Abstract
The development and growth of renal glomeruli is regulated by specific angiogenic growth factors, including vascular endothelial growth factor (VEGF) and the angiopoietins (ANGPT1 and ANGPT2). The expression of these factors has already been studied during metanephric glomerulogenesis, but it remains to be elucidated during the development of the embryonic mesonephros, which can function as an interesting model for glomerular development and senescence. In this study, the presence of the angiogenic growth factors was studied in developing porcine mesonephroi, using IHC and real-time RT-qPCR on laser capture microdissected glomeruli. In addition, mesonephric glomerular growth was measured by using stereological methods. ANGPT2 remained upregulated during maturation of glomeruli, which may be explained by the continuous growth of the glomeruli, as observed by stereological examination. The mRNA for VEGFA was expressed in early developing and in maturing glomeruli. The VEGF receptor VEGFR1 was stably expressed during the whole lifespan of mesonephric glomeruli, whereas VEGFR2 mRNA was only upregulated in early glomerulogenesis, suggesting that VEGFR2 is important for the vascular growth but that VEGFR1 is important for the maintenance of endothelial fenestrations. (J Histochem Cytochem 58:1045–1056, 2010)
Keywords: mesonephros, kidney, intussusceptive angiogenesis, fenestrations, KDR, FLT1, TEK, real-time PCR, 3D reconstruction, micro-CT
During mammalian embryogenesis, the urinary system develops through three successive stages, each characterized by a distinct pair of excretory organs. Initially, the pronephros is formed, but it only remains vestigial and regresses quickly as the mesonephros develops. The latter becomes a functional excretory organ in embryos of a number of species such as pigs, sheep, rabbits, and presumably also in man (Leeson and Baxter 1957; Ludwig and Landmann 2005). In the early fetal stage, the mesonephros regresses and the metanephros, i.e., the definitive kidney, starts to develop. The normal physiological regression of the mesonephros can serve as a model for the study of pathologically degenerating renal glomeruli because the morphological characteristics of developing mesonephric and metanephric glomeruli are highly similar (Gersh 1937; Leeson and Baxter 1957; Tiedemann and Egerer 1984; Smith and Mackay 1991; Nacher et al. 2006,2010). However, the molecular mechanisms of mesonephric glomerulogenesis are not well-known.
Glomeruli form the basic filtration units of the kidney. They allow the passive filtration of blood into primary urine. The glomerulus is composed of three different cell types, i.e., endothelial cells, podocytes, and mesangial cells. The flattened and highly fenestrated endothelial cell layer is entirely enveloped by podocytes that belong to the visceral layer of the Bowman's capsule. The mesangial cells are situated near the hilus of the glomerulus. They provide structural support and act as specialized pericytes for the glomerular capillaries (Quaggin and Kreidberg 2008). Glomerular development starts with the formation of “S-shaped bodies” that connect to collecting tubules. In the metanephros, these collecting tubules arise from the ureteric bud, whereas the collecting tubules of the mesonephros are formed by the Wolffian duct. The S-shaped bodies bear a layer of podocytes at their proximal end, which forms the proximal cleft. Angioblasts migrate into this cleft and form the glomerular capillaries (Gersh 1937; Leeson and Baxter 1957; Smith and Mackay 1991).
The development of the glomerular vasculature starts with the formation of a few capillary loops through the process of vasculogenesis, i.e., the development of new blood vessels “de novo” from endothelial progenitor cells (Quaggin and Kreidberg 2008; Kassmeyer et al. 2009). The further growth proceeds through a process called angiogenesis, i.e., the formation of new blood vessels from an already existing capillary plexus. During angiogenesis, new capillaries are formed either through sprouting angiogenesis or through intussusceptive branching (Djonov et al. 2002). During sprouting angiogenesis, a capillary sprout grows on an existing vessel and forms a new capillary branch. This mechanism is initiated by the destabilization of the blood vessels and of the endothelial basement membrane, followed by migration and proliferation of the endothelial cells and eventually by the formation of the new capillary (Carmeliet 2000; Papetti and Herman 2002). Intussusceptive angiogenesis is a process in which an existing blood vessel splits up into two new vessels through the growth of an intraluminal tissue pillar (Patan et al. 1993). During this process, the endothelial cells do not necessarily proliferate, but they increase in volume and become thinner (Kurz et al. 2003). This type of angiogenesis is considered as a faster process that requires a lower metabolic cost than sprouting angiogenesis. Moreover, no blind-ending capillaries are formed (Djonov et al. 2002). In developing glomeruli, sprouting angiogenesis mainly takes place during the initial growth of the glomerular vasculature and is supervened by intussusceptive angiogenesis in the later stages of development (Makanya et al. 2005; Vaughan and Quaggin 2008). This switch in angiogenic growth facilitates the expansion of glomeruli without interfering with their ability to filter the circulating blood.
Vascular endothelial growth factor A (VEGFA) is currently known as the major angiogenic growth factor. By acting through its receptors VEGFR1 (FLT1) and VEGFR2 (KDR), it initiates vasculogenesis, induces endothelial migration, functions as a survival factor for endothelial cells, and enhances vessel permeability through the formation of endothelial fenestrations and interendothelial gaps (Otrock et al. 2007). In the developing metanephric kidney, VEGFA is expressed during glomerulogenesis as well as in mature glomeruli. It is thought to initiate angioblast migration, initial vasculogenesis, and further capillary growth (Eremina and Quaggin 2004). In mature glomeruli, VEGFA secures the maintenance of the endothelial fenestrations (Kamba et al. 2006; Satchell et al. 2006).
The angiopoietins also have a major influence on endothelial cells, but their function in glomerulogenesis is still elusive (Augustin et al. 2009). Angiopoietin-1 (ANGPT1), acting through its receptor TIE2 (TEK), is a blood vessel stabilization factor. It promotes endothelial quiescence, tightens interendothelial bonds, and acts as a survival factor. ANGPT2 is described as an antagonist of ANGPT1. By competitively binding on TIE2, it inhibits ANGPT1-mediated receptor activation (Maisonpierre et al. 1997). Depending on the presence or absence of VEGF, ANGPT2 induces angiogenesis or vascular degeneration, respectively (Lobov et al. 2002; Scharpfenecker et al. 2005). TIE1 is another endothelium-specific receptor, showing a high degree of structural similarity to the TIE2 receptor. Although its role is unclear and TIE1 is not activated by the angiopoietins, recent evidence shows that it may play an important regulatory role during ANGPT–TIE2 activation (Seegar et al. 2010). The angiopoietins and TIE receptors are present in glomeruli of human, murine, and porcine developing kidneys, but their role in glomerulogenesis is still unclear (De Spiegelaere et al. in press).
