Abstract
This work uses cross-innervation of respiratory muscles of different developmental origins to probe myogenic and neurogenic mechanisms regulating their fiber types. The thyroarytenoid (TA) originates from the sixth branchial arch, whereas the sternohyoid (SH) is derived from somitic mesoderm. Immunohistochemical analysis using highly specific monoclonal antibodies to myosin heavy chain (MyHC) isoforms reveals that normal rat SH comprises slow, 2a, 2x, and 2b fibers, as in limb fast muscles, whereas the external division of the TA has only 2b/eo fibers coexpressing 2B and extraocular (EO) MyHCs. Twelve weeks after cross-innervation with the recurrent laryngeal nerve, the SH retained slow and 2a fibers, greatly increased the proportion of 2x fibers, and their 2b fibers failed to express EO MyHC. In the cross-innervated TA, the SH nerve failed to induce slow and 2A MyHC expression and failed to suppress EO MyHC expression in 2b/eo fibers. However, 2x fibers amounting to 4.2% appeared de novo in the external division of the TA. We conclude that although MyHC gene expression in these muscles can be modulated by neural activity, the patterns of response to altered innervation are largely myogenically determined, thus supporting the idea that SH and TA differ in muscle allotype. (J Histochem Cytochem 58:1057–1065, 2010)
Keywords: muscle fiber type, myosin heavy chain, muscle allotype, larynx, thyroarytenoid, sternohyoid, respiratory muscle, cross-innervation
Limb muscles may express slow, 2A, 2X, or 2B myosin heavy chain (MyHC) in slow, 2a, 2x, and 2b fibers, respectively. What a mature fiber actually expresses is a function of the developmental origin as primary or secondary myotube, the thyroid hormone level, and innervation (Pette and Vrbová 1985; Zhong et al. 2010). Muscle fiber types in the adult are plastic and are subject to neural regulation. Cross-innervation between a muscle with primarily fast fibers and another with primarily slow fibers results in reciprocal changes in myosin gene expression in these muscles (Hoh 1975; Hoh et al. 1980).
Muscles in the craniofacial region differ from those of the limb and trunk in fiber types and in their repertoire for MyHC expression. Craniofacial muscles may express MyHC isoforms found in limb muscles, but in addition, may also express the extraocular (EO) (Lucas et al. 1995; Briggs and Schachat 2000), α-cardiac and masticatory (Hoh 2002), embryonic, neonatal (Lucas and Hoh 2003), and slow-tonic (Bormioli et al. 1979) MyHCs. These differences are associated with the different developmental origins of these muscles and their diverse pathways leading to myoblast specification (Kang et al. 2010). The limb and trunk muscles are derived from myoblasts originating from the somites, whereas the craniofacial muscles are derived mainly from the somitomeres associated with the developing head. In particular, laryngeal muscles are derived from the branchial arches: the fourth arch giving rise to the cricothyroid (CT) muscle and the sixth arch giving rise to the thyroarytenoid (TA) and other muscles innervated by the recurrent laryngeal nerve (RLN) (Sperber 1989). These observations have given rise to the idea that the craniofacial muscles are allotypically distinct from limb muscles, with their myogenic cells being preprogrammed to express a specific suite of myofibrillar proteins during myogenesis (Hoh and Hughes 1988; Lucas et al. 1995; Pedrosa-Domellöf et al. 2000; Rhee et al. 2004).
Direct experimental support for the muscle allotype concept has been obtained in cross-transplantation experiments between cat jaw and limb muscles and in tissue culture experiments. Cat jaw muscle can potentially express masticatory or slow MyHC. When cat jaw muscle fibers are transplanted into a limb fast muscle bed, only masticatory fibers are found in the regenerated jaw muscle innervated by the limb fast muscle nerve. However, when transplanted into the slow muscle bed and innervated by the slow muscle nerve, both slow and masticatory fibers are initially found, but in the long term, most fibers are slow (Hoh and Hughes 1988). These results suggest that myoblasts from the jaw and limb muscles have distinct programs for MyHC expression during myogenesis. This has now been confirmed: myotubes derived from cat jaw satellite cells express masticatory-specific isoforms of MyHC, myosin-binding protein-C, and tropomyosin, but not myotubes from limb satellite cells (Kang et al. 2010).
The intrinsic laryngeal muscles innervated by the RLN are thought to belong to a distinct muscle allotype in view of their unique developmental origin and their capacity to express EO MyHC (Lucas et al. 1995; Rhee et al. 2004), but there has been no experimental evidence to support this idea. The TA muscle in the rat has two divisions, a minor vocalis division and a major external division, with different fiber types and functions. The vocalis division contains fibers that coexpress 2B and EO MyHCs (2b/eo fibers) and 2x fibers, whereas the external division is composed of purely 2b/eo fibers (Rhee et al. 2004). When the RLN is severed and allowed to reinnervate laryngeal muscle fibers at random, 16.5% of 2b/eo fibers in the reinnervated external division of the TA progressively transform into 2x fibers, showing that EO MyHC expression can be suppressed by an appropriate nerve (Rhee et al. 2004). This raises the question as to whether expression of EO MyHC in 2b/eo fibers can be suppressed when cross-innervated by nerve fibers normally innervating pure 2b fibers. Also in question is whether the absence of slow and 2a fibers in the TA muscle is an intrinsic allotypic property, or due to the absence of nerve fibers in the RLN capable of supporting slow and 2a fibers.
