Abstract
Postoperative C5 palsy is a common complication after cervical spine decompression surgery. However, the incidence, prognosis, and etiology of C5 palsy after anterior decompression with spinal fusion (ASF) have not yet been fully established. In the present study, we analyzed the clinical and radiological characteristics of patients who developed C5 palsy after ASF for cervical degenerative diseases. The cases of 199 consecutive patients who underwent ASF were analyzed to clarify the incidence of postoperative C5 palsy. We also evaluated the onset and prognosis of C5 palsy. The presence of high signal changes (HSCs) in the spinal cord was analyzed using T2-weighted magnetic resonance images. C5 palsy occurred in 17 patients (8.5%), and in 15 of them, the palsy developed after ASF of 3 or more levels. Among ten patients who had a manual muscle test (MMT) grade ≤2 at the onset, five patients showed incomplete or no recovery. Sixteen of the 17 C5 palsy patients presented neck and shoulder pain prior to the onset of muscle weakness. In the ten patients with a MMT grade ≤2 at the onset, nine patients showed HSCs at the C3–C4 and C4–C5 levels. The present findings demonstrate that, in most patients with severe C5 palsy after ASF, pre-existing asymptomatic damage of the anterior horn cells at C3–C4 and C4–C5 levels may participate in the development of motor weakness in combination with the nerve root lesions that occur subsequent to ASF. Thus, when patients with spinal cord lesions at C3–C4 and C4–C5 levels undergo multilevel ASF, we should be alert to the possible occurrence of postoperative C5 palsy.
Keywords: C5 palsy, Cervical spine, Anterior surgery, Decompression, Fusion
Introduction
Postoperative C5 palsy is well known as a common complication after decompression surgery at the cervical spine [12]. Previous reports have indicated that C5 palsy occurs not only after posterior cervical decompression surgery, such as laminoplasty [1, 2, 5, 6, 8, 10, 13, 16–19], but also after anterior surgery [3–5, 7, 14, 15, 18, 19]. However, the number of studies analyzing C5 palsy after anterior cervical surgery is smaller than that for after posterior cervical surgery. Thus, the incidence, prognosis, and etiology of the C5 palsy after anterior cervical surgery have not yet been fully established. The aim of the present study was to investigate the incidence and prognosis of C5 palsy after anterior cervical surgery and discuss the mechanism of development of this disorder.
Materials and methods
Patient population
Between 1996 and 2004, 199 patients underwent anterior decompression surgery and spinal fusion (ASF) for cervical degenerative diseases in our institute (Table 1). Their cervical lesions were cervical spondylotic myelopathy (CSM) in 113 cases, cervical ossification of the posterior longitudinal ligament (OPLL) in 62 cases, cervical spondylotic amyotrophy (CSAM) in 16 cases, cervical spondylotic radiculopathy in 6 cases, and disc herniation in 2 cases. The number of fused levels was 1 for 33 cases, 2 for 40 cases, 3 for 46 cases, 4 for 71 cases, and 5 for 9 cases (Table 2). One level fusion surgery was performed by anterior cervical discectomy and fusion, and two or more levels of fusion surgery were performed by anterior cervical corpectomy and arthrodesis. Autologous iliac bone was grafted for one- or two-level fusions and an autologous fibula strut was grafted for fusion surgery of three or more levels [11]. In cases of one or two level fusion, a cervical collar was used postoperatively. In cases where three or more levels were fused, patients were immobilized with a halo vest for 8 weeks after surgery. No spinal instrumentation such as an anterior cervical plate system was used in the present series.
Table 1.
Cervical lesions and incidence of postoperative C5 palsy
| Cervical lesions | No. of cases | Cases of palsy (%) | Patients with MMT grade ≤2 (%) |
|---|---|---|---|
| Cervical spondylotic myelopathy | 113 | 9 (7.9) | 5 (4.4) |
| OPLL | 62 | 6 (9.7) | 5 (8.1) |
| Disc herniation | 2 | 0 | 0 |
| Cervical spondylotic amyotrophy | 16 | 2 (12.5) | 0 |
| Cervical spondylotic radiculopathy | 6 | 0 | 0 |
| Total | 199 | 17 (8.5) | 10 (5.0) |
MMT Manual muscle test, OPLL ossification of posterior longitudinal ligament
Table 2.