In this study, the expression patterns of the angiopoietins and VEGFA were studied during mesonephric glomerulogenesis of porcine embryos. The porcine mesonephros is a good model to study glomerular degeneration because this organ is known to become extremely large and to remain functional for an extended period during porcine embryonic development (Tiedemann and Egerer 1984). In addition, the growth of mesonephric glomeruli was measured using stereology and vascular corrosion casting.
Materials and Methods
Materials
Porcine embryonic specimens were obtained at a local slaughterhouse. Their approximate age in embryonic days postconception (E) was deduced from the crown–rump lengths (CRL) (Evans and Sack 1973). Specimens were fixed for 48 hr in zinc salt fixative (ZSF) (Gonzalez et al. 2001) or in modified methacarn (eight parts methanol, one part acetic acid) depending on the downstream applications. After fixation, the specimens were processed with an STP 420D Tissue Processor (Microm; Prosan, Merelbeke, Belgium), paraffin embedded with the embedding center EC 350-1 (Microm; Prosan), and sectioned with a rotary microtome HM 360 (Microm; Prosan).
Stereology
The purpose of the stereological measurements was to estimate the average volume of morphologically intact mesonephric glomeruli at different ages of development. Specimens fixed in ZSF were serially sectioned at 8 μm thickness. The glomerular volume was measured by a point-counting procedure, in which only those points that fell on top of glomerular tissue were counted. This procedure was applied on 7–15 sections per specimen, segregated by a fixed interval (T) according to the Cavalieri method (Casteleyn et al. 2007). Total glomerular volume was calculated using the equation 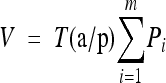 (where V is the total volume, T is the interval, a/p is the area per point, and Pi is the number of points counted on the ith section; Casteleyn et al. 2007). The precision of these volume estimates, i.e., the coefficient of error (CE), was calculated as previously described (Gundersen and Jensen 1987; Casteleyn et al. 2007).
(where V is the total volume, T is the interval, a/p is the area per point, and Pi is the number of points counted on the ith section; Casteleyn et al. 2007). The precision of these volume estimates, i.e., the coefficient of error (CE), was calculated as previously described (Gundersen and Jensen 1987; Casteleyn et al. 2007).
Because of the large variation observed in the sizes of glomeruli from single mesonephric kidneys, the mean glomerular volume was calculated by dividing the total glomerular volume of one mesonephric kidney with the total number of glomeruli in that kidney. The total number of glomeruli was calculated according to the physical dissector method (Sterio 1984), which relies on the principle that a particle is counted when its transect is seen in one section, but not in the next. Because the apical glomeruli start to show signs of regression, beginning around 35 days postgestation, glomeruli that showed signs of regression, i.e., capillary collapse and the presence of apoptotic bodies, were excluded from the stereological measurements.
Immunohistochemistry
Immunohistochemical staining was performed as previously described (De Spiegelaere et al. 2010). Nonspecific staining was blocked by immersing the slides in 30% rabbit serum in PBS for 30 min at room temperature and subsequently incubating in a humidified chamber for 1 hr at 37C, with anti-ANGPT1 at 1/100 (sc-6319; Santa Cruz Biotechnology, Tebu-Bio, Boechout, Belgium) or anti-ANGPT2 at 1/100 (sc-7016; Santa Cruz Biotechnology, Tebu-Bio) as primary antibody. Endogenous peroxidase activity was quenched with 5% H2O2 in methanol, and the slides were covered for 30 min at 37C with biotinylated rabbit anti-goat IgG 1/400 (E0466; Dako, Heverlee, Belgium) as secondary antibody. The slides were incubated for 1 hr at room temperature in a conjugated streptavidin solution 1/3000 (P0397; Dako). The reaction was visualized by staining with the Liquid DAB Substrate Chromogen System (K3468; Dako), and a nuclear counterstaining was performed using Mayer's hematoxylin. Negative control stainings were carried out using the same procedure except that the primary antibody was omitted or replaced by non-immunized goat serum.
Laser Capture Microdissection
The modified methacarn solution was preferred as a fixative for laser capture microdissection and RT-qPCR because it preserves an adequate RNA quality without compromising the morphological quality of the tissue sections (Buesa 2008; Cox et al. 2008). Samples consisted of two embryos with a CRL of 4 mm, six with a CRL of 9 mm, three with a CRL of 23 mm, five with a CRL of 35 mm, two with a CRL of 66 mm, and five with a CRL of 85 mm. Sections were cut at 10 μm thickness and immersed in a 0.7% gelatin solution in water for adhesion to glass slides. They were left on a hot plate at 60C for 1 min, deparaffinized by immersing them two times for 3 min in xylene, and rehydrated in decreasing alcohol series. The sections were consecutively stained with a droplet of hematoxylin and a droplet of eosin. Subsequently, they were dehydrated by placing the slides in 70% and 95% ethanol for 30 sec, followed by dehydration in 100% isopropanol for 5 min and two times in xylene for 3 min. Samples were isolated using the Arcturus Pixcell IIe laser capture microdissection device (Molecular Devices; Berkshire, UK), with the laser pulse power at 38 mW and a threshold voltage of 145 mV. Each sample consisted of ∼20 glomeruli.