The sternohyoid (SH) muscle is one of the upper airway dilator muscles that ensure pharyngeal patency to airflow. The SH muscle in the rat has been shown by myosin ATPase and succinic dehydrogenase histochemistry to resemble a limb fast muscle, with 67% of 2b, 28% of 2a, and 5% of slow fibers (Bracher et al. 1997). In the present work using IHC, we show that this muscle also has 2x fibers but does not express EO MyHC. This muscle is innervated by a branch of the ansa cervicalis nerve, which should thus be a rich source of nerve fibers supporting pure 2b fibers as well as slow and 2a fibers.
The aim of the present experiments is to ascertain the extent to which fiber types in TA and SH muscles are under neural and myogenic control. We surgically allow the nerve to the SH to cross-innervate the TA and the RLN to cross-innervate the SH. The hypothesis that TA and SH are allotypically distinct predicts that the nerve to the SH will neither induce slow and 2A MyHC expression nor suppress EO MyHC expression in the TA, and the RLN will not induce EO MyHC expression in the SH.
Materials and Methods
Animals and Surgery
Eight 10-week-old female Sprague–Dawley rats were operated on under general anesthesia (ketamine hydrochloride 35 mg/kg and xylazine hydrochloride 5 mg/kg, administered IM). In four of these animals, the nerve to the SH muscle, a branch of the ansa cervicalis nerve, was used to cross-innervate the TA, and in the other four animals, the RLN was used to cross-innervate the SH.
All procedures were performed on the left side only to minimize laryngeal dysfunction in conformity with ethical requirements. Muscles on the right served as controls. The larynx was exposed by a midline incision. Aided by a dissecting microscope, the left ansa cervicalis nerve was first located and its branch to the SH was sectioned between two ties with fine silk. The RLN was dissected, similarly tied and cut. To cross-innervate the TA, the proximal stump of the SH branch of the ansa cervicalis nerve was joined end-to-end to the distal stump of the RLN sectioned below the larynx. The proximal stump of the RLN was moved as far caudally as possible and securely embedded by sutures in the denervated SH muscle to encourage the nerve stump to invade and terminate therein, thereby preventing its reinnervation of laryngeal muscles. To cross-innervate the SH, the RLN was sectioned closer to the larynx, thus allowing the longer proximal stump to be united to the distal stump of the nerve to SH without undue tension. The proximal stump of the nerve to the SH was deeply embedded by suturing to traumatized neighboring muscles to allow regenerating fibers to act as a sink for reinnervation. The strategy of achieving cross-innervated SH and TA muscles separately in different animals and of embedding the unwanted proximal nerve stump in denervated and traumatized neighboring muscles had been successfully used in earlier works (Hoh and Salafsky 1971; Hoh, 1975) for reducing the chance of unintended self-reinnervation in cross-innervation experiments.
Operated rats were studied 12 weeks after surgery as earlier work on reinnervation of laryngeal muscle by the RLN showed that most fiber type changes occurred by 4 weeks, with little further changes between 6 and 12 weeks (Rhee et al. 2004). The animals were euthanized by anesthetic overdose, the RLN and nerve to the SH were visually checked for successful cross-union, and control and cross-innervated SH muscles or whole larynges were removed. Excised muscles and larynges were mounted on cork with Tissue-Tek (Miles Scientific; Elkhart, IN), frozen in isopentane cooled in liquid nitrogen, then stored at −80C till used. All surgery and handling were performed in accordance with the guidelines of the Animal Research Act and the 1997 Australian Code of Practice for the Care and Use of Animals for Scientific Purposes of the National Health and Medical Research Council, and were approved by the Animal Care and Ethics Review Committee of the University of Sydney.
Immunohistochemistry
Serial sections of the larynges, control, and cross-innervated SH muscles were cut at 10 μm in a cryostat maintained at −20C. Whole larynges were cut at right angles to the midline, so that normal and cross-innervated TA muscles appeared in the same section and were treated the same way. Sections from the mid-regions of these muscles that contain the full complement of fibers were stained immunohistochemically.
Indirect immunoperoxidase histochemistry was carried out as previously described (Hoh et al. 1988). Primary antibodies used were monoclonal antibody (MAb) NOQ7-5-4D, specific to slow MyHC (Hoh et al. 1988); MAb SC-71, specific to 2A MyHC (Schiaffino et al. 1989); MAbs 6H1 and 10F5 (available from Developmental Studies Hybridoma Bank; University of Iowa, IA), specific to 2X and 2B MyHCs, respectively (Lucas et al. 2000); and MAb 4A6, specific to EO MyHC (Lucas et al. 1995). The secondary antibodies used were horseradish peroxidase (HRP)-labeled rabbit anti-mouse immunoglobulin antibody (Dako; Carpinteria, CA) for MAbs 4A6 and NOQ7-5-4D and HRP-labeled goat anti-mouse IgM antibody (Sigma-Aldrich; St Louis, MO) for MAbs 6H1 and 10F5.