Fused levels and C5 palsy incidence
| No. of levels fused | Fused level | No. of patients | Cases of palsy (%) | Patients with MMT grade ≤2 (%) |
|---|---|---|---|---|
| 1 | C3–C4 | 13 | 0 | 0 |
| C4–C5 | 5 | 0 | 0 | |
| C5–C6 | 11 | 0 | 0 | |
| C6–C7 | 3 | 0 | 0 | |
| C7–T1 | 1 | 0 | 0 | |
| Subtotal | 33 | 0 | 0 | |
| 2 | C3–C5 | 14 | 0 | 0 |
| C4–C6 | 14 | 2 (14.3) | 1 (7.1) | |
| C5–C7 | 12 | 0 | 0 | |
| Subtotal | 40 | 2 (5.0) | 1 (2.5) | |
| 3 | C2–C5 | 1 | 0 | 0 |
| C3–C6 | 27 | 4 (14.8) | 2 (7.4) | |
| C4–C7 | 15 | 0 | 0 | |
| C5–T1 | 3 | 0 | 0 | |
| Subtotal | 46 | 4 (8.7) | 2 (4.3) | |
| 4 | C2–C6 | 6 | 2 (33.3) | 2 (33.3) |
| C3–C7 | 63 | 9 (14.3) | 5 (7.9) | |
| C4–T1 | 2 | 0 | 0 | |
| Subtotal | 71 | 11 (15.5) | 7 (9.9) | |
| 5 | C2–C7 | 9 | 0 | 0 |
Clinical assessment
We defined C5 palsy as when patients showed a deterioration in muscle power of the deltoid or biceps brachii by at least one grade in the manual muscle test (MMT) without aggravation of lower extremity function. We evaluated the laterality of C5 palsy, onset of pain, onset of weakness, and time course of any MMT grade change. The Japanese Orthopaedic Association (JOA) score was used to evaluate the severity of myelopathy [9].
Radiological assessment
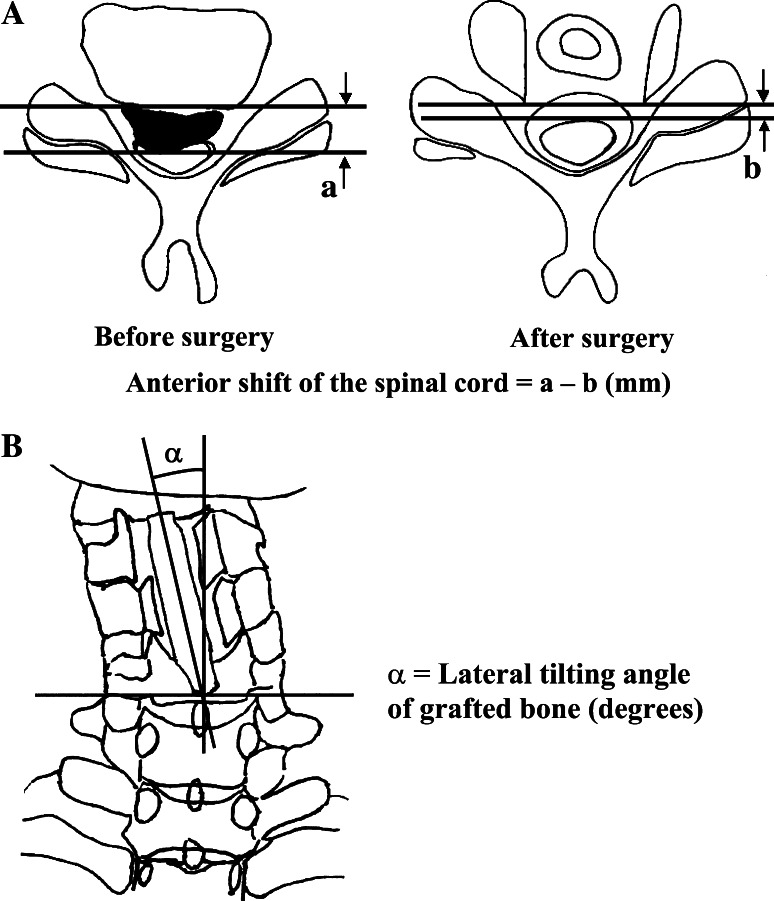
Using lateral cervical radiographs and images from computed tomography (CT), CT myelography, and magnetic resonance (MR), we identified the most stenotic level of the spinal column. High signal changes (HSCs) of spinal cord were assessed on preoperative and postoperative T2-weighted MR images. Anterior shift of the spinal cord after surgery at the most stenotic level of the spinal column was calculated on axial images from preoperative and postoperative CT myelography (Fig. 1a). Lateral tilting of grafted bone was assessed on postoperative cervical anterior–posterior radiographs (Fig. 1b).
Fig. 1.
Schematic drawings of anterior shift of the spinal cord (a) and lateral tilting of the grafted bone (b)
Statistical analysis
Fisher’s exact probability test was applied for statistical analysis. P < 0.05 was considered significant.
Results
Postoperative C5 palsy was found in 17 of 199 cases (8.5%). In CSM cases, 9 of 113 patients (7.9%) developed C5 palsy. Similarly, 6 of 62 OPLL patients (9.7%) and 2 of 16 CSAM patients (12.5%) developed C5 palsy. No patients with cervical spondylotic radiculopathy and disc herniation developed C5 palsy (Table 1). When the degree of paralysis was restricted to MMT grade ≤2, ten patients (5.0%) showed paralysis. They included five CSM cases (4.4%) and five OPLL cases (8.1%) (Table 1).
No patient showed an incidence of C5 palsy where there was only one level fusion. C5 palsy was found in 2 (5.0%) of 40 patients who underwent 2 level fusion, 4 (8.7%) of 46 patients with 3 level fusion, and 11 (15.5%) of 71 patients with 4 level fusion. No case of C5 palsy was found in nine patients with 5 level fusion (Table 2). To summarize, 2 of 73 patients (2.7%) developed C5 palsy after fusion at 1 or 2 levels, while 15 of 126 patients (11.9%) developed the palsy after 3 or more levels of fusion. The incidence of the palsy after fusion at three or more levels was significantly higher than that when only one or two levels were fused.