RT-qPCR
RNA was isolated with the PicoPure RNA isolation kit (Molecular Devices), including the RNase-Free DNase Set (Qiagen; Venlo, The Netherlands). Genomic contamination was tested with the primers for YWHAZ for which the amplicon spans an intron of 80 bp. RNA quantity was measured with the NanoDrop electrophotometer (Thermo Fisher Scientific; Doornik, Belgium), and RNA integrity was analyzed, based on the 28S/18S ratio, with the Experion Automated Electrophoresis System, with the software version 2.0 (Bio-Rad; Nazareth, Belgium). Reverse transcription to cDNA was performed with 10 μl RNA isolate, using the Qscript cDNA Supermix (Quanta Biosciences, VWR; Heverlee, Belgium) that contained a manufacturer-defined mix of oligo-dT and random hexamer primers. To clear the cDNA product from PCR inhibitors, the cDNA was purified with the GenElute PCR Cleanup kit (Sigma-Aldrich; Bornem, Belgium) (De Spiegelaere et al. 2008). To prevent RNase contamination, all recipients were washed with RNase AWAY (Sigma-Aldrich), the water was ultrapurified using the Modulab Ultra Clear system with an RNase retention filter (Eurowater; Nazareth, Belgium), and only RNase-free products were used.
RT-qPCR was conducted on the iCycler iQ Real-Time PCR Detection System (Bio-Rad) using the Perfecta SYBR Green Fastmix (Quanta Biosciences, VWR) with gene-specific primers and 1 μl of cDNA to a total volume of 25 μl. Primers were selected from literature or designed with Primer3 software (Rozen and Skaletsky 2000) (Table 1). Special care was taken to minimize the amplicon size between the primer pairs because small amplicons are less susceptible to RNA degradation (Fleige and Pfaffl 2006). Amplicons from genes with known splice variants were chosen from the regions expressed by all the splice variants. The cycling conditions of the qPCR comprised 10 min of polymerase activation at 95C followed by 40 cycles at 95C for 15 sec, an annealing step at a primer-specific temperature (Table 1) for 30 sec, and an extension step at 72C for 1 min during which fluorescence was measured. Finally, a melting curve was constructed by heating the PCR product from 70C to 95C in steps of 0.5C per 10 sec. A serial dilution of cDNA, obtained from a whole embryonic tissue lysate, was used as a standard curve and analyzed with the iCycler iQ software (Bio-Rad). The details of these assays are stored in a free online database, RTPrimerDB (Lefever et al. 2009).
Table 1.
Information on the primer pairs, used for RT-qPCR
| Gene | Primer sequence (5′–3′) | Amplicon length (bp) | Ta (C) | Genbank acc. no./reference | RTPrimerDB ID |
|---|---|---|---|---|---|
| ANGPT1 | GACAGCAGGAAAACAGAGCA | 130 | 60 | NM_213959 | 7836 |
| GCCACAAGCATCAAACCAC | |||||
| ANGPT2 | AAGCGGCATCAGAAACCA | 73 | 57 | NM_213808 | 8156 |
| TGGCTCAGGGTCCATCAA | |||||
| TIE1 | TCAACCTTCTGGGAGCCTGT | 190 | 60 | AJ867846 | 7836 |
| GCAGCATCACTGGCAAAAC | |||||
| TIE2 | TGGAACCTCGAACAGAATATGA | 118 | 57 | AY616678 | 8155 |
| GGAGGAGGAAGACCGATAGAA | |||||
| VEGFA | CATTGGAGCCTTGCCTTG | 90 | 60 | AF318502.1 | 8154 |
| TTCGTGGGGTTTCTGGTCT | |||||
| VEGFR 1 | TTGGACTGTTGGCACAAAGAC | 141 | 60 | Ribeiro et al. 2007 | 7835 |
| GCTGTTGCTCGTCAGAATGG | |||||
| VEGFR2 | GAACGAGTGGAGGTGACAGA | 142 | 62 | EU714326 | 8153 |
| GAACGTACACATAGACGACAGAGG | |||||
| ACTB* | TCTGGCACCACACCTTCT | 114 | 60 | Erkens et al. 2006 | 8160 |
| TGATCTGGGTCATCTTCTCAC | |||||
| B2M* | AAACGGAAAGCCAAATTACC | 178 | 60 | Erkens et al. 2006 | 3607 |
| ATCCACAGCGTTAGGAGTGA | |||||
| GAPDH* | ACTCACTCTTCTACCTTTGATGCT | 100 | 57 | Erkens et al. 2006 | 3608 |
| TGTTGCTGTAGCCAAATTCA | |||||
| HMBS* | CTGTTTACCAAGGAGCTGGAAC | 100 | 59 | Erkens et al. 2006 | 3609 |
| TGAAGCCAGGAGGAAGCA | |||||
| LOC448984* | GGCTACCACATCCAAGGAAG | 149 | 60 | Kuijk et al. 2007 | 7838 |
| TCCAATGGATCCTCGCGGAA | |||||
| TBP* | GATGGACGTTCGGTTTAGG | 124 | 59 | Erkens et al. 2006 | 8157 |
| AGCAGCACAGTACGAGCAA | |||||
| TOPOII* | AACTGGATGATGCTAATGATGCT | 137 | 60 | Van Poucke et al. 2001 | 8138 |
| TGGAAAAACTCCGTATCTGTCTC | |||||
| YWHAZ* | ATGCAACCAACACATCCTATC | 178 | 60 | Erkens et al. 2006 | 8159 |
| GCATTATTAGCGTGCTGTCTT |
The reference genes are marked with an asterisk.
Normalization was performed with internal reference genes (Tables 1 and 2) (Vandesompele et al. 2002; Erkens et al. 2006). Gene expression stability was measured for eight genes that are frequently used as reference genes in porcine tissue (Tables 1 and 2). The gene expression stability of these genes was measured over all samples using the GeNorm algorithm (Vandesompele et al. 2002). From these results, the three most stable genes were selected for further normalization of the samples. Finally, gene expression analysis was performed using the qBase software (Hellemans et al. 2007).
Table 2.