Quantification of Muscle Fiber Type Distribution and Fiber Cross-sectional Areas
The percentages of fibers expressing slow, 2A, 2X, 2B, and EO MyHCs present in the normal and cross-innervated SH and TA muscles were calculated based on counts using photomicrographs of tissue sections taken at the mid-belly of the muscles stained by MyHC IHC. All fibers of the muscles visible in representative photomicrographs were counted, amounting to ∼1600 per muscle. Fibers were classified as hybrid if they were clearly stained by more than one anti-MyHC MAb in serial sections. Fiber cross-sectional areas were measured using ImageJ (version 1.33u; National Institutes of Health, Bethesda, MD). All normal and cross-innervated SH muscles and a randomly chosen representative normal TA and cross-innervated TA were analyzed. Statistical comparisons were performed using unpaired two-tailed Student's t-test and were noted as being significant when p<0.05.
Results
Fiber Types in Normal and Cross-innervated SH
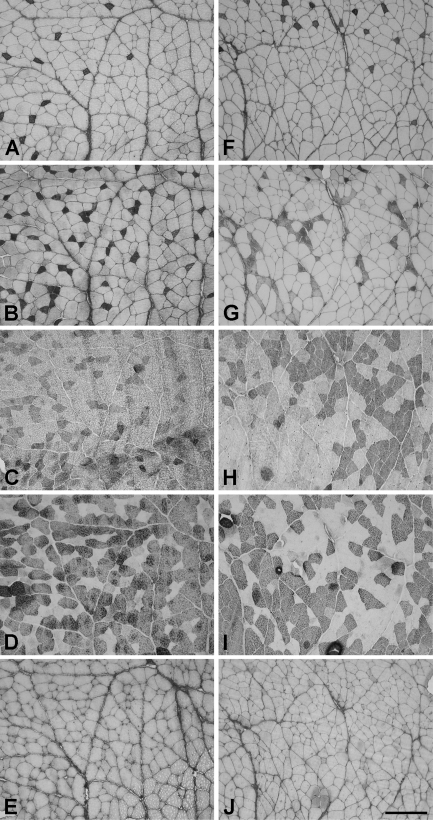
Figures 1A–1E show serial cross-sections of an SH muscle stained with MAbs to slow (Figure 1A), 2A (Figure 1B), 2X (Figure 1C), 2B (Figure 1D), and EO (Figure 1E) MyHCs. All antibodies with the exception of anti-EO MyHC stain fibers in this tissue, as in rat limb fast (Lucas et al. 2000) and CT (Rhee et al. 2004) muscles. The average percentages of various fiber types based on the four normal SH muscles are presented in Table 1. It can be seen that the SH is rich in 2b (44.2%), 2x (20.8%), and 2a (24.2%) fibers and has a moderate component of slow fibers (9.0%). Hybrid fibers coexpressing two or more MyHCs (slow/2a, 2a/2x, and 2x/2b fibers) are present, but together formed less than 2% of total fibers.
Figure 1.
Photomicrographs of immunoperoxidase-stained semiserial sections of the normal sternohyoid (SH; A–E) and cross-innervated (F–J) SH muscles stained with anti-slow (A,F), anti-2A (B,G), anti-2X (C,H), anti-2B (D,I), and anti-extraocular (anti-EO; E,J) myosin heavy chain monoclonal antibodies (MyHC MAbs). Bar = 200 μm.
Table 1.
Distribution of fiber types in normal and cross-innervated sternohyoid (SH) and thyroarytenoid (TA) muscles
| SH |
TA (external division) |
|||||
|---|---|---|---|---|---|---|
| Fiber type | Normal | Cross-innervated | Normal | Cross-innervated | ||
| Slow | 9.0 ± 0.4 | 6.7 ± 1.0 | 0 | 0 | ||
| Slow/2a | 1.1 ± 0.2 | 1.9 ± 0.2 | 0 | 0 | ||
| 2a | 24.2 ± 3.8 | 17.2 ± 0.8 | 0 | 0 | ||
| 2a/2x | 0.5 ± 0.5 | 1.2 ± 0.1 | 0 | 0 | ||
| 2x | 20.8 ± 2.4 | 37.8 ± 1.9** | 0 | 4.2 ± 1.3* | ||
| 2x/2b | 0 | 0.5 ± 0.3 | 0 | 0.2 ± 0.1 | ||
| 2b | 44.4 ± 2.5 | 34.9 ± 3.2 | 0 | 0.4 ± 0.2 | ||
| 2b/eo | 0 | 0 | 100 ± 0 | 95.3 ± 1.4* | ||
Symbols indicate statistically significant differences between cross-innervated and normal muscles (*p<0.02, **p<0.002, t-tests). Values are mean percentages ± SEM, n=4.