No additional surgery was performed for the postoperative C5 palsy cases. When C5 palsy developed, we stimulated the patient’s deltoid muscle using low-frequency sound waves and performed both active and passive shoulder range of motion (ROM) exercises to prevent contracture of the patient’s shoulder.
JOA scores of 17 patients who developed C5 palsy are shown in Table 3. All of the 17 patients showed a recovery from their myelopathy. Their extent of recovery ranged from 27.6 to 100% (average 71.2%) (Table 3).
Table 3.
JOA score of 17 patients who developed C5 palsy
| Patient no. | Age (years)/sex | Lesion | Levels fused (n) | FU period | JOA score | Recovery rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-op | At FU | ||||||||||
| Total | M-UE | M-LE | Total | M-UE | M-LE | ||||||
| MMT grade ≤2 | |||||||||||
| 1 | 37/F | CSM | C4–C6 (2) | 9y 3m | 16 | 4 | 4 | 17 | 4 | 4 | 100 |
| 2 | 26/M | CSM | C3–C6 (3) | 10y 10m | 13 | 3 | 3 | 17 | 4 | 4 | 100 |
| 3 | 50/M | CSM | C3–C6 (3) | 1y | 13 | 3 | 3 | 15 | 3 | 4 | 50 |
| 4 | 73/F | CSM | C2–C6 (4) | 9y 10m | 8 | 1 | 1 | 15 | 4 | 3 | 77.8 |
| 5 | 62/M | OPLL | C3–C7 (4) | 4y 4m | 7 | 1 | 1 | 16 | 4 | 3 | 90 |
| 6 | 52/M | CSM | C3–C7 (4) | 5y 1m | 10 | 2 | 3 | 15 | 3 | 4 | 71.4 |
| 7 | 61/M | OPLL | C3–C7 (4) | 1y | 2.5 | 0 | 0 | 6.5 | 1 | 1 | 27.6 |
| 8 | 70/F | OPLL | C3–C7 (4) | 5y 6m | 10 | 3 | 2 | 12.5 | 3.5 | 2.5 | 35.7 |
| 9 | 71/M | OPLL | C2–C6 (4) | 5y 6m | 8.5 | 2 | 1.5 | 13 | 4 (−2) | 2 | 64.7 |
| 10 | 64/M | OPLL | C3–C7 (4) | 10y | 10.5 | 2.5 | 2 | 13 | 4 (−2) | 3 | 38.5 |
| MMT grade ≥3 | |||||||||||
| 11 | 73/M | CSAM | C4–C6 (2) | 9y 10m | 15 | 4 (−2) | 2 | 17 | 4 | 4 | 100 |
| 12 | 70/M | CSAM | C3–C6 (3) | 5y 2m | 16 | 4 (−1) | 4 | 17 | 4 | 4 | 100 |
| 13 | 65/F | CSM | C3–C7 (4) | 10y 3m | 12 | 4 | 4 | 17 | 4 | 4 | 100 |
| 14 | 44/F | CSM | C3–C7 (4) | 10y | 13 | 3 | 3 | 15 | 4 | 4 | 50 |
| 15 | 64/M | CSM | C3–C7 (4) | 5y 5m | 10.5 | 2 | 2 | 13 | 3 | 2 | 38.5 |
| 16 | 48/M | CSM | C3–C7 (4) | 4y 6m | 14 | 3 | 3 | 16 | 4 | 4 | 66.7 |
| 17 | 65/M | OPLL | C3–C7 (4) | 4y 2m | 13.5 | 3 | 3 | 17 | 4 | 4 | 100 |
| Mean ± SD | 11.3 ± 3.5 | 2.4 ± 1.1 | 2.5 ± 1.1 | 14.8 ± 2.7 | 3.4 ± 0.9 | 3.3 ± 1.0 | 71.2 ± 27.0 | ||||
JOA score Japanese Orthopaedic Association score for cervical myelopathy, FU follow-up, M-UE motor function score of upper extremity, M-LE motor function score of lower extremity, CSM cervical spondylotic myelopathy, CSAM cervical spondylotic amyotrophy
The onset and prognosis of C5 palsy in the 17 patients are summarized in Table 4. Sixteen of 17 patients had radiating neck and shoulder pain prior to their muscle weakness. Pain was recognized 1–7 days (average 3.6 days) after surgery. Muscle weakness developed 2–23 days (average 7.2 days) after surgery. Twelve patients completely recovered from their C5 palsy. However, the recovery was incomplete in three patients, and two patients with OPLL showed no recovery. No patient showed preoperative weakness of the deltoid or biceps brachii. All seven patients who had an MMT grade ≥3 at the onset of their C5 palsy showed full recovery. Among ten patients with MMT grade ≤2 at the onset, five showed recovery to MMT grade 5, and three to MMT grade 4; two patients did not show any recovery and still had an MMT grade ≤2 at their follow-up. There was 0.8–15 months to maximum recovery (average 2.8 months). No patient with C5 palsy developed a new sensory deficit at the C5 dermatome area.
Table 4.