Full gene names
| Official symbol | Alias | Name |
|---|---|---|
| ANGPT1 | ANG1 | Angiopoietin-1 |
| ANGPT2 | ANG2 | Angiopoietin-2 |
| Tie-1 | TIE1 | tyrosine kinase with immunoglobulin-like and EGF-like domains 1 |
| TEK | TIE2 | TEK tyrosine kinase, endothelial |
| VEGFA | VEGF | Vascular endothelial growth factor A |
| FLT1 | VEGFR1 | fms-related tyrosine kinase 1 |
| KDR | VEGFR2 | kinase insert domain protein receptor |
| ACTB* | ACTB | actin, β |
| B2M* | B2M | β-2-microglobulin |
| GAPDH* | GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| HMBS* | HMBS | hydroxymethylbilane synthase |
| LOC448984* | S18 | ribosomal protein S18 |
| TBP* | TBP | TATA box binding protein |
| TOPOII* | TOPOII | topoisomerase (DNA) II β |
| YWHAZ* | YWHAZ | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, β-polypeptide |
The reference genes are marked with an asterisk.
Vascular Corrosion Casting of Glomeruli and Micro-computed Tomographic Analysis of Casted Glomeruli
The vasculature of porcine embryos was corrosion casted with Mercox II casting resin (Ladd Research, ETS Edouard Defrance; Wemmel, Belgium). Casted glomeruli were excised out of the vascular casts and studied with scanning electron microscopy (SEM) or micro-computed tomography (micro-CT). For SEM, the samples were examined with a JEOL JSM 5600 LV scanning electron microscope (Jeol; Zaventem, Belgium). For micro-CT, the corrosion casts of the glomeruli were scanned using high-resolution X-ray tomography. An in-house-developed CT system was used to scan the glomeruli. The system consisted of a Hamamatsu nanofocus X-ray tube (Hamamatsu; Louvain-La-Neuve, Belgium) with transmission target operated at 70 kV and a Photonic Science 16MP CCD digital detector with Gadox scintillator (Photonic Science; St Etienne de St Geoirs, France) operated in binning 4 mode (1000 × 1000 pixels with 30 μm pixel pitch). During the scan, 1000 projection images were recorded at 0.36° rotation interval with 3-sec exposure per image and a magnification of 30×. These projection images were reconstructed using Octopus software (InCT; Zwijnaarde, Belgium) (Vlassenbroeck et al. 2007) and resulted in a dataset of 1000 × 1000 × 1000 isotropic voxels of 1 μm. Finally, a three-dimensional (3D) reconstruction was obtained from the dataset, using the Amira 3D reconstruction software (version 4.0.1.; Mercury Computer Systems, Mérignac Cedex, France).
Results
Stereological Assessment
The porcine mesonephros consisted of ∼60 glomeruli (Figure 1). This number started to decline in embryos with a CRL of 35 mm (E35) when the cranial glomeruli start regressing. However, the caudal glomeruli remained morphologically intact until the embryos attained a CRL of 95 mm (E55). Statistical analysis of the volume estimates revealed a strong, positive correlation of the mean glomerular volume with the estimated embryonic age of the embryos (R2 = 0.7837, p<0.0001) (Figure 2). In addition, a correlation of the glomerular volume with the embryonic age was weak but significant (R2 = 0.4157, p=0.0029). The mean CE for these volume estimates was 3%.
Figure 1.
Sagittal section through an embryo with a crown–rump length (CRL) of 4 mm (E18) stained with hematoxylin–eosin (E18: 18 embryonic days postconception). The mesonephros (Mn) is in an early stage of development. It is situated caudodorsally and contains mesonephric tubules (arrowheads) and glomeruli (arrows). The developing brain (Br), spinal cord (Nt), atrium (At), and ventricle (V) can also be seen. Bar = 1000 μm.
Figure 2.
Mesonephric glomerular volume in function of the increasing CRL of porcine embryos.
Immunohistochemistry
Immunohistochemical staining for ANGPT1 was not observed in mesonephric glomeruli of embryos with a CRL of 4 mm (E18) (Figure 3). The glomeruli of these embryos were still in an early stage of development. Capillary structures were not observed, and presumptive podocytes were still arranged as a monolayer of cuboidal epithelial cells positioned apically on the glomerulus. In contrast, ANGPT2 immunostaining was observed in mesonephric glomeruli of embryos with a CRL of 4 mm (E18), but was restricted to the podocytes (Figure 4).
Figure 3.
Angiopoietin-1 (ANGPT1) immunostaining in porcine mesonephric glomeruli of embryos with a CRL of 4 mm (E18; A), 9 mm (E20; B), 13 mm (E23; C), 23 mm (E29; D), and 85 mm (E50; E). No immunostaining was observed in early developing glomeruli that have presumptive podocytes (arrowheads) situated in the periphery and endothelial cells (asterisks) in their core. The insert in C shows a higher magnification image illustrating the immunostaining in endothelial cells (black arrows), in mesangial cells (white arrows), and to a lesser extent in podocytes (arrowheads). ANGPT1 immunostaining was also recorded in the cranial degenerating glomeruli of embryos with a CRL of 85 mm (E50; F). Bars: A = 20 μm; B–D = 100 μm (insert in C = 20 μm); E,F = 200 μm.
Figure 4.
ANGPT2 immunostaining of porcine mesonephric glomeruli of embryos with a CRL of 4 mm (E18; A), 9 mm (E20; B), 13 mm (E23; C), 23 mm (E29; D), and 85 mm (E50; E). Immunostaining of ANGPT2 was observed in the presumptive podocytes (arrowheads) enveloping the early developing glomeruli. The insert in C shows a higher magnification image illustrating the immunostaining in the podocytes (arrowheads), in endothelial cells (black arrows), and also in mesangial cells (white arrows). ANGPT2 immunostaining was also observed in degenerating glomeruli of embryos with a CRL of 85 mm (E50; E,F). Bars: A = 20 μm; B–D = 100 μm (insert in C = 20 μm); E,F = 200 μm.
The glomeruli of embryos with a CRL of 9 mm (E20) show abundant capillaries in which the podocytes could not be discerned from the underlying endothelial cells. ANGPT1 staining (Figure 3) and a strong ANGPT2 staining (Figure 4) were observed in these glomeruli.