The mean cross-sectional areas of the various types of fibers in the SH muscles are presented in Table 2. In the normal SH, slow fibers are the smallest in cross-sectional area and 2a fibers are not significantly larger than slow fibers. The 2x fibers are significantly larger than 2a fibers (p<0.005, t-test), whereas 2b fibers are significantly larger than 2x fibers (p<0.005, t-test).
Table 2.
Cross-sectional areas of the different types of fibers of normal and cross-innervated SH muscles
| Fiber type | Normal SH (μm2) | Cross-innervated SH (μm2) |
|---|---|---|
| Slow | 656 ± 71 | 707 ± 34 |
| 2a | 711 ± 48 | 890 ± 24* |
| 2x | 1317 ± 128** | 1470 ± 38** |
| 2b | 2334 ± 122** | 2374 ± 129** |
The differences between 2a and 2x and between 2x and 2b fibers in both normal and cross-innervated muscles are statistically significant (**p<0.005, t-test), whereas 2a fibers in cross-innervated SH muscles are significantly larger than those of normal SH muscles (*p<0.05, t-test). Values are mean areas ± SEM, n=4.
Figures 1F–1J show serial sections of a representative cross-innervated SH muscle at 12 weeks stained with the various MAbs. The full spectrum of MyHC isoforms found in normal SH is expressed, and EO MyHC is absent. Fiber type grouping is evident in the cross-innervated muscles, particularly in sections stained for 2A MyHC (Figure 1G), 2X MyHC (Figure 1H), and 2B MyHC (Figure 1I). The percentages of slow, 2a, 2x, and 2b fibers in the four cross-innervated SH muscles are shown in Table 1. Cross-innervation results in a significant increase in percentage of 2x fibers from 20.8% to 37.8% (p<0.002, t-test) at the expense of 2a and 2b fiber types. Small percentages of hybrid fibers are also present. However, none of the four cross-innervated SH muscles shows any trace of EO MyHC expression.
Table 2 compares the mean cross-sectional areas of the various types of fibers in cross-innervated SH muscles with those of normal SH muscles. Cross-innervation results in a 25% increase in 2a fiber cross-sectional area (p<0.05, t-test), whereas the differences in relative sizes between 2a, 2x, and 2b fibers remain as for normal muscles.
Fiber Types in Normal and Cross-innervated TA muscles
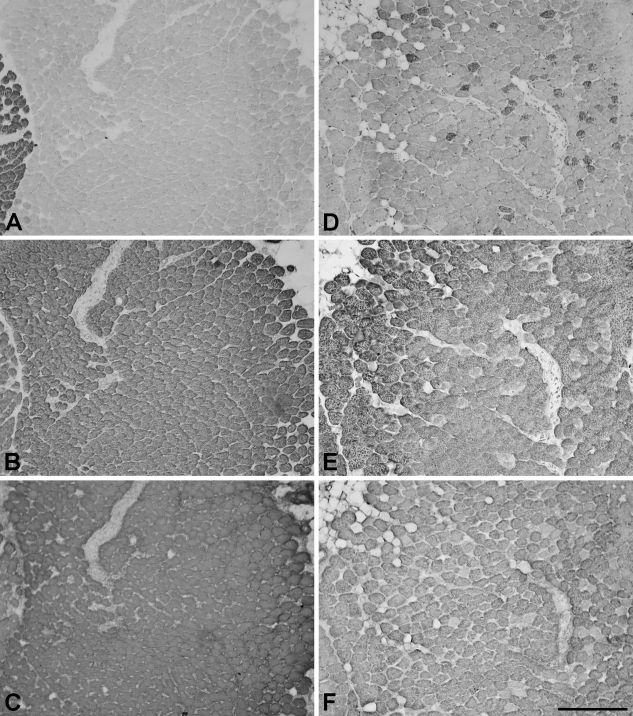
Figure 2 shows serial sections of a representative normal TA (Figures 2A–2C) and a cross-innervated TA (Figures 2D–2F) stained with anti-2X (Figures 2A and 2D), anti-2B (Figures 2B and 2E), and anti-EO (Figures 2C and 2F) MAbs. The vocalis division of the TA is just visible at the left edge of the photomicrographs of normal TA. Examination of all normal TA muscles confirms the earlier report (Rhee et al. 2004) that the external division of TA comprises a homogenous population of hybrid 2b/eo fibers, whereas the vocalis division consists of a mixture of 2x and 2b/eo fibers, 2a and slow fibers being absent. The external division of TA muscle thus serves as an ideal background for the detection of changes in fiber type following cross-innervation.
Figure 2.
Photomicrographs of immunoperoxidase-stained semiserial sections of the normal thyroarytenoid (TA; A–C) and cross-innervated TA (D–F) muscles. The sections were stained with anti-2X (A,D), anti-2B (B,E), and anti-EO (C,F) MyHC MAbs. Bar = 200 μm.