Onset and prognosis of C5 palsy
| Patient no. | Impaired muscle | Laterality | Onset of pain (days) | Onset of weakness (days) | MMT grade | Months to recovery | Degree of recovery | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre-op | At onset | At FU | |||||||
| MMT grade ≤2 | |||||||||
| 1 | D, B | Rt | 2 | 13 | 5 | 2 | 5 | 3 | Complete |
| 2 | D, B | Rt | 3 | 4 | 5 | 2 | 5 | 1.8 | Complete |
| 3 | D, B | Blt | 3 | 8 | 5 | 2 | 5 | 0.8 | Complete |
| 4 | D, B | Rt | 3 | 6 | 5 | 2 | 5 | 2 | Complete |
| 5 | D, B | Lt | 3 | 4 | 5 | 2 | 5 | 1 | Complete |
| 6 | D, B | Lt | 7 | 12 | 5 | 2 | 4 | 6 | Incomplete |
| 7 | D, B | Rt | 3 | 4 | 5 | 2 | 4 | 1.8 | Incomplete |
| 8 | D, B | Lt | – | 2 | 5 | 2 | 4 | 15 | Incomplete |
| 9 | D, B | Blt | 1 | 5 | 5 | 2 | 2 | NR | No |
| 10 | D, B | Lt | 5 | 5 | 5 | 1 | 1 | NR | No |
| MMT grade ≥3 | |||||||||
| 11 | D, B | Rt | 3 | 7 | 5 | 4 | 5 | 1 | Complete |
| 12 | D, B | Lt | 2 | 3 | 5 | 3 | 5 | 1.3 | Complete |
| 13 | D, B | Rt | 7 | 23 | 5 | 4 | 5 | 2 | Complete |
| 14 | D, B | Lt | 1 | 2 | 5 | 4 | 5 | 1 | Complete |
| 15 | D, B | Lt | 3 | 10 | 5 | 3 | 5 | 0.8 | Complete |
| 16 | D, B | Lt | 5 | 5 | 5 | 4 | 5 | 1 | Complete |
| 17 | D, B | Rt | 7 | 10 | 5 | 3 | 5 | 3 | Complete |
| Mean ± SD | 3.6 ± 2.0 | 7.2 ± 5.3 | 5.0 ± 0 | 2.6 ± 0.9 | 4.4 ± 1.2 | 2.8 ± 3.6 | |||
D Deltoid, B biceps, Rt right, Lt left, Blt bilateral, NR not recovered
The radiological characteristics of the 17 patients are summarized in Table 5. In all of the 17 patients, the most stenotic level of their spinal column included the C3–C4 or C4–C5 levels. On MR images, HSCs were found at C3–C4 or C4–C5 levels in 12 patients. When restricted to the ten patients who had an MMT grade ≤2 at the onset, nine of the ten had such HSCs (Table 5). Anterior shift of the spinal cord ranged 0.8–5.2 mm. Tilting of the grafted bone ranged from 0° to 14° (Table 5).
Table 5.
Radiological characteristics of 17 patients who developed the C5 palsy
| Case no. | Most stenotic level | T2W HSC | Anterior shift of spinal cord (mm) | Lateral tilting of grafted bone (°) |
|---|---|---|---|---|
| MMT ≤2 | ||||
| 1 | C4/5 | – | NA | 1 |
| 2 | C4/5, C5/6 | C4/5 | 1.9 | 14 |
| 3 | C3/4 | C3/4 | 3.4 | 2 |
| 4 | C4/5 | C4/5 | NA | 11 |
| 5 | C4/5 | C4/5 | 3.6 | 2 |
| 6 | C3/4, C4/5 | C3/4, 4/5 | 1.0 | 1 |
| 7 | C4/5, C5/6 | C4/5, 5/6 | NA | NA |
| 8 | C3/4 | C3/4 | 1.2 | 0 |
| 9 | C3/4, C4/5 | C3/4, 4/5 | 5.2 | 2 |
| 10 | C3/4 | C3/4, 4/5 | 1.0 | 6 |
| MMT ≥3 | ||||
| 11 | C4/5 | – | 2.0 | 1 |
| 12 | C4/5 | – | 1.9 | 1 |
| 13 | C4/5, C5/6 | – | NA | 6 |
| 14 | C4/5, C5/6 | C4/5, 5/6 | 0.8 | 0 |
| 15 | C4/5 | C4/5 | 1.9 | 1 |
| 16 | C3/4 | C3/4 | 4.3 | 1 |
| 17 | C3/4, C4/5 | – | 3.6 | 2 |
T2W HSC High signal change in T2-weighted magnetic resonance images, NA not assessed
Case presentation
Case 2
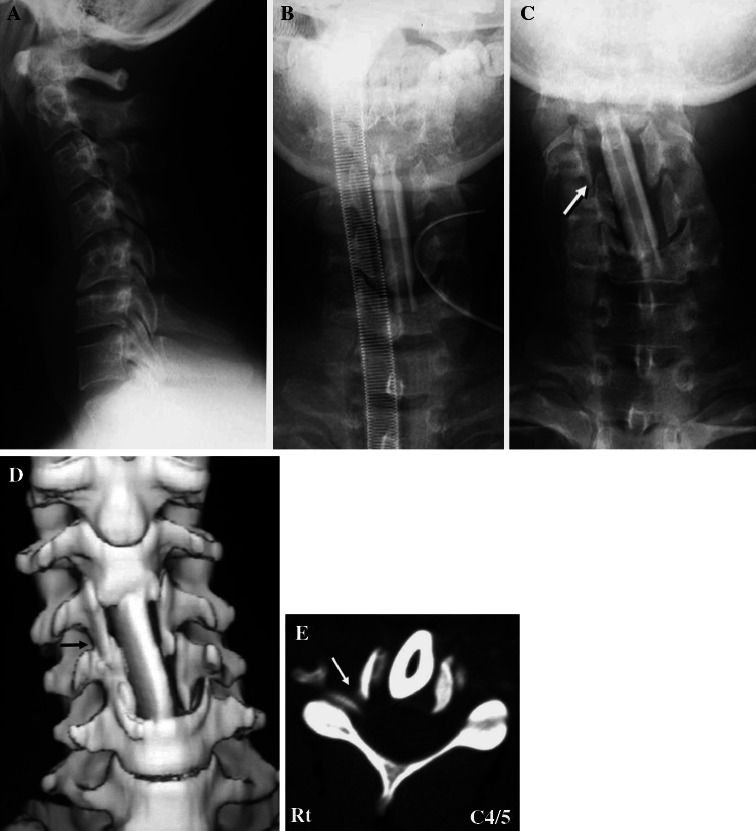
