Abstract
Autotypic tight junctions are formed by tight junction–like structures in three regions of myelinating Schwann cells, the paranodal loops, Schmidt–Lanterman incisures, and outer/inner mesaxons, and various tight junction molecules, including claudin-19 and junctional adhesion molecule (JAM)-C. Our findings demonstrate the identification and subcellular distribution of a novel tricellular tight junction protein, tricellulin (TRIC), in the autotypic tight junctions of mouse myelinating Schwann cells, compared with the autotypic adherens junction protein E-cadherin and the autotypic tight junction protein JAM-C, which are expressed in the paranodal loops, Schmidt–Lanterman incisures, and mesaxons. In real-time RT-PCR, the expression level of TRIC mRNA was about 10-fold higher in the sciatic nerve than in the spinal cord or cerebrum. In immunostaining, TRIC signals were completely restricted to the peripheral nervous system (PNS) and strongly concentrated at the paranodal loops, Schmidt–Lanterman incisures, and mesaxons of myelinating Schwann cells. In addition, TRIC was expressed in the thin region of the paranode and there was a gap between TRIC and the Na+ channel. Furthermore, TRIC was more distally located from the node than E-cadherin and was colocalized with JAM-C. It is possible that TRIC may be a component to maintain the integrity for PNS myelin function and morphology. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 58:1067–1073, 2010)
Keywords: paranode, node of Ranvier, Schmidt–Lanterman incisure, mesaxon, non-compact myelin, myelin sheath
The myelin membrane is divided into two structurally and biochemically distinct regions, compact myelin and non-compact myelin (Poliak et al. 2002; Ryu et al. 2008). Compact myelin forms many layers composed of the major dense line and the intraperiod line. Non-compact myelin regions are found in the paranodal loops, Schmidt–Lanterman incisures, and the inner and outer mesaxons.
Areas of non-compact myelin contain several types of specialized junctions, including tight, gap, and adherens junctions, which are found in epithelial cells (Mugnaini and Schnapp 1974; Fannon et al. 1995; Balice-Gordon et al. 1998; Poliak et al. 2002; Spiegel and Peles 2002). These junctions are found between membrane lamellae of the same cell and are termed autotypic tight, gap, and adherens junctions, respectively (Trapp et al. 1989; Fannon et al. 1995; Scherer et al. 1995; Gumbiner 2000; Altevogt et al. 2002).
Autotypic tight junctions are observed as tight junction strands between adjacent cell membranes in the inner and outer mesaxon, paranodal loops, and Schmidt–Lanterman incisures in the peripheral myelin sheath by freeze-fracture electron microscopy (Sandri et al. 1977; Tetzlaff 1978,1982). They are proposed to function as a mechanical link and as a permeability barrier separating the extracellular space outside the myelin sheath from the intramyelinic space between the lamellae (Hall and Williams 1969; Revel and Hamilton 1969; Mugnaini and Schnapp 1974; Tabira et al. 1978; MacKenzie et al. 1984). The autotypic tight junctions present in different components of non-compact myelin contain distinct junctional complexes including the paranodal loops, Schmidt–Lanterman incisures, and mesaxons (Poliak et al. 2002).
Tight junctions in endothelial and epithelial cells consist of not only the integral membrane proteins claudins (Cldns), occludin, and junctional adhesion molecule (JAMs) but also many peripheral membrane proteins, including the scaffold PDZ-domain expression proteins zonula occludens (ZO)-1, ZO-2, ZO-3, multi-PDZ domain protein-1 (MUPP1) and membrane-associated guanylate kinase with inverted orientation (MAGI)-1, MAGI-2, MAGI-3, and cell polarity molecules ASIP/PAR-3, PAR-6, PALS-1, and PALS-1-associated tight junction (PATJ) and the non–PDZ-expressing proteins, cingulin, symplekin, ZONAB, GEF-H1, aPKC, PP2A, Rab3b, Rab13, PTEN, and 7H6 (Tsukita et al. 2001; Sawada et al. 2003; Schneeberger and Lynch 2004). More recently, tricellulin (TRIC) was identified as the first marker of the tricellular tight junction in epithelial cells. The loss of TRIC affects the organization of the tricellular tight junction and the barrier function of epithelial cells (Ikenouchi et al. 2005). Autotypic tight junctions of myelinating Schwann cells are also composed of various transmembrane and peripheral cytoplasmic tight junction proteins, including Cldn-19 and JAM-C (Miyamoto et al. 2005; Scheiermann et al. 2007). However, in the autotypic tight junctions of myelinating Schwann cells, little is known about the expression and localization of TRIC, which may play a crucial role in bicellular and tricellular tight junctions of epithelial cells.