Figure 2D shows that after cross-innervation, 2x fibers appear de novo in the external division of TA and there is a corresponding decrease in fibers coexpressing 2B (Figure 2E) and EO (Figure 2F) MyHCs. Staining for slow and 2A MyHCs is not found in any of the four cross-innervated TA muscles (data not shown). The percentages of various fiber types in the external division of the cross-innervated TA muscles are shown in Table 1. It shows the presence of 4.2% of 2x fibers and very small percentages (totaling 0.6%) of 2b fibers and hybrid 2x/2b fibers. There is apparently no fiber type grouping in both divisions of the TA, nor is there significant change in 2b/eo fiber size. The mean cross-sectional areas of 2b/eo fibers in a representative normal and a cross-innervated TA, 574 ± 69 (SEM) μm2 and 607 ± 93 (SEM) μm2, respectively, are not significantly different. Neither are 2x fibers [486 ± 106 (SEM) μm2)] in cross-innervated TA significantly different in size from the 2b/eo fibers of normal and cross-innervated TA muscles. It is noteworthy that EO MyHC continues to be extensively expressed in the 2b/eo fibers of cross-innervated TA, which constitute 95.3% of total fibers.
Discussion
Fiber Types of Rat SH Muscle and Functional Significance
We characterized the fiber types present in the rat SH muscle using a comprehensive battery of highly specific MAbs to MyHCs and showed that rat SH has four fiber types, slow, 2a, 2x, and 2b, identical to those of rat limb fast (Lucas et al. 2000) and CT (Rhee et al. 2004) muscles, and also does not express EO MyHC. The abundance of slow and 2a fibers closely agrees with earlier immunohistochemical (Petrof et al. 1992) and histochemical (Bracher et al. 1997) analyses on this muscle. However, neither of these earlier studies reported the presence of 2x fibers. The histochemical methods used by Bracher et al. (1997) apparently did not resolve 2x fibers from 2b fibers, thus explaining their higher content (67%) of 2b fibers compared with our 44.4%. Using an MAb specific to 2X MyHC, we showed that 20.8% of fibers in the SH are 2x fibers. The relationship between fiber type and relative fiber size in the SH muscle conforms with general observations in eutherian and marsupial limb muscles. Slow and 2a fibers are the smallest, 2x fibers are intermediate, and 2b fibers are the largest (Rivero et al. 1998; Lucas et al. 2000; Zhong et al. 2001), reflecting common adaptations to the metabolic styles of these fiber types.
The SH has contractile characteristics of a moderately fast muscle (McGuire et al. 2001), consistent with its fiber type distribution. The SH together with the genioglossus, geniohyoid, thyrohyoid, and sternothyroid are a group of upper airway dilator muscles that regulate the patency of the upper airway. The contraction of these muscles opposes the tendency for the upper airway to collapse due to negative pressure generated during inspiration. They thereby provide structural stability to the upper airway, particularly in the presence of an obstruction (Deegan and McNicholas 1995). In humans, these muscles are implicated in the pathogenesis of snoring and obstructive sleep apnea syndrome (Strohl 1981; Block et al. 1984). Phasic inspiratory electromyographic activity has been recorded in rabbit SH, sternothyroid, and genioglossus muscles during tidal breathing, which progressively increases with airway occlusion (Roberts et al. 1984). The moderate presence of slow (9%) and 2a (24.2%) fibers in the rat SH would be appropriate for such functions, whereas the 2x and 2b fibers provide strong contractions in an emergency. A similar histochemical profile of fiber types has been reported in the cat SH (Dick and van Lunteren 1990).
Plasticity of the SH After Cross-innervation With the RLN
There are convincing signs of reinnervation of the SH following cross-innervation by the RLN. First, there is fiber type grouping of 2a, 2x, and 2b fibers. The contiguous fibers of the same type are presumably reinnervated by the same regenerating nerve fiber, which exerts the same influence on MyHC gene expression (Gunn 1972; Rhee et al. 2009). This shows that the SH muscle is subject to neural regulation. Second, there is no fiber atrophy to suggest the presence of denervation; in fact, 2x fibers in cross-innervated SH are 25% larger compared with normal 2x fibers. Cross-innervation of the SH with the RLN has been reported in the guinea pig (Heaton et al. 2000), but the focus of that work was not on a possible allotypic difference between laryngeal and somitic muscles. Following such surgery, motor signals of the RLN in relation to respiration and in response to various types of stimuli that elicit laryngeal motor responses could be recorded from the cross-innervated SH, showing that fibers in the RLN retain their laryngeal muscle-specific efferent impulse activity following cross-innervation. It is thus likely that rat RLN also retains laryngeal muscle-specific efferent impulse activity, and so has the potential to transform fiber types in the SH.