A 26-year-old man presented with myelopathy due to cervical spondylosis and a preoperative JOA score of 13 points. A preoperative roentgenogram showed kyphotic alignment of his cervical spine (Fig. 2a). Anterior corpectomy of C4 and C5 and arthrodesis at C3–C6 were performed using an autologous fibula strut. After surgery, the fibula strut tilted toward his right side. The lateral tilt angle of the grafted fibula was 5° immediately after surgery (Fig. 2b), and 14° on the seventh day (Fig. 2c). On the seventh day, subluxation of the right uncovertebral joint of C4–C5 was shown on an anterior–posterior roentgenogram (Fig. 2c, arrow). Postoperative CT 8 weeks after surgery showed foraminal stenosis of C4–C5 on the right side (Fig. 2d, e, arrows). On the third day after surgery, he had neck and right-shoulder pain. On the fourth day, his right deltoid muscle began to deteriorate to MMT grade 2. On the 70th postoperative day, the palsy naturally recovered to MMT grade 5. In this case, we speculated that the cause of the C5 palsy was foraminal stenosis at the right C4–C5.
Fig. 2.
Case 2. A preoperative lateral cervical radiograph showing kyphotic alignment of the cervical spine (a). Anterior–posterior views of cervical radiographs just after anterior corpectomy of C4 and C5 and arthrodesis at C3–6 (b) and on the seventh day after surgery (c). c The lateral tilting angle of the grafted fibula was 14° and the right C4–C5 uncovertebral joint was subluxed (arrow). Front view of three-dimensional CT (d) and axial CT images at the level of C4–C5 (e). CT 8 weeks postoperatively showing a subluxed right C4–C5 uncovertebral joint and stenosis of the right C4–C5 foramen (arrows)
Case 9
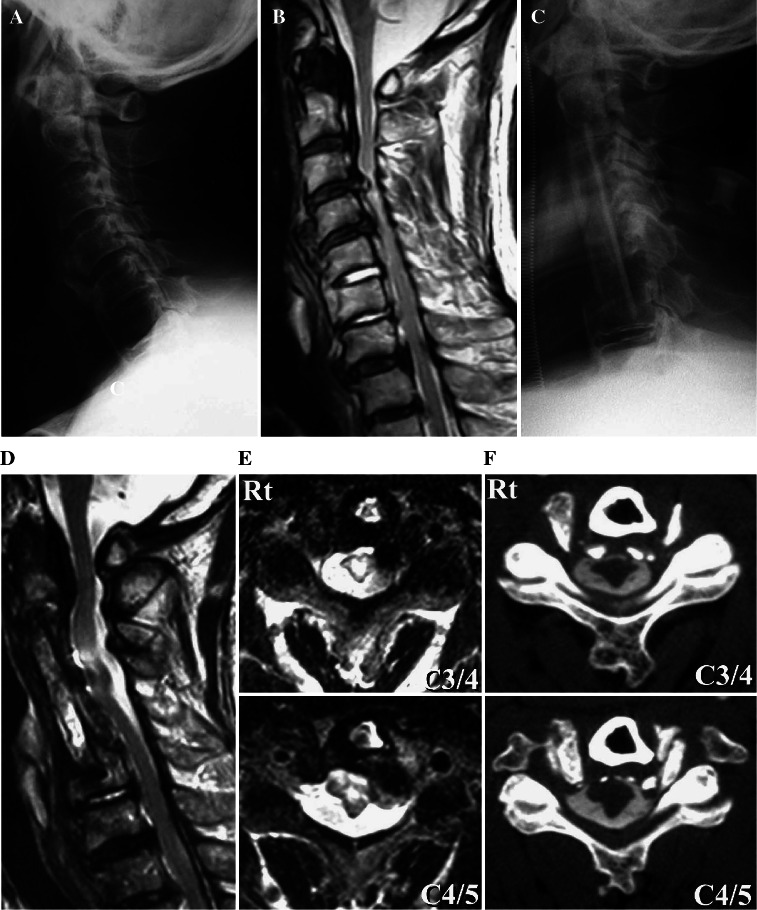
A 71-year-old man presented with cervical myelopathy caused by OPLL. A preoperative roentgenogram showed mixed type OPLL from C1 to C6 (Fig. 3a). His preoperative JOA score was 8.5 points. Preoperative T2-weighted MR images showed compression of the spinal cord and HSCs at C3–C4 and C4–C5 levels (Fig. 3b). He underwent anterior corpectomy of C3, C4, and C5 and arthrodesis using an autologous fibula strut from C2 to C6 (Fig. 3c). On the first day after surgery, he complained of left shoulder pain. On the fifth day, his left deltoid muscle power had deteriorated to MMT grade 2. On the 22nd postoperative day, his right deltoid muscle power had deteriorated to MMT grade 2. Eight months later, muscle power in his right upper extremities recovered to MMT grade 5. However, his left deltoid did not show any recovery, and was still MMT grade 2 at a final follow-up of 5 years and 6 months after surgery. Postoperative T2-weighted MR images showed HSCs at C3–C4 and C4–C5 levels (Fig. 3d, e). High intensity signal changes were detected in the gray matter of the spinal cord. Axial views of a CT myelogram revealed an excessive 5.2 mm anterior shift of the spinal cord (Fig. 3f). In this case, we suggest that the cause of C5 palsy was an excessive anterior shift of the spinal cord and tethering of nerve roots. In addition, we speculate that there was asymptomatic damage of the anterior horn cells in the spinal cord, which had existed before surgery and may have also participated in the development of C5 palsy.
Fig. 3.
Case 9. A preoperative lateral cervical radiograph (a) showing mixed type OPLL from C1 to C6. A midsagittal T2-weighted MR image (b) showing severe compression of the spinal cord and HSCs at C3–C4 and C4–C5 levels. A postoperative lateral cervical radiograph shows anterior corpectomy of C3, C4, and C5 and arthrodesis at C2–C6 (c). T2-weighted MR midsagittal (d) and axial views at C3–C4 and C4–C5 (e) and a CT myelogram (f) showing an excessive anterior shift of the spinal cord at C3–C5. e HSCs in the gray matter at the C3–C4 and C4–C5 levels