ANGPT1 and ANGPT2 immunostaining was further observed in mesonephric glomeruli of embryos with a CRL of 13 mm (E24), 22 mm (E29), and 35 mm (E35). A strong ANGPT1 staining was present in both normal and degenerating glomeruli of embryos with a CRL of 85 mm (E50) (Figure 3). However, considerable interindividual variation was observed for the ANGPT2 staining, independent of the developmental stage (Figure 4). At higher magnification, ANGPT1 immunostaining was observed in endothelial cells, in mesangial cells, and to a lesser extent in podocytes (Figure 3C) and ANGPT2 immunostaining was observed in podocytes, endothelial cells, and mesangial cells (Figure 4C).
RT-qPCR
The isolation of 20 glomeruli with laser capture microdissection (Figure 5) resulted in ∼10–15 μg of total RNA. The 28S/18S ratio of the RNA varied around 0.5. The most stable reference genes across all the samples were ACTB, B2M, and HMBS (Table 3). These were used to calculate the normalization factors of the individual samples and for subsequent gene expression analysis with qBase.
Figure 5.
Laser capture microdissection of glomeruli from a histological section of a porcine mesonephros, showing the section before capture (A), after capture (B), and the glomerular tissue adhering to the bottom of the cap (C). Bar = 200 μm.
Table 3.
Coefficient of variance (CV) and M value (M) for the chosen reference genes as measured with the GeNorm software
| CV (%) | M | |
|---|---|---|
| ACTB | 47.80 | 11.676 |
| B2M | 57.96 | 12.149 |
| HMBS | 49.55 | 11.882 |
| Mean | 51.77 | 1.902 |
VEGFA mRNA transcripts were detected in the glomerular isolates from all age stages. They were slightly upregulated (p=0.0414) during early glomerulogenesis in embryos measuring 4–9 mm (E18–20), but remained relatively stable in the later stages (Figure 6). ANGPT1 mRNA was almost undetectable in glomeruli of embryos with a CRL of 4 mm (E18) and was upregulated in the later stages of glomerulogenesis (Figure 6). ANGPT2 mRNA was found in all glomeruli, showing a large variation among all samples both in and between the age groups. A slight upregulation could be observed in embryos with a CRL of 85 mm, but it was not significant (p=0.5281) (Figure 6). VEGFR1 and TIE2 mRNA transcripts were stably expressed during the complete lifespan of the mesonephros (Figure 7). Glomerular TIE1 expression was stable during the entire lifespan of the mesonephros, but it was slightly upregulated during early glomerulogenesis (p<0.0002) in embryos with a CRL of 4 mm (E18) and also during glomerular regression (p<0.0007) in embryos with a CRL of 85 mm (E50). VEGFR2 mRNA was highly upregulated in early developing glomeruli, whereas dropping to basal levels in mesonephric glomeruli of embryos with a CRL of 35 mm (E35) and remaining downregulated in the later stages (p<0.0001) (Figure 7).
Figure 6.
Gene expression measurements from RT-qPCR of mRNA for ANGPT1, ANGPT2, and vascular endothelial growth factor A (VEGFA) in porcine glomeruli at different stages of development.
Figure 7.
Gene expression measurements of mRNA for TIE1, TIE2, VEGFR1, and VEGFR2 in porcine glomeruli at different stages of development.
Evaluation of the Corrosion-casted Glomeruli
The mesonephric glomeruli were characterized by a large number of capillary loops (Figure 8A). The diameter of these capillaries showed a considerable variation. Small casting gaps with a diameter of ∼1–10 μm were recorded in the capillary casts of some mesonephric glomeruli (Figures 8B and 8C). These gaps were also present in the inner capillaries of the 3D reconstructions of glomeruli scanned with micro-CT (Figures 8D–8F).
Figure 8.
Micrographs of mesonephric glomeruli obtained with scanning electron microscopy (A–D) and three-dimensional reconstruction of micro-computed tomography (D–F). Small gaps, which are a sign of active intussusceptive angiogenesis (arrowheads), were frequently encountered. The internal surface of capillaries is marked with asterisks (E).
Discussion
The porcine mesonephros is a transient embryonic organ that becomes functional during embryonic development (Egerer et al. 1984). Although the morphology of this organ has extensively been described, no morphometrical study has been conducted on the growth of mesonephric glomeruli. The stereological data of this work reveal that the mean glomerular volume of mesonephric glomeruli increases significantly during the complete lifespan of the organ, implying that mesonephric glomerular capillaries undergo a continuous growth. Interestingly, these mesonephric glomeruli kept growing, even when the cranial part of the organ, including the cranial glomeruli, had started to regress. This phenomenon may be explained by the fact that the growing embryo requires a sufficiently large glomerular filtration surface. In the absence of a well-developed functioning metanephric kidney, the mesonephros of porcine embryos has to maintain its excretory function (Patten 1948). Consequently, the total glomerular surface is maintained and slightly increased due to the growth of individual glomeruli.
Intussusceptive angiogenesis plays a major role during glomerular growth in the chick meso- and metanephros and in adult rat glomeruli during vascular repair after induced Thy1.1 nephritis (Nyengaard 1993; Notoya et al. 2003; Makanya et al. 2005). The casting gaps that were recorded in the corrosion casts of porcine mesonephric glomeruli are reminiscent of the gaps that have previously been described in a range of vascular beds and are considered as proof for active intussusceptive angiogenesis (Kurz et al. 2003). This suggests that intussusceptive angiogenesis is also active during porcine mesonephric glomerulogenesis.