In the cross-innervated SH, the four fiber types of the normal SH are still present. However, the proportion of 2x fibers increased significantly (p<0.002) from 20.8% in the normal SH to 37.8% at the expense of 2b and 2a fibers. This change in fiber type proportions suggests that nerve fibers in the RLN, most likely those originally innervating 2x fibers in the TA, have reinnervated and transformed 2a and 2b fibers in the SH into 2x fibers. The RLN arguably contains a sufficiently large population of nerve fibers that normally innervate 2x fibers to account for this increase in 2x fibers in the cross-innervated SH, because the principal muscles it normally innervates, the TA and posterior cricoarytenoid, have 2X MyHC contents of 20% and 27–37%, respectively (DelGaudio et al. 1995; Wu et al. 2000).
The RLN fails to induce 2b/eo fibers in any of the cross-innervated SH muscles, which has 34.9% pure 2b fibers. This failure to induce EO MyHC expression is highly significant as fibers in the RLN supporting EO MyHC expression are numerous, 2b/eo fibers being very prevalent in rat laryngeal muscles. The result suggests that the nerve fibers in the RLN normally innervating 2b/eo fibers can only support 2B MyHC expression in the cross-innervated SH. The inability of these nerve fibers to induce EO MyHC expression in the SH suggests that SH and TA are allotypically distinct. The alternative scenario is that these muscles are allotypically identical; the RLN does have the capacity to induce EO MyHC expression in the SH, but cross-innervation completely fails, the 2b fibers being reinnervated by their original nerve fibers. However, as the methods used are sensitive enough to detect a single 2b/eo motor unit, this hypothesis would require that none of the numerous nerve fibers in the RLN innervating 2b/eo fibers manages to cross-innervate the SH in all four animals. This is extremely improbable in view of the visual integrity of the nerve cross-union and the strategy used to avoid self-reinnervation (see Materials and Methods). These results favor the interpretation that SH and TA are different muscle allotypes.
The proportions of slow (6.7%) and 2a (17.2%) fibers in the cross-innervated SH are not significantly diminished compared with normal SH, even though it appears likely that the RLN has few nerve fibers innervating slow and 2a muscle fibers. These two fiber types are absent in the TA (Wu et al. 2000; Rhee et al. 2004), and in the posterior cricoarytenoid, slow and 2A MyHCs constitute little more than 10% of total MyHCs (DelGaudio and Sciote 1997; Wu et al. 2000). The possibility thus arises that some of the slow and 2a fibers in the SH are cross-innervated by nerve fibers in the RLN that do not normally innervate slow and 2a fibers in laryngeal muscles. In other words, the neural signals required to induce a particular MyHC expression in allotypically different muscles may be different.
In summary, these results on cross-innervated SH reveal that the RLN is capable of influencing the expression of MyHCs in the SH muscle, but only within the phenotypic options (slow, 2A, 2X, and 2B) available to the SH muscle allotype. Our results in the rat differ substantially from those of Heaton et al. (2000) in the guinea pig. These authors showed that the SH in normal guinea pig had 30% of 2a and 2x fibers, together with some slow fibers and an abundance of 2b fibers, but they did not study guinea pig laryngeal muscles. Laryngeal muscles generally express faster MyHCs in small animals than in larger ones. EO MyHC is expressed in the TA of the rat and rabbit (Lucas et al. 1995; Briggs and Schachat 2000) but not in the TA of the cat and baboon, whereas 2B MyHC is expressed in the TA of the cat but not in the TA of larger animals such as baboon (Rhee and Hoh 2008), human (Sciote et al. 2002; Li et al. 2004), or horse (Rhee et al. 2009). It thus seems rather unlikely that 2b fibers would be absent in the TA of the relatively small animal, the guinea pig. In the cross-innervated guinea pig SH, 2a and 2x fibers dramatically increased to 83%, but there were little or no 2b fibers. This is in sharp contrast to our results showing an abundance of 2b fibers (34.9%) in cross-innervated rat SH. The reason for this difference is not apparent, but may be due to a failure to detect 2B MyHC expression histochemically.
Plasticity of the TA After Cross-innervation With the Nerve to the SH
Following cross-innervation of the TA with the nerve to the SH, 2x fibers amounting to 4.2% of total fibers appeared de novo in the external division of the TA. This change is not a result of denervation, as the mean cross-sectional area of these 2x fibers is not significantly different from that of normal 2b/eo fibers. The presence of 2x fibers is most likely due to the reinnervation of 2b/eo fibers by the nerve to the SH. As the external division of the normal TA is homogeneously 2b/eo, there has been a 2b/eo → 2x fiber type transformation following cross-innervation, similar to the more extensive change reported earlier following self-reinnervation of the TA by the RLN (Rhee et al. 2004). This fiber type transformation consolidates the earlier conclusion that laryngeal muscle fibers are subject to regulation by their nerve supply.