Discussion
Sakaura et al. [12] have reviewed the literature regarding postoperative C5 palsy published from 1986 to 2002. They reported that the incidence of C5 palsy after cervical posterior decompression surgery was 4.7% on average, ranging from 0 to 30.0%. On the other hand, its incidence after ASF for cervical lesions was 4.3% on average, ranging from 1.6 to 12.1%, although the number of reports regarding anterior surgery was smaller than that for posterior surgery. To the best of our knowledge, there have been only five large studies on C5 palsy that analyzed more than 100 cases of cervical anterior surgeries [4, 5, 7, 14, 19]. We have summarized their data in Table 6. According to these studies, the incidence of C5 palsy after anterior surgery differed considerably from 3.2 to 9.1%, depending on the institution where they were conducted. The major difference seems to be because a unified definition of C5 palsy has not yet been clearly made. In the present study, we defined C5 palsy as a deterioration of upper extremity motor function by at least one grade in a standard MMT without aggravation of lower extremity function. According to our criteria, the incidence of C5 palsy after the surgeries described was 8.5% in our institute. When the palsy was restricted to a MMT grade ≤2, the incidence was 5.0%. When reporting C5 palsy, therefore, it is essential to define it clearly.
Table 6.
Incidence and prognosis of C5 palsy after anterior decompression surgery for cervical lesions
| Report (year) | No. of patients | No. of cases of palsy (% of patients) | Cases of palsy with MMT grade ≤2 (% of all cases) | 1 and 2 levels fusion | 3 and more levels fusion | Recovery of the palsy (n) | |||
|---|---|---|---|---|---|---|---|---|---|
| No. of cases | Cases of palsy (%) | No. of cases | Cases of palsy (%) | Complete | Incomplete and none | ||||
| Yonenobu (1991) | 204 | 8 (3.9) | 8 (3.9) | ND | 0 | ND | 8 | 0 | 8 |
| Saunders (1995) | 176 | 16 (9.1) | ND | 20 | 1 (5.0) | 156 | 15 (9.6) | ND | ND |
| Greiner-Perth (2005) | 121 | 10 (8.2) | 3 (2.5) | 65 | 3 (4.6) | 56 | 7 (12.5) | 8 | 2 |
| Ikenaga (2005) | 563 | 18 (3.2) | 7 (1.2) | 201 | 0 (0) | 362 | 18 (5.0) | 11 | 7 |
| Hasegawa (2007) | 424 | 22 (5.2) | ND | ND | ND | ND | ND | ND | ND |
| Present report | 199 | 17 (8.5) | 10 (5.0) | 73 | 2 (2.7) | 126 | 15 (11.9) | 12 | 5 |
ND Not described
Regarding the correlation between the fused levels and the incidence of C5 palsy, Ikenaga et al. [7] described that no patient developed C5 palsy after fusion of 1 or 2 levels, and 18 of 362 patients (5.0%) developed the palsy after fusion of 3 or more levels. Greiner-Perth et al. [4] described that 3 of 65 (4.6%) patients developed C5 palsy after fusion of 1 or 2 levels, and 7 of 56 patients (12.5%) developed the palsy after fusion of 3 or more levels. In the present study, we found that 2 of 73 patients (2.7%) developed C5 palsy after fusion of 1 or 2 levels, and 15 of 126 patients (11.9%) developed the palsy after fusion of 3 or more levels. The present results, taken together with those previous reports collectively indicate that the more levels involved in anterior cervical decompression, the more likely the occurrence of C5 palsy.