In this study, our findings demonstrate the identification and subcellular distribution of the novel tricellular tight junction protein TRIC in autotypic tight junctions of mouse myelinating Schwann cells, compared with the autotypic adherens junction protein E-cadherin and the autotypic tight junction protein JAM-C, which are expressed in the paranodal loops, Schmidt–Lanterman incisures, and mesaxons.
Materials and Methods
Animals
C57BL/6 mice were purchased from CREA Japan (Tokyo, Japan). Adult mice (6–8 weeks old) were used. All animal experiments conformed to the regulations of the Sapporo Medical University Animal Care Committee (accession number 09-014) and were carried out in accordance with National Institute of Health Guidelines on Animal Care. All efforts were made to minimize the number of animals used and their suffering.
Antibodies
Rabbit polyclonal anti-TRIC, anti-Cldn-11, and rat monoclonal and rabbit polyclonal anti-JAM-C antibodies were purchased from Zymed Laboratories (San Francisco, CA). A mouse monoclonal anti-E-cadherin antibody was purchased from BD Transduction Laboratories (San Jose, CA). Mouse monoclonal anti-pan Na+ channel (NaCh) and mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibodies were purchased from Sigma-Aldrich (St Louis, MO). A mouse monoclonal anti-neurofilament protein (NFP) antibody was purchased from Sternberger Monoclonals (Baltimore, MD). Rabbit polyclonal anti-myelin protein zero (MPZ) was a gift from Dr. N. Tachi. The secondary antibodies, goat anti-mouse IgG, anti-rat IgG, and anti-rabbit IgG, labeled with Alexa Fluor 488 or 594, were purchased from Invitrogen (Carlsbad, CA).
Preparation of Teased Nerve Fibers
Under deep anesthesia, mice were fixed by cardiac perfusion of 4% paraformaldehyde (PFA) dissolved in a 0.1 M PBS (pH 7.4) and sciatic nerves were isolated. The sciatic nerves were permeabilized in 4% PFA for 30 min at 4C. After washing with PBS, the sciatic nerves were teased on MAS-coated glass slides (Matsunami; Osaka, Japan) using 30G needles and air-dried for 1 hr at room temperature. The teased sciatic nerves were stored frozen at −80C until use. The samples were fixed in acetone/ethanol for 20 min at −20C before use.
Preparation of Dorsal Roots and Transverse Sections
Under deep anesthesia, mice were fixed by cardiac perfusion with 4% PFA and spinal cords with dorsal roots were isolated. They were then immersed in the same solution overnight at 4C. Then the specimens were put in 0.01 M PBS with 10% sucrose for 2 hr at 4C (three times), with 15% sucrose for 2 hr at 4C (one time), and finally with 20% sucrose overnight at 4C (one time) for cryoprotection. The specimens were embedded in Neg-50 frozen section medium (Richard-Allan Scientific; Kalamazoo, MI). Serial sections, each 12-μm thick, were cut with a cryostat (HM 5050E; Microm, Heidelberg, Germany) and placed on MAS-coated slides (Matsunami). After being cut, the specimens were air-dried for 30 min at room temperature and washed in 0.01 M PBS before use.