The localization of the angiopoietins, VEGFA, and their respective receptors has already been studied extensively in developing metanephric kidneys of various species (Simon et al. 1998; Robert et al. 2000; Bevan et al. 2008; De Spiegelaere et al. in press). ANGPT1 mRNA transcripts have already been detected in the different cell types of mouse metanephric glomeruli, with the highest expression in podocytes (Yuan et al. 1999). In this study, the ANGPT1 protein was also observed in the three different cell types of porcine mesonephric glomeruli, but podocytes showed a weaker staining in comparison to the staining in the endothelial and mesangial cells. The ANGPT2 protein has been detected in mesangial cells of mouse metanephric glomeruli (Yuan et al. 2000) and also in podocytes of rat and porcine metanephric glomeruli (Câmpean et al. 2008; De Spiegelaere et al. in press). In this study, ANGPT2 was restricted to podocytes during early glomerulogenesis and was detected at high concentrations in the three cell types of maturing glomeruli. Although the exact function of these growth factors during renal organogenesis is still unclear, it is hypothesized that ANGPT2 and VEGFA are important for the initial vascular growth of the glomeruli and that ANGPT1 and VEGFA regulate the subsequent quiescence and formation of endothelial fenestrations during vascular maturation (Satchell et al. 2004). This study reveals that mRNA of VEGFA is indeed expressed throughout the complete lifespan of mesonephric glomeruli and that ANGPT1 is not expressed in early glomerulogenesis, but becomes upregulated during the later maturation of glomeruli. Although the expression of ANGPT1 and ANGPT2 detected with IHC seemed to be upregulated in degenerating glomeruli, this upregulation was not detected with RT-qPCR. In the case of ANGPT2, a higher protein concentration could be explained by the accumulation of the protein in Weibel–Palade bodies of endothelial cells (Fiedler et al. 2004). However, performing a quantitative analysis of the immunostaining in degenerating glomeruli is hardly possible because the tissue in these glomeruli is highly collapsed, which may cause a higher staining intensity. The expression pattern of VEGFA and ANGPT1 in mesonephric glomeruli is in accordance with their expression pattern in mouse, porcine, and human developing metanephric glomeruli (Tufro et al. 1999; Yuan et al. 1999; De Spiegelaere et al. in press).
In contrast to ANGPT1 and VEGFA, the expression pattern of ANGPT2 is somewhat different from what has been recorded in developing metanephric glomeruli (De Spiegelaere et al. in press). According to Woolf et al. (2009), ANGPT2 is expressed in podocytes of early forming glomeruli, but its expression decreases in more mature glomeruli, only to become upregulated in pathological situations (Woolf et al. 2009). Although ANGPT2 expression showed a lot of variation, both the immunohistochemical staining and RT-qPCR revealed that ANGPT2 is expressed in glomeruli throughout the whole lifespan of the organ. This continuous expression of ANGPT2 could cause the persisting growth of mesonephric glomeruli, as ANGPT2 inhibits the vessel-maturing actions of ANGPT1 and renders the endothelial cells more active, making them more prone for angiogenesis (Thomas and Augustin 2009). ANGPT2 is also known to act as a vascular regression factor when the balance of ANGPT1/ANGPT2 and VEGFA/ANGPT2 decreases (Lobov et al. 2002). This change is observed during the physiological regression of blood vessels such as the distal portion of the hyaloid artery in the prenatal eye, which regresses before birth, and the blood vessels in the cyclic ovary (Hackett et al. 2002; Augustin et al. 2009). In contrast, in the regressing glomeruli, no significant changes were detected with RT-qPCR for both the ANGPT1/ANGPT2 and VEGFA/ANGPT2 balance. This suggests that factors other than ANGPT2 may trigger the physiological regression of the mesonephric glomerular vasculature. Recently, TIE1 has been identified as an inhibitor of TIE2 activation (Seegar et al. 2010). The upregulation of TIE1 in regressing glomeruli may inhibit the survival effects by ANGPT1 through TIE2 activation, thus directing the glomerular endothelial cells toward regression.
The expression of both ANGPT1 and ANGPT2 may indicate that these factors regulate intussusceptive angiogenesis. This is the main mechanism of glomerular growth in maturing glomeruli (Vaughan and Quaggin 2008). There are indeed clear indications that the angiopoietins play an important role during intussusceptive angiogenesis (Augustin 2001; Kurz et al. 2003). Targeted deletion of TIE2 expression leads to deficient pillar formation (Patan 1998). Mice overexpressing ANGPT1 show a vascular phenotype of enlarged vessels with abundant small invaginations that are reminiscent of intussusceptive angiogenesis (Thurston et al. 1999; Burri and Djonov 2002). In addition, overexpression of ANGPT2 has been described to influence ongoing intussusceptive angiogenesis in the chick chorioallantoic membrane (Winnik et al. 2009).
Currently, VEGFA is recognized as the most important factor for both angiogenesis and the formation of endothelial fenestrations (Satchell and Braet 2009). However, the role of its receptors in these processes is unclear. Among the VEGF receptors, VEGFR2 is the most potent angiogenic receptor. VEGFR1 binds VEGFA with higher affinity than VEGFR2, but VEGFR1 receptor activation does not lead to an increase in angiogenesis. VEGFR1 is thought to be a decoy receptor, regulating VEGFR2 expression by capturing VEGFA molecules, but the exact function of VEGFR1 is still elusive (Maharaj and D'Amore 2007). There is little data on the expression of the VEGFA receptors during glomerulogenesis. According to the literature, both VEGFR2 and VEGFR1 are present during metanephric development, whereas VEGFR1 is mainly present in the adult metanephric kidney (Simon et al. 1998; Tufro et al. 1999). The results of this study show a high expression of VEGFR2 mRNA in early mesonephric glomerulogenesis and a decrease in glomeruli of older embryos, whereas the expression of VEGFR1 mRNA remains stable during the entire lifespan of the mesonephros. The upregulation of VEGFR2 mRNA and its sudden drop in the later stages of glomerulogenesis may indicate that VEGFR2 mainly regulates sprouting angiogenesis, which takes place during early glomerulogenesis. Both VEGFR1 and VEGFR2 are able to induce vascular permeability, but VEGFR1 has been described to be able to induce endothelial permeability without the activation of VEGFR2 (Maharaj and D'Amore 2007; Vogel et al. 2007). This could indicate that VEGFR1 may be important for the formation and maintenance of glomerular fenestrations.
In conclusion, the expression pattern of the major angiogenic growth factors in the mesonephric glomeruli shows many similarities with their expression pattern as described in metanephric glomeruli. Therefore, the mesonephros presents a promising model organ for the study of glomerulogenesis and glomerular degeneration.
Acknowledgments
This work was financially supported by the Bijzonder OnderzoeksFonds project (05B01906) of Ghent University.