Previous reports showed that C5 palsy generally had a good prognosis for neurologic and functional recovery. However, irreversible cases of C5 palsy were also reported. Ikenaga et al. [7] reported 7 cases of partial recovery in 18 patients with cases of C5 palsy, and Greiner-Perth et al. [4] reported two cases of partial recovery in ten patients with cases of C5 palsy. In the present study, among 17 patients with cases of C5 palsy, all 7 cases with an MMT grade ≥3 at the onset showed complete recovery. However, only five out of ten patients with cases of C5 palsy and an MMT grade ≤2 palsy showed complete recovery, three patients recovered incompletely, and two patients remained unchanged at the latest follow-up. For the two palsy patients with no recovery, both had OPLL, and deteriorated to MMT grades 1 and 2 at the onset, and had HSCs on T2-weighted MR images at both C3–C4 and C4–C5 levels. This suggests that, when patients with compression myelopathy and an ossified mass at C3–C4 and C4–C5 levels develop C5 palsy with an MMT grade ≤2 after ASF, favorable recovery from their palsy may not necessarily be expected. We speculate that certain pre-existing asymptomatic damage to the anterior horn cells in the gray matter of the spinal cord at the C3–C4 and C4–C5 levels may participate in the poor neurological improvement.
Over the past few years, hypotheses regarding the etiology of postoperative C5 palsy have been proposed by many authors. Most of the speculation regarding the etiology is roughly classified into two groups. One hypothesis is that C5 palsy is caused by nerve root damage. Some authors proposed that this might be caused by direct injury to the nerve root [6]. They proposed that surgical instruments could injure neural tissue. However, such a hypothesis cannot explain why many cases of C5 palsy develop several days after the surgery. Others have presumed that tethering of nerve roots might cause C5 palsy as the result of a shift of the spinal cord in association with anchoring of the nerve root [6, 16]. Saunders [14] hypothesized that narrowing of the width of anterior decompression decreased the anterior shift of the spinal cord and the traction of the roots, and consequently prevented the development of radiculopathy after corpectomy. Ikenaga et al. [7] reported the possibility that the extent of anterior dural expansion might have enhanced the incidence of C5 palsy after anterior surgery.
The other hypothesis is that C5 palsy might be caused by a spinal cord disorder. Chiba et al. [1] analyzed C5 palsy after laminoplasty, and proposed that postoperative upper extremity paresis might be caused by a deterioration of the gray matter and proposed that local reperfusion injury in the spinal cord could be the pathomechanism. Hasegawa et al. [5] analyzed C5 palsy after laminoplasty and anterior decompression surgery, and commented that postoperative upper extremity palsy following cervical decompression surgery might result from a transient and localized spinal cord lesion caused by the decompression of a chronic compressive cervical cord disorder. However, both theories regarding the nerve root and spinal cord remain hypothetical because of non-availability of reliable evidence for verifying their proposed hypothetical mechanism.
In the present study, 16 of 17 patients with cases of C5 palsy presented neck and shoulder pain prior to the onset of muscle weakness. This finding supports the theory that postoperative C5 palsy is caused by a certain nerve root lesion. In Case 9, excessive anterior shift of the spinal cord was observed. Because shoulder pain was detected prior to the muscle weakness in this case, it is possible that tethering of C5 nerve roots occurred bilaterally at the posterior edge of excavated vertebra, and that this caused the bilateral C5 palsy. However, the presence of HSCs on T2-weighted MR images at the C3–C4 and C4–C5 levels also provided the possibility of a spinal cord disorder as the etiology of the C5 palsy in this case.
In the present study, it is of particular interest that in all 17 patients with C5 palsy, the most stenotic level of their spinal canal included C3–C4 or C4–C5 levels. In addition, in 12 out of the 17 cases, HSCs were detected at C3–C4 or C4–C5 levels. When restricted to the ten cases of palsy with an MMT grade ≤2, nine cases showed such HSCs. Because all of the 17 patients with C5 palsy had normal muscle power of deltoid and biceps preoperatively, we suggest that asymptomatic damage of anterior horn cells at the gray matter of the spinal cord pre-existed in these cases. Taking all these findings into account, we propose a “double lesion” hypothesis for the development of C5 palsy after ASF for cervical lesion as follows: the pre-existing asymptomatic damage at the anterior horn cells may contribute to the development of postoperative C5 palsy, in combination with nerve root lesions caused by the foraminal stenosis or the excessive anterior shift of the spinal cord after the anterior decompression procedure. Further studies using preoperative and intraoperative electrodiagnostic techniques, such as preoperative EMG and intraoperative neuromonitoring, should provide us with useful information to support this hypothesis.