Reverse Transcriptase–Polymerase Chain Reaction
Total RNA was isolated from the sciatic nerve, spinal cord, and cerebrum using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. The isolated total RNA was used for synthesis of cDNA by SuperScript III reverse transcriptase primed with Random hexamer (Invitrogen). To confirm that there was no contamination, RNA for which reverse transcription was omitted was also forward transcribed as a negative control (NC). The synthesized cDNA was used for RT-PCR. The RT-PCR was performed with Takara standard Taq polymerase (TAKARA; Tokyo, Japan) in 50 μl of reaction mixture that contained 2 μl of synthesized cDNA, 5 μl of 10× Taq buffer (TAKARA), 1 μl of deoxynucleotide triphosphates mixture (TAKARA), 0.2 μM forward and reverse primers, and 0.4 μl of rTaq polymerase (TAKARA). The sequences of the primers were as follows: 5′-ACCACAGTCCATGCCATCAC-3′ (forward)/5′-TCCACCACCCTGTTGCTGTA-3′ (reverse) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 5′-AAGACCCCCTTCGTACTCGT-3′ (forward)/5′-GAACATCGCATTCATTGGTG-3′ (reverse) for TRIC. PCR was performed for 25 cycles of 30 sec at 94C, 30 sec at 58C, and 45 sec at 72C for GAPDH and 40 cycles of 10 sec at 98C, 30 sec at 55C, and 1 min at 72C. The PCR products were loaded in 1.5% agarose gels and visualized via ethidium bromide staining. The gel results obtained were captured on a computer using an ultraviolet irradiation system. The samples without the addition of RNA (NC) had no detectable product.
Quantitative Real-time RT-PCR
Quantitative real-time RT-PCR was performed using a TaqMann Gene Expression Assay kit (Applied Biosystems; Foster City, CA). The amount of 18S rRNA (Hs99999901) mRNA in each sample was used as housekeeping gene to standardize the quantity of mRNA for TRIC (Mm00617924_m1) mRNA expression. Relative levels of gene expression were estimated by a comparative Ct method. Levels were expressed by the formula 2 − (Ct TRIC gene − Ct rRNA), which was described previously (Livak and Schmittgen 2001).
Immunofluorescence and Myelin Staining
The prepared specimens were washed with 0.01 M PBS containing 0.3% Triton X-100 (PBST) and blocked with 5% goat serum in PBS. After blocking, the specimens were incubated with primary antibodies at 4C overnight. The samples were then washed thoroughly with PBST and reacted with appropriate secondary antibodies for 2 hr at room temperature. FluoroMyelin Red (Invitrogen) was used according to manufacturer's protocol to label myelin. After washing with PBS, the specimens were mounted using 5% 1,4-diazabicyclo[2,2,2]octane and 50% glycerol dissolved in PBS. As NCs, the primary antibodies and/or secondary antibodies were omitted, with PBS as a substitute. We observed the images captured using a fluorescence inverted system microscope (IX71; Olympus, Tokyo, Japan), or fluorescence microscope (BZ-9000; KEYENCE, Osaka, Japan).
Results
Expression of TRIC in Mouse Nervous System
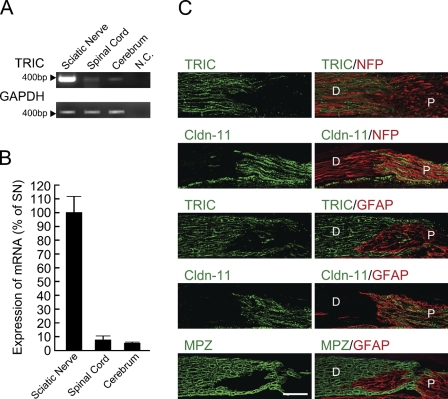
To investigate expression patterns of TRIC in the mouse nervous system, RT-PCR and real-time RT-PCR for TRIC were performed for sciatic nerves, spinal cord, and cerebrum. In RT-PCR, the expression of TRIC mRNA was strongly detected in the sciatic nerve compared with the spinal cord or cerebrum (Figure 1A). In real-time RT-PCR, the expression level of TRIC mRNA was about 10-fold higher in the sciatic nerve than in the spinal cord or cerebrum (Figure 1B).
Figure 1.