Apart from the appearance of 2x fibers, the rest of the fibers in the cross-innervated TA remained unchanged as 2b/eo fibers, a result similar to the effect of reinnervation of the TA by the RLN (Rhee et al. 2004). Although the nerve to the SH contains abundant nerve fibers innervating 2b muscle fibers (which constitute 44.4% of total SH fibers), there are practically no pure 2b fibers in the cross-innervated TA. The 2b/eo fibers in the cross-innervated TA are presumably cross-innervated by nerve fibers, which normally innervate 2b fibers in the SH, where they do not support EO MyHC expression. They permit 2B MyHC expression in the TA, but fail to suppress EO MyHC expression in this muscle, further supporting a difference in allotype between TA and SH. The alternative scenario is that TA and SH do not differ in allotype, but the cross-innervation completely fails, with the TA being reinnervated by the RLN. However, the virtual complete absence of pure 2b fibers in the cross-innervated TA requires that none of the 2b-innervating nerve fiber in the SH nerve manages to cross-innervate the TA in each of the four animals. This again is extremely unlikely in view of the visual integrity of the nerve cross-union and the strategy used to avoid self-reinnervation.
Another significant finding is that the nerve to the SH fails to induce the expression of slow and 2A MyHCs in any of the cross-innervated TA muscles examined, even though this nerve supports slow and 2a fibers that amount to one third of all fibers in the normal SH. This suggests that nerve fibers normally innervating slow and 2a muscle fibers in the SH are incompetent at inducing the slow and 2A MyHCs in the TA. This failure indicates that the rat TA is intrinsically unable to express these isoforms, or different patterns of stimulation are needed to induce them in the TA, adding further support for an allotypic difference between TA and SH.
In conclusion, the TA and SH muscles in the rat are physiologically plastic and fiber types in both muscles are subject to regulation by their nerve supply. However, the SH cannot be induced to express EO MyHC, nor can TA be induced to suppress it, by cross-innervation with nerve fibers having these capacities. The ability of these muscles to respond to a foreign nerve is limited by their intrinsic characteristics, which reflect their diverse developmental origins. These results support the idea that they differ in muscle allotype.
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Block AJ, Faukner JA, Huges RL, Remmers JE, Thach BT (1984) Factors influencing upper airway closure. Chest 86:114–122 [DOI] [PubMed] [Google Scholar]
- Bormioli SP, Torresan P, Sartore S, Moschini GB, Schiaffino S (1979) Immunohistochemical identification of slow-tonic fibers in human extrinsic eye muscles. Invest Ophthalmol Vis Sci 18:303–306 [PubMed] [Google Scholar]
- Bracher A, Coleman R, Schnall R, Oliven A (1997) Histochemical properties of upper airway muscles: comparison of dilator and nondilator muscles. Eur Resp J 10:990–993 [DOI] [PubMed] [Google Scholar]
- Briggs MM, Schachat F (2000) Early specialization of the superfast myosin in extraocular and laryngeal muscles. J Exp Biol 203:2485–2494 [DOI] [PubMed] [Google Scholar]
- Deegan PC, McNicholas WT (1995) Pathophysiology of obstructive sleep apnoea. Eur Resp J 8:1161–1178 [DOI] [PubMed] [Google Scholar]
- DelGaudio JM, Sciote JJ (1997) Changes in myosin expression in denervated laryngeal muscle. Ann Otol Rhinol Laryngol 106:1076–1081 [DOI] [PubMed] [Google Scholar]
- DelGaudio JM, Sciote JJ, Carroll WR, Escalmado RM (1995) Atypical myosin heavy chain in rat laryngeal muscle. Ann Otol Rhinol Laryngol 104:237–245 [DOI] [PubMed] [Google Scholar]
- Dick TE, van Lunteren E (1990) Fiber subtype distribution of pharyngeal dilator muscles and diaphragm in the cat. J Appl Physiol 68:2237–2240 [DOI] [PubMed] [Google Scholar]
- Gunn HM (1972) Histochemical observations on laryngeal skeletal muscle fibres in ‘normal’ horses. Equine Vet J 4:144–148 [DOI] [PubMed] [Google Scholar]
- Heaton JT, Kobler JB, Goldstein EA, McMahon TA, Barry DT, Hillman RE (2000) Recurrent laryngeal nerve transposition in guinea pigs. Ann Otol Rhinol Laryngol 109:972–980 [DOI] [PubMed] [Google Scholar]
- Hoh JFY (1975) Neural regulation of mammalian fast and slow muscle myosins: an electrophoretic analysis. Biochemistry 14:742–747 [DOI] [PubMed] [Google Scholar]
