Abstract
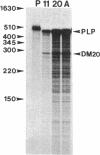
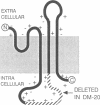
Proteolipid protein (PLP) is the major myelin membrane protein of the central nervous system. We have isolated a copy of an alternatively spliced PLP gene transcript from a mouse brain cDNA library that was screened for PLP-related sequences. The encoded 241-amino acid protein differs from PLP by an internal deletion of 35-amino acid residues (116-150) from the major hydrophilic domain. This PLP variant is identical with the DM-20 protein of myelin, previously described as a brain-specific myelin component and known to be related to PLP. We determined the corresponding nucleotide sequence of the rat PLP gene and found that DM-20 mRNA results when a second 5' splice site, located 105 nucleotides within the third exon of the primary PLP transcript, is utilized in precursor mRNA (pre-mRNA) splicing. This demonstrates that alternative 5' splice site selection can determine the protein product of a cellular gene. DM-20 mRNA is expressed in rat brain with approximately 50% abundance relative to PLP mRNA and appears to be developmentally coregulated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Agrawal H. C., Burton R. M., Fishman M. A., Mitchell R. F., Prensky A. L. Partial characterization of a new myelin protein component. J Neurochem. 1972 Sep;19(9):2083–2089. doi: 10.1111/j.1471-4159.1972.tb05118.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brow M. A., Pesin R., Sutcliffe J. G. The tetracycline repressor of pSC101. Mol Biol Evol. 1985 Jan;2(1):1–12. doi: 10.1093/oxfordjournals.molbev.a040334. [DOI] [PubMed] [Google Scholar]
- Dautigny A., Alliel P. M., d'Auriol L., Pham Dinh D., Nussbaum J. L., Galibert F., Jollès P. Molecular cloning and nucleotide sequence of a cDNA clone coding for rat brain myelin proteolipid. FEBS Lett. 1985 Aug 19;188(1):33–36. doi: 10.1016/0014-5793(85)80869-3. [DOI] [PubMed] [Google Scholar]
- Dautigny A., Mattei M. G., Morello D., Alliel P. M., Pham-Dinh D., Amar L., Arnaud D., Simon D., Mattei J. F., Guenet J. L. The structural gene coding for myelin-associated proteolipid protein is mutated in jimpy mice. 1986 Jun 26-Jul 2Nature. 321(6073):867–869. doi: 10.1038/321867a0. [DOI] [PubMed] [Google Scholar]
- Diehl H. J., Schaich M., Budzinski R. M., Stoffel W. Individual exons encode the integral membrane domains of human myelin proteolipid protein. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9807–9811. doi: 10.1073/pnas.83.24.9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eperon L. P., Estibeiro J. P., Eperon I. C. The role of nucleotide sequences in splice site selection in eukaryotic pre-messenger RNA. Nature. 1986 Nov 20;324(6094):280–282. doi: 10.1038/324280a0. [DOI] [PubMed] [Google Scholar]
- Gardinier M. V., Macklin W. B., Diniak A. J., Deininger P. L. Characterization of myelin proteolipid mRNAs in normal and jimpy mice. Mol Cell Biol. 1986 Nov;6(11):3755–3762. doi: 10.1128/mcb.6.11.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood M. M., Gilbert W. R., Agrawal H. C. In vivo acylation of proteolipid protein and DM-20 in myelin and myelin subfractions of developing rat brain: immunoblot identification of acylated PLP and DM-20. Neurochem Res. 1983 May;8(5):649–659. doi: 10.1007/BF00964704. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L. D., Berndt J. A., Puckett C., Kozak C. A., Lazzarini R. A. Aberrant splicing of proteolipid protein mRNA in the dysmyelinating jimpy mutant mouse. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1454–1458. doi: 10.1073/pnas.84.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollès J., Nussbaum J. L., Jollès P. Enzymic and chemical fragmentation of the apoprotein of the major rat brain myelin proteolipid. Biochim Biophys Acta. 1983 Jan 12;742(1):33–38. doi: 10.1016/0167-4838(83)90355-2. [DOI] [PubMed] [Google Scholar]
- Kühne T., Wieringa B., Reiser J., Weissmann C. Evidence against a scanning model of RNA splicing. EMBO J. 1983;2(5):727–733. doi: 10.1002/j.1460-2075.1983.tb01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C., Brow M. A., Nave K. A., Noronha A. B., Quarles R. H., Bloom F. E., Milner R. J., Sutcliffe J. G. Two forms of 1B236/myelin-associated glycoprotein, a cell adhesion molecule for postnatal neural development, are produced by alternative splicing. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4337–4341. doi: 10.1073/pnas.84.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen R. A., Samiullah M., Lees M. B. The structure of bovine brain myelin proteolipid and its organization in myelin. Proc Natl Acad Sci U S A. 1984 May;81(9):2912–2916. doi: 10.1073/pnas.81.9.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees M. B., Chao B. H., Lin L. F., Samiullah M., Laursen R. A. Amino acid sequence of bovine white matter proteolipid. Arch Biochem Biophys. 1983 Oct 15;226(2):643–656. doi: 10.1016/0003-9861(83)90334-x. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. J., Lai C., Nave K. A., Lenoir D., Ogata J., Sutcliffe J. G. Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell. 1985 Oct;42(3):931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- Morello D., Dautigny A., Pham-Dinh D., Jollès P. Myelin proteolipid protein (PLP and DM-20) transcripts are deleted in jimpy mutant mice. EMBO J. 1986 Dec 20;5(13):3489–3493. doi: 10.1002/j.1460-2075.1986.tb04674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith A. L., Hoffman-Chudzik E., Tsui L. C., Riordan J. R. Study of the expression of myelin proteolipid protein (lipophilin) using a cloned complementary DNA. Nucleic Acids Res. 1985 Oct 25;13(20):7413–7425. doi: 10.1093/nar/13.20.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K. A., Lai C., Bloom F. E., Milner R. J. Jimpy mutant mouse: a 74-base deletion in the mRNA for myelin proteolipid protein and evidence for a primary defect in RNA splicing. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9264–9268. doi: 10.1073/pnas.83.23.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S., Kitamura K., Campagnoni A. T. Identification of a cDNA coding for a fifth form of myelin basic protein in mouse. Proc Natl Acad Sci U S A. 1987 Feb;84(3):886–890. doi: 10.1073/pnas.84.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. A role for exon sequences and splice-site proximity in splice-site selection. Cell. 1986 Aug 29;46(5):681–690. doi: 10.1016/0092-8674(86)90343-0. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Skalidis G., Trifilieff E., Luu B. Selective extraction of the DM-20 brain proteolipid. J Neurochem. 1986 Jan;46(1):297–299. doi: 10.1111/j.1471-4159.1986.tb12962.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Hillen H., Schröder W., Deutzmann R. The primary structure of bovine brain myelin lipophilin (proteolipid apoprotein). Hoppe Seylers Z Physiol Chem. 1983 Oct;364(10):1455–1466. doi: 10.1515/bchm2.1983.364.2.1455. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Roach A., Teplow D. B., Prusiner S. B., Hood L. Cloning and characterization of the myelin basic protein gene from mouse: one gene can encode both 14 kd and 18.5 kd MBPs by alternate use of exons. Cell. 1985 Aug;42(1):139–148. doi: 10.1016/s0092-8674(85)80109-4. [DOI] [PubMed] [Google Scholar]
- Trifilieff E., Luu B., Nussbaum J. L., Roussel G., Espinosa de los Monteros A., Sabatier J. M., Van Rietschoten J. A specific immunological probe for the major myelin proteolipid. Confirmation of a deletion in DM-20. FEBS Lett. 1986 Mar 31;198(2):235–239. doi: 10.1016/0014-5793(86)80412-4. [DOI] [PubMed] [Google Scholar]
- Trifilieff E., Skalidis G., Hélynck G., Lepage P., Sorokine O., Van Dorsselaer A., Luu B. Données structurales sur le protéolipide de la myéline de masse moléculaire apparente 20 kDa (DM-20). C R Acad Sci III. 1985;300(7):241–246. [PubMed] [Google Scholar]
- Vacher-Leprêtre M., Nicot C., Alfsen A., Jollès J., Jollès P. Study of the apoprotein of Folch-Pi bovine proteolipid. II. Characterization of the components isolated from sodium dodecyl sulfate solutions. Biochim Biophys Acta. 1976 Feb 20;420(2):323–331. doi: 10.1016/0005-2795(76)90324-x. [DOI] [PubMed] [Google Scholar]
- Waehneldt T. V., Matthieu J. M., Jeserich G. Major central nervous system myelin glycoprotein of the African lungfish (Protopterus dolloi) cross-reacts with myelin proteolipid protein antibodies, indicating a close phylogenetic relationship with amphibians. J Neurochem. 1986 May;46(5):1387–1391. doi: 10.1111/j.1471-4159.1986.tb01752.x. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Riordan J. R. Assignment of the gene for myelin proteolipid protein to the X chromosome: implications for X-linked myelin disorders. Science. 1985 Nov 22;230(4728):940–942. doi: 10.1126/science.3840606. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- de Ferra F., Engh H., Hudson L., Kamholz J., Puckett C., Molineaux S., Lazzarini R. A. Alternative splicing accounts for the four forms of myelin basic protein. Cell. 1985 Dec;43(3 Pt 2):721–727. doi: 10.1016/0092-8674(85)90245-4. [DOI] [PubMed] [Google Scholar]