Tricellulin (TRIC) expression is abundant in peripheral nerves. (A) TRIC mRNA expression levels in mouse sciatic nerve, spinal cord, and cerebrum revealed by RT-PCR. Glyceraldehyde-3-phosphate dehydrogenase is used as an internal control. (B) Quantitative real-time RT-PCR shows expression levels of mRNA in mouse sciatic nerve, spinal cord, and cerebrum as percentage of sciatic nerve (% of SN). (C) The spinal root (peripheral nervous system)–spinal cord (central nervous system) transitional zone is shown by longitudinal sections of mouse dorsal roots at the distal (D) and proximal (P) sides. The left panels show immunoreactivities for TRIC (green), claudin-11 (Cldn-11; green), or myelin protein zero (MPZ; green). The right panels represent merged images with neurofilament proteins (NFP; red) or glial fibrillary acidic protein (GFAP; red) in the same line. N.C., negative control. Bar = 50 μm.
In contrast to TRIC, Cldn-11 was reported to be expressed in the central nervous system (CNS), but not in the peripheral nervous system (PNS) (Gow et al. 1999; Morita et al. 1999). We examined the detailed expression patterns of TRIC in the mouse nervous system compared with Cldn-11 by using immunofluorescence microscopy (Figure 1C). We used anti-NFP, anti-GFAP, and anti-MPZ antibodies as markers of neurofilaments, astrocytes, and myelinating Schwann cells, respectively. Transverse frozen sections of the mouse dorsal root were stained with anti-TRIC or anti-Cldn-11 antibodies. As shown in Figure 1C, the TRIC and Cldn-11 immunoreactivities were completely restricted to the MPZ-positive distal side (i.e., PNS) and GFAP-positive proximal side (i.e., CNS), respectively.
TRIC Is Expressed in Junctional Regions of Schwann Cells in Mouse Peripheral Nerves
We examined the detailed distribution of TRIC in mouse peripheral nerves by using single fibers teased from sciatic nerves and whole-mount staining with anti-TRIC, anti-E-cadherin, anti-JAM-C, and anti-NaCh antibodies.
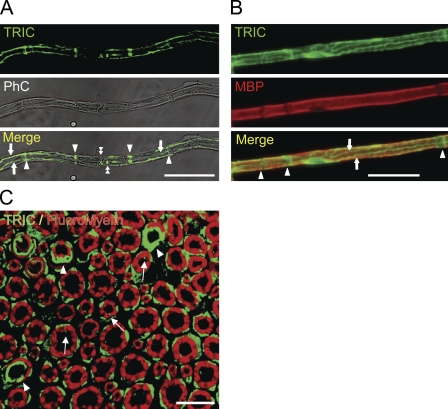
The whole-mount sciatic nerves costained with anti-TRIC antibodies showed the specific localization patterns of TRIC. Characteristic staining patterns were observed in the non-compact regions of the myelinating nerve fibers (Figures 2A and 2B). The transverse sections revealed that TRIC expression was observed in the outside of myelin sheaths (cytoplasm of Schwann cells), the inner mesaxons, and paranodal regions or Schmidt–Lanterman incisures. The distinction between the spots of outer mesaxon and cytoplasm of Schwann cells is difficult (Figure 2C). In a recent study, TRIC expressions were detected in the membrane and also in the cytoplasm of cultured epithelial cells (Kojima et al. in press).
Figure 2.
(A) Panels show teased mouse sciatic nerve fibers stained with anti-TRIC antibodies (TRIC) and phase contrast (PhC). The marginal image is shown in bottom (Merge). TRIC immunoreactivities are localized to the paranode (double arrowheads), mesaxon (arrows), and Schmidt–Lanterman incisures (arrowheads). (B) Panels show teased sciatic nerve fibers stained with anti-TRIC (TRIC) and anti-MBP (MBP) antibodies. The bottom panel is marginal image of TRIC and MBP (Merge). TRIC immunoreactivities are localized to mesaxons (arrows) and Schmidt–Lanterman incisures (arrowheads). (C) Transverse sections of adult mouse sciatic nerve fibers are stained with antibody against TRIC (green) and FluoroMyelin Red (red). TRIC immunoreactivities are localized to the outside of myelin sheaths (cytoplasm of Schwann cells), the inner mesaxons (arrows), and paranodal regions or Schmidt–Lanterman incisures (arrowheads). The distinction between the spots of outer mesaxon and cytoplasm of Schwann cells is difficult. Bars: A,B = 50 μm; C = 10 μm.