- Hoh JFY (2002) ‘Superfast’ or masticatory myosin and the evolution of jaw-closing muscles of vertebrates. J Exp Biol 205:2203–2210 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S (1988) Myogenic and neurogenic regulation of myosin gene expression in cat jaw-closing muscles and regenerating in fast and slow limb muscle beds. J Muscle Res Cell Motil 9:59–72 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S, Hale PT, Fitzsimons RB (1988) Immunocytochemical and electrophoretic analyses of changes in myosin gene expression in cat limb fast and slow muscles during postnatal development. J Muscle Res Cell Motil 9:30–47 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Kwan BTS, Dunlop C, Kim BH (1980) Effects of nerve cross-union and cordotomy on myosin isoenzymes in fast-twitch and slow-twitch muscles of the rat. In Pette D, ed. Plasticity of Muscle. Berlin, Walter de Gruyter, 339–352
- Hoh JFY, Salafsky B (1971) Effects of nerve cross-union on rat intracellular potassium in fast-twitch and slow-twitch rat muscles. J Physiol 216:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang LH, Rughani A, Walker ML, Bestak R, Hoh JFY (2010) Expression of masticatory-specific isoforms of myosin heavy chain, myosin binding protein-C, and tropomyosin in muscle fibers and satellite cell cultures of cat masticatory muscle. J Histochem Cytochem 58:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZB, Lehar M, Nakagawa H, Hoh JFY, Flint PW (2004) Differential expression of myosin heavy chain isoforms between abductor and adductor muscles in the human larynx. Otolaryngol Head Neck Surg 130:217–222 [DOI] [PubMed] [Google Scholar]
- Lucas CA, Hoh JFY (2003) Distribution of developmental myosin heavy chains in adult rabbit extraocular muscle: identification of a novel embryonic isoform absent in fetal limb. Invest Ophthalmol Vis Sci 44:2450–2456 [DOI] [PubMed] [Google Scholar]
- Lucas CA, Kang LH, Hoh JFY (2000) Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Commun 272:303–308 [DOI] [PubMed] [Google Scholar]
- Lucas CA, Rughani A, Hoh JFY (1995) Expression of extraocular myosin heavy chain in rabbit laryngeal muscle. J Muscle Res Cell Motil 16:368–378 [DOI] [PubMed] [Google Scholar]
- McGuire M, Dumbleton M, MacDermott M, Bradford A (2001) Contractile and electrical properties of sternohyoid muscle in streptozotocin diabetic rats. Clin Exp Pharmacol Physiol 28:184–187 [DOI] [PubMed] [Google Scholar]
- Pedrosa-Domellöf F, Holmgren Y, Lucas CA, Hoh JFY, Thornell LE (2000) Human extraocular muscles: unique pattern of myosin heavy chain expression during myotube formation. Invest Ophthalmol Vis Sci 41:1608–1616 [PubMed] [Google Scholar]
- Petrof BJ, Kelly AM, Rubinstein NA, Pack AI (1992) Effect of hypothyroidism on myosin heavy chain expression in rat pharyngeal dilator muscles. J Appl Physiol 73:179–187 [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbová G (1985) Neural control of phenotypic expression in mammalian muscle fibres. Muscle Nerve 8:676–689 [DOI] [PubMed] [Google Scholar]
- Rhee HS, Hoh JFY (2008) Immunohistochemical analysis of myosin heavy chain expression in laryngeal muscles of the rabbit, cat, and baboon. J Histochem Cytochem 56:929–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Lucas CA, Hoh JFY (2004) Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J Histochem Cytochem 52:581–590 [DOI] [PubMed] [Google Scholar]
- Rhee HS, Steel CM, Derksen FJ, Robinson NE, Hoh JFY (2009) Immunohistochemical analysis of laryngeal muscles in normal horses and horses with subclinical recurrent laryngeal neuropathy. J Histochem Cytochem 57:787–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero JL, Talmadge RJ, Edgerton VR (1998) Fibre size and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. J Muscle Res Cell Motil 19:733–742 [DOI] [PubMed] [Google Scholar]
- Roberts JL, Reed WR, Thach BT (1984) Pharyngeal airway-stabilizing function of sternohyoid and sternothyroid muscles in the rabbit. J Appl Physiol 57:1790–1795 [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, et al. (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10:197–205 [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Morris TJ, Brandon CA, Horton MJ, Rosen C (2002) Unloaded shortening velocity and myosin heavy chain variations in human laryngeal muscle fibers. Ann Otol Rhinol Laryngol 111:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber GH (1989) Craniofacial Embryology. 4th ed. London, Wright
- Strohl KP (1981) Upper airway muscles of respiration. Am Rev Resp Dis 124:211–213 [DOI] [PubMed] [Google Scholar]
- Wu YZ, Baker MJ, Crumley RL, Caiozzo VJ (2000) Single-fiber myosin heavy-chain isoform composition of rodent laryngeal muscle: modulation by thyroid hormone. Arch Otolaryngol Head Neck Surg 126:874–880 [DOI] [PubMed] [Google Scholar]
- Zhong WW, Lucas CA, Kang LH, Hoh JFY (2001) Electrophoretic and immunochemical evidence showing that marsupial limb muscles express the same fast and slow myosin heavy chains as eutherians. Electrophoresis 22:1016–1020 [DOI] [PubMed] [Google Scholar]
- Zhong WW, Withers KW, Hoh JF (2010) Effects of hypothyroidism on myosin heavy chain composition and fibre types of fast skeletal muscles in a small marsupial, Antechinus flavipes. J Comp Physiol B 180:531–544 [DOI] [PubMed] [Google Scholar]