In conclusion, when patients having spinal cord lesions at C3–C4 and C4–C5 levels undergo multilevel ASF, we should be alert to the possible occurrence of postoperative C5 palsy. To avoid the development of C5 palsy, further improvement of surgical techniques is required to minimize the damage to the nerve roots that may occur subsequent to anterior decompression procedures.
Acknowledgments
This work was supported by the Health Labour Science Research Grant of Japan.
References
- 1.Chiba K, Toyama Y, Matsumoto M, et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine. 2002;27:2108–2115. doi: 10.1097/00007632-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Edwards CC, 2nd, Heller JG, Silcox DH., 3rd T-Saw laminoplasty for the management of cervical spondylotic myelopathy: clinical and radiographic outcome. Spine. 2000;25:1788–1794. doi: 10.1097/00007632-200007150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Epstein N. Anterior approaches to cervical spondylosis and ossification of the posterior longitudinal ligament: review of operative technique and assessment of 65 multilevel circumferential procedures. Surg Neurol. 2001;55:313–324. doi: 10.1016/S0090-3019(01)00464-5. [DOI] [PubMed] [Google Scholar]
- 4.Greiner-Perth R, ElSaghir E, Böhm H, et al. The incidence of C5–C6 radiculopathy as a complication of extensive cervical decompression: own results and review of literature. Neurosurg Rev. 2005;28:137–142. doi: 10.1007/s10143-004-0352-7. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa K, Homma T, Chiba Y, et al. Upper extremity palsy following cervical decompression surgery results from a transient spinal cord lesion. Spine. 2007;32:E197–E202. doi: 10.1097/01.brs.0000257576.84646.49. [DOI] [PubMed] [Google Scholar]
- 6.Hirabayashi K, Toyama Y, Chiba K. Expansive laminoplasty for myelopathy in ossification of the posterior longitudinal ligament. Clin Orthop. 1999;359:35–48. doi: 10.1097/00003086-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Ikenaga M, Shikata J, Tanaka C, et al. Radiculopathy of C-5 after anterior decompression for cervical myelopathy. J Neurosurg Spine. 2005;3:210–217. doi: 10.3171/spi.2005.3.3.0210. [DOI] [PubMed] [Google Scholar]
- 8.Komagata K, Nishiyama M, Endo K, et al. Prophylaxis of C5 palsy after cervical expansive laminoplasty by bilateral partial foraminotomy. Spine J. 2004;4:650–655. doi: 10.1016/j.spinee.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20:7–13. doi: 10.1097/01.bsd.0000211260.28497.35. [DOI] [PubMed] [Google Scholar]
- 10.Minoda Y, Nakamura H, Konishi S, et al. Palsy of the nerve root after midsagittal-splitting laminoplasty of the cervical spine. Spine. 2003;28:1123–1127. doi: 10.1097/00007632-200306010-00008. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki M, Aiba A, Hashimoto M, et al. Cervical myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg Spine. 2009;10:122–128. doi: 10.3171/2008.10.SPI08480. [DOI] [PubMed] [Google Scholar]
- 12.Sakaura H, Hosono N, Mukai Y, et al. C5 palsy after decompression surgery for cervical myelopathy. Review of the literature. Spine. 2003;28:2447–2451. doi: 10.1097/01.BRS.0000090833.96168.3F. [DOI] [PubMed] [Google Scholar]
- 13.Satomi K, Nishu Y, Kohno T, et al. Long-term follow-up studies of open door expansive laminoplasty for cervical stenotic laminoplasty. Spine. 1994;19:507–510. doi: 10.1097/00007632-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Saunders RL. On the pathogenesis of the radiculopathy complicating multilevel corpectomy. Neurosurgery. 1995;37:408–413. doi: 10.1227/00006123-199509000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Shinomiya K, Kurosa Y, Fuchioka M, et al. Clinical study of dissociated motor weakness following anterior cervical decompression surgery. Spine. 1989;14:1211–1214. doi: 10.1097/00007632-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Tsuzuki N, Abe R, Saiki K, et al. Paralysis of the arm after posterior decompression of the cervical spinal cord. II. Analyses of clinical findings. Eur Spine J. 1993;2:197–202. doi: 10.1007/BF00299446. [DOI] [PubMed] [Google Scholar]
- 17.Uematsu Y, Tokuhashi Y, Matsuzaki H. Radiculopathy after laminoplasty of the cervical spine. Spine. 1998;23:2057–2062. doi: 10.1097/00007632-199810010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Wada E, Suzuki S, Kanazawa A, et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term-follow-up study over 10 years. Spine. 2001;26:1443–1448. doi: 10.1097/00007632-200107010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Yonenobu K, Hosono N, Iwasaki M, et al. Neurologic complications of surgery for cervical compression myelopathy. Spine. 1991;16:1277–1282. doi: 10.1097/00007632-199111000-00006. [DOI] [PubMed] [Google Scholar]