The authors thank Prof. Paul Simoens for his constructive comments and Jurgen De Craene, Lobke De Bels, Liliane Standaert, Patrick Vervaet, and Eric Maes for their excellent technical assistance.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Augustin HG (2001) Tubes, branches, and pillars: the many ways of forming a new vasculature. Circ Res 89:645–647 [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10:165–177 [DOI] [PubMed] [Google Scholar]
- Bevan HS, van den Akker NM, Qiu Y, Polman JA, Foster RR, Yem J, Nishikawa A, et al. (2008) The alternatively spliced anti-angiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol 110:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buesa RJ (2008) Histology without formalin? Ann Diagn Pathol 12:387–396 [DOI] [PubMed] [Google Scholar]
- Burri PH, Djonov V (2002) Intussusceptive angiogenesis: the alternative to capillary sprouting. Mol Aspects Med 23:S1–27 [DOI] [PubMed] [Google Scholar]
- Câmpean V, Karpe B, Haas C, Atalla A, Peters H, Rupprecht H, Liebner S, et al. (2008) Angiopoietin 1 and 2 gene and protein expression is differentially regulated in acute anti-Thy1.1 glomerulonephritis. Am J Physiol Renal Physiol 294:F1174–1184 [DOI] [PubMed] [Google Scholar]
- Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395 [DOI] [PubMed] [Google Scholar]
- Casteleyn C, Van den Broeck W, Simoens P (2007) Histological characteristics and stereological volume assessment of the ovine tonsils. Vet Immunol Immunopathol 120:124–135 [DOI] [PubMed] [Google Scholar]
- Cox ML, Eddy SM, Stewart ZS, Kennel MR, Man MZ, Paulauskis JD, Dunstan RW (2008) Investigating fixative-induced changes in RNA quality and utility by microarray analysis. Exp Mol Pathol 84:156–172 [DOI] [PubMed] [Google Scholar]
- De Spiegelaere W, Cornillie P, Van den Broeck W (2010) Localization of erythropoietin in and around growing cartilage. Mol Cell Biochem 337:287–291 [DOI] [PubMed] [Google Scholar]
- De Spiegelaere W, Cornillie P, Van den Broeck W (In Press) Immunohistochemical detection of the angiopoietins during porcine metanephric kidney development. Acta Histochem. Published online June 30, 2010 (DOI: 10.1016/j.acthis.2010.05.001) [DOI] [PubMed]
- De Spiegelaere W, Erkens T, De Craene J, Burvenich C, Peelman L, Van den Broeck W (2008) Elimination of amplification artifacts in real-time reverse transcription PCR using laser capture microdissected samples. Anal Biochem 382:72–74 [DOI] [PubMed] [Google Scholar]
- Djonov VG, Kurz H, Burri PH (2002) Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev Dyn 224:391–402 [DOI] [PubMed] [Google Scholar]
- Egerer G, Taugner R, Tiedemann K (1984) Renin immunohistochemistry in the mesonephros and metanephros of the pig embryo. Histochemistry 81:385–390 [DOI] [PubMed] [Google Scholar]
- Eremina V, Quaggin SE (2004) The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens 13:9–15 [DOI] [PubMed] [Google Scholar]
- Erkens T, Van Poucke M, Vandesompele J, Goossens K, Van Zeveren A, Peelman LJ (2006) Development of a new set of reference genes for normalization of real-time RT-PCR data of porcine backfat and longissimus dorsi muscle, and evaluation with PPARGC1A. BMC Biotechnol 6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HE, Sack WO (1973) Prenatal development of domestic and laboratory animals: growth curves, external features and selected references. Zentralbl Veterinarmed C 2:11–45 [DOI] [PubMed] [Google Scholar]
- Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Thurston G, et al. (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103:4150–4156 [DOI] [PubMed] [Google Scholar]
- Fleige S, Pfaffl M (2006) RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med 27:126–139 [DOI] [PubMed] [Google Scholar]
- Gersh I (1937) The correlation of structure and function in the developing mesonephros and metanephros. Contrib Embryol 26:33–58 [Google Scholar]
- Gonzalez L, Anderson I, Deane D, Summers C, Buxton D (2001) Detection of immune system cells in paraffin wax-embedded ovine tissues. J Comp Pathol 125:41–47 [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB (1987) The efficiency of systematic-sampling in stereology and its prediction. J Microsc 147:229–263 [DOI] [PubMed] [Google Scholar]
- Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA (2002) Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol 192:182–187 [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamba T, Tam BYY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, et al. (2006) VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290:H560–H576 [DOI] [PubMed] [Google Scholar]
- Kassmeyer S, Plendl J, Custodis P, Bahramsoltani M (2009) New insights in vascular development: vasculogenesis and endothelial progenitor cells. Anat Histol Embryol 38:1–11 [DOI] [PubMed] [Google Scholar]
- Kuijk EW, du Puy L, van Tol HT, Haagsman HP, Colenbrander B, Roelen BA (2007) Validation of reference genes for quantitative RT-PCR studies in porcine oocytes and preimplantation embryos. BMC Dev Biol 7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz H, Burri PH, Djonov VG (2003) Angiogenesis and vascular remodeling by intussusception: from form to function. News Physiol Sci 18:65–70 [DOI] [PubMed] [Google Scholar]
- Leeson TS, Baxter JS (1957) The correlation of structure and function in the mesonephros and metanephros of the rabbit. J Anat 91:383–390 [PMC free article] [PubMed] [Google Scholar]
- Lefever S, Vandesompele J, Speleman F, Pattyn F (2009) RTPrimerDB: the portal for real-time PCR primers and probes. Nucleic Acids Res 37:D942–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA (2002) Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA 99:11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig KS, Landmann L (2005) Early development of the human mesonephros. Anat Embryol (Berl) 209:439–447 [DOI] [PubMed] [Google Scholar]
- Maharaj AS, D'Amore PA (2007) Roles for VEGF in the adult. Microvasc Res 74:100–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand S, Radziejewski C, Compton D, et al. (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60 [DOI] [PubMed] [Google Scholar]
