflammation by itself did not induce ATF-3 expression, and direct injury of nerve induced ATF3 expression [1, 11, 24, 28]. Therefore, nerve injury occurred in the current model, and the nerve injury was able to induce pain-related behavior and molecular change in DRG neurons.
After peripheral nerve injury, CGRP, which is not produced in medium and large DRG neurons under physiological conditions, begins to be expressed in these neurons [20, 23]. The expression of CGRP in DRG neurons is induced by mechanical allodynia [20]. These results indicate that a subpopulation of DRG neurons express CGRP in response to peripheral nerve injury, and transport this peptide to laminae III and IV in the spinal dorsal horn. The increase in CGRP in the deep laminae may affect the excitability of postsynaptic neurons, and may have a role in neuronal plasticity following peripheral nerve injury [20]. Considering the previous reports and current study, the upregulation of CGRP in DRG neurons in this current model seems to be correlated with pain-related behavior.
NF-κB and p-p38 expression were respectively upregulated in nuclei and cytoplasm of DRG neurons in the current nerve-injury model. We demonstrated that nerve injury produces mechanical allodynia and thermal hyperalgesia, and NF-κB decoy was transduced into nuclei of DRG neurons in vivo leading to a decrease of mechanical allodynia and thermal hyperalgesia in rats [25]. On the other hand, we demonstrated that nerve injury caused by lumbar disc herniation produces pain-related behavior, and that a specific p38 inhibitor decreased mechanical allodynia and thermal hyperalgesia in rats [12]. Therefore, we concluded that upregulation of NF-κB and p-p38, which are cell-signaling molecules, was correlated with pain-related behavior in the current injured-nerve model.
Some authors have reported that sciatic nerve-pinch rat models show pain-related behavior; however, the pattern of pain-related behavior varied as suggested in the introduction. Bester et al. [5] reported that a rat sciatic nerve pinch for 30 s resulted in an inconsistent, but marked tactile allodynia manifesting first at 3 weeks and persisting for up to 52 weeks. Kato et al. [14] reported that a rat sciatic nerve pinch for 5 s induced mechanical hyperalgesia that was evident during 12 days. These models showed a transient mechanical hypoalgesia that usually develops for a brief period and then is replaced by mechanical hyperalgesia that persists for weeks. We therefore selected the current model. We pinched sciatic nerves once for 2 s with 2-mm-wide smooth forceps. These animals did not show hypoalgesia and show pain-related behavior immediately after injury. The temporal difference in pain-related behavior among studies appears to be dependent on the different study designs. In the clinic, slight nerve compression induces short-term pain without hypoalgesia; for example, post-surgical pain caused by nerve compression during lumbar surgery and acute leg pain caused by mild compression of a nerve root. We consider that the current model is analogous to such clinical situations.
There were some limitations to this study and the current model. First, this model only produced pain-related behavior for up to 8 days and it is therefore not suitable for long-term studies, compared with the chronic constriction injury model. Second, we did not examine other molecules that may be related to pain in DRG neurons. Third, we did not examine the effect of varying the period of pinch and type of forceps used to create different pinch models. Fourth, we did not measure the force of the pinch using a calibrated device. Therefore, further study is needed to strengthen our current hypothesis.
In conclusion, a simple, sciatic nerve-pinch model produced pain-related behavior. CGRP, ATF-3, NF-κB, and p-p38 expression were upregulated in the nerve-injury model, and thus we concluded that this could be used as a model of short-term pain.