- Makanya AN, Stauffer D, Ribatti D, Burri PH, Djonov V (2005) Microvascular growth, development, and remodeling in the embryonic avian kidney: the interplay between sprouting and intussusceptive angiogenic mechanisms. Microsc Res Tech 66:275–288 [DOI] [PubMed] [Google Scholar]
- Nacher V, Carretero A, Navarro M, Armengol C, Llombart C, Rodriguez A, Herrero-Fresneda I, et al. (2006) The quail mesonephros: a new model for renal senescence? J Vasc Res 43:581–586 [DOI] [PubMed] [Google Scholar]
- Nacher V, Carretero A, Navarro M, Ayuso E, Ramos D, Luppo M, Rodriguez A, et al. (2010) Endothelial cell transduction in primary cultures from regressing mesonephros. Cells Tissues Organs 191:84–95 [DOI] [PubMed] [Google Scholar]
- Notoya M, Shinosaki T, Kobayashi T, Sakai T, Kurihara H (2003) Intussusceptive capillary growth is required for glomerular repair in rat Thy-1.1 nephritis. Kidney Int 63:1365–1373 [DOI] [PubMed] [Google Scholar]
- Nyengaard JR (1993) Number and dimensions of rat glomerular capillaries in normal development and after nephrectomy. Kidney Int 43:1049–1057 [DOI] [PubMed] [Google Scholar]
- Otrock ZK, Makarem JA, Shamseddine AI (2007) Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis 38:258–268 [DOI] [PubMed] [Google Scholar]
- Papetti M, Herman IM (2002) Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol 282:C947–970 [DOI] [PubMed] [Google Scholar]
- Patan S (1998) TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res 56:1–21 [DOI] [PubMed] [Google Scholar]
- Patan S, Haenni B, Burri PH (1993) Evidence for intussusceptive capillary growth in the chicken chorioallantoic membrane (CAM). Anat Embryol (Berl) 187:121–130 [DOI] [PubMed] [Google Scholar]
- Patten BM (1948) Embryology of the Pig. 3rd ed. New York, McGraw-Hill
- Quaggin SE, Kreidberg JA (2008) Development of the renal glomerulus: good neighbors and good fences. Development 135:609–620 [DOI] [PubMed] [Google Scholar]
- Ribeiro LA, Bacci ML, Seren E, Tamanini C, Forni M (2007) Characterization and differential expression of vascular endothelial growth factor isoforms and receptors in swine corpus luteum throughout estrous cycle. Mol Reprod Dev 74:163–171 [DOI] [PubMed] [Google Scholar]
- Robert B, Zhao XM, Abrahamson DR (2000) Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am J Physiol Renal Physiol 279:F275–282 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In Krawetz S, Misener S, eds. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, Humana Press, 365–386 [DOI] [PubMed]
- Satchell SC, Anderson KL, Mathieson PW (2004) Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol 15:566–574 [DOI] [PubMed] [Google Scholar]
- Satchell SC, Braet F (2009) Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol 296:F947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O'Hare MJ, et al. (2006) Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int 69:1633–1640 [DOI] [PubMed] [Google Scholar]
- Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG (2005) The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 118:771–780 [DOI] [PubMed] [Google Scholar]
- Seegar T, Eller B, Tzvetkova-Robev D, Kolev M, Henderson S, Nikolov D (2010) Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol Cell 37:643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Rockl W, Hornig C, Grone EF, Theis H, Weich HA, Fuchs E, et al. (1998) Receptors of vascular endothelial growth factor vascular permeability factor (VEGF/VPF) in fetal and adult human kidney: localization and [125I]VEGF binding sites. J Am Soc Nephrol 9:1032–1044 [DOI] [PubMed] [Google Scholar]
- Smith C, Mackay S (1991) Morphological development and fate of the mouse mesonephros. J Anat 174:171–184 [PMC free article] [PubMed] [Google Scholar]
- Sterio DC (1984) The unbiased estimation of number and sizes of arbitrary particles using the dissector. J Microsc 134:127–136 [DOI] [PubMed] [Google Scholar]
- Thomas M, Augustin HG (2009) The role of the angiopoietins in vascular morphogenesis. Angiogenesis 12:125–137 [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM (1999) Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286:2511–2514 [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Egerer G (1984) Vascularization and glomerular ultrastructure in the pig mesonephros. Cell Tissue Res 238:165–175 [DOI] [PubMed] [Google Scholar]
- Tufro A, Norwood VF, Carey RM, Gomez RA (1999) Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol 10:2125–2134 [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034 [DOI] [PMC free article] [PubMed]
- Van Poucke M, Yerle M, Tuggle C, Piumi F, Genet C, Van Zeveren A, Peelman LJ (2001) Integration of porcine chromosome 13 maps. Cytogenet Cell Genet 93:297–303 [DOI] [PubMed] [Google Scholar]
- Vaughan MR, Quaggin SE (2008) How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol 19:24–33 [DOI] [PubMed] [Google Scholar]
- Vlassenbroeck J, Dierick M, Masschaele B, Cnudde V, Hoorebeke L, Jacobs P (2007) Software tools for quantification of X-ray microtomography. Nucl Instrum Methods Phys Res A 580:442–445 [Google Scholar]
- Vogel C, Bauer A, Wiesnet M, Preissner KT, Schaper W, Marti HH, Fischer S (2007) Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-K/Akt pathway. J Cell Physiol 212:236–243 [DOI] [PubMed] [Google Scholar]
- Winnik S, Klinkert M, Kurz H, Zoeller C, Heinke J, Wu YX, Bode C, et al. (2009) Hoxb5 induces endothelial sprouting in vitro and modifies intussusceptive angiogenesis in vivo involving angiopoietin-2. Cardiovasc Res 83:558–565 [DOI] [PubMed] [Google Scholar]
- Woolf AS, Gnudi L, Long DA (2009) Roles of angiopoietins in kidney development and disease. J Am Soc Nephrol 20:239–244 [DOI] [PubMed] [Google Scholar]
- Yuan HT, Suri C, Yancopoulos GD, Woolf AS (1999) Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney maturation. J Am Soc Nephrol 10:1722–1736 [DOI] [PubMed] [Google Scholar]
- Yuan HT, Yang SP, Woolf AS (2000) Hypoxia up-regulates angiopoietin-2, a Tie-2 ligand, in mouse mesangial cells. Kidney Int 58:1912–1919 [DOI] [PubMed] [Google Scholar]