References
- 1.Averill S, Michael GJ, Shortland PJ, Leavesley RC, King VR, Bradbury EJ, McMahon SB, Priestley JV. NGF and GDNF ameliorate the increase in ATF3 expression which occurs in dorsal root ganglion cells in response to peripheral nerve injury. Eur J Neurosci. 2004;19:1437–1445. doi: 10.1111/j.1460-9568.2004.03241.x. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 4.Bennett GJ. An animal model of neuropathic pain: a review. Muscle Nerve. 1993;16:1040–1048. doi: 10.1002/mus.880161007. [DOI] [PubMed] [Google Scholar]
- 5.Bester H, Beggs S, Woolf CJ. Changes in tactile stimuli-induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve pinch. J Comp Neurol. 2000;428:45–61. doi: 10.1002/1096-9861(20001204)428:1<45::AID-CNE5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Chan CF, Sun WZ, Lin JK, Lin-Shiau SY. Activation of transcription factors of nuclear factor kappa B, activator protein-1 and octamer factors in hyperalgesia. Eur J Pharmacol. 2000;402:61–68. doi: 10.1016/S0014-2999(00)00431-3. [DOI] [PubMed] [Google Scholar]
- 7.Delcroix JD, Averill S, Fernandes K, Tomlinson DR, Priestley JV, Fernyhough P. Axonal transport of activating transcription factor-2 is modulated by nerve growth factor in nociceptive neurons. J Neurosci RC. 1999;24:1–7. doi: 10.1523/JNEUROSCI.19-18-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George A, Buehl A, Sommer C. Wallerian degeneration after pinch injury of rat sciatic nerve increases endo- and epineurial tumor necrosis factor-alpha protein. Neurosci Lett. 2004;372:215–219. doi: 10.1016/j.neulet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 9.Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/S0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- 10.Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-U. [DOI] [PubMed] [Google Scholar]
- 11.Inoue G, Ohtori S, Aoki Y, Ozawa T, Doya H, Saito T, Ito T, Akazawa T, Moriya H, Takahashi K. Exposure of the nucleus pulposus to the outside of the anulus fibrosus induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine. 2006;31:1433–1438. doi: 10.1097/01.brs.0000219946.25103.db. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Ozawa T, Saito T, Moriya H, Takahashi K. Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine. 2007;32:159–167. doi: 10.1097/01.brs.0000251437.10545.e9. [DOI] [PubMed] [Google Scholar]
- 13.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/S0896-6273(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 14.Kato K, Kikuchi S, Shubayev VI, Myers RR. Distribution and tumor necrosis factor-alpha isoform binding specificity of locally administered etanercept into injured and uninjured rat sciatic nerve. Neurosci. 2009;160:492–500. doi: 10.1016/j.neuroscience.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami M, Tamaki T, Weinstein JN, Hashizume H, Nishi H, Meller ST. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine. 1996;21:2101–2107. doi: 10.1097/00007632-199609150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Bae JC, Kim JY, Lee HL, Lee KM, Kim DS, Cho HJ. Activation of p38MAP kinase in the rat dorsal root ganglia and spinal cord following peripheral inflammation and nerve injury. Neuroreport. 2002;13:2483–2486. doi: 10.1097/00001756-200212200-00021. [DOI] [PubMed] [Google Scholar]
- 18.Ma W, Bisby MA. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res. 1998;797:243–254. doi: 10.1016/S0006-8993(98)00380-1. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy PW, Lawson SN. Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience. 1990;34:623–632. doi: 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- 20.Miki K, Fukuoka T, Tokunaga A, Noguchi K. Calcitonin gene-related peptide increase in the rat spinal dorsal horn and dorsal column nucleus following peripheral nerve injury: upregulation in a subpopulation of primary afferent sensory neurons. Neuroscience. 1998;82:1243–1252. doi: 10.1016/S0306-4522(97)00258-3. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima T, Obata K, Yamanaka H, Dai Y, Fukuoka T, Tokunaga A, Mashimo T, Noguchi K. Activation of p38 MAPK in primary afferent neurons by noxious stimulation and its involvement in the development of thermal hyperalgesia. Pain. 2005;113:51–60. doi: 10.1016/j.pain.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 22.Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/S0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- 23.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Phenotypic inflammation switch in rats shown by calcitonin gene-related peptide immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints. Spine. 2001;26:1009–1013. doi: 10.1097/00007632-200105010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Inoue G, Gemba T, Watanabe T, Ito T, Koshi T, Yamauchi K, Yamashita M, Orita S, Eguchi Y, Ochiai N, Kishida S, Takaso M, Aoki Y, Takahashi K, Ohtori S. Nuclear factor-kappa B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur Spine J. 2009;18:1001–1007. doi: 10.1007/s00586-009-0940-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 27.Takasaki I, Andoh T, Shiraki K, Kuraishi Y. Allodynia and hyperalgesia induced by herpes simplex virus type-1 infection in mice. Pain. 2000;86:95–101. doi: 10.1016/S0304-3959(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang R, Guo W, Ossipov MH, Vanderah TW, Porreca F, Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/S0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 29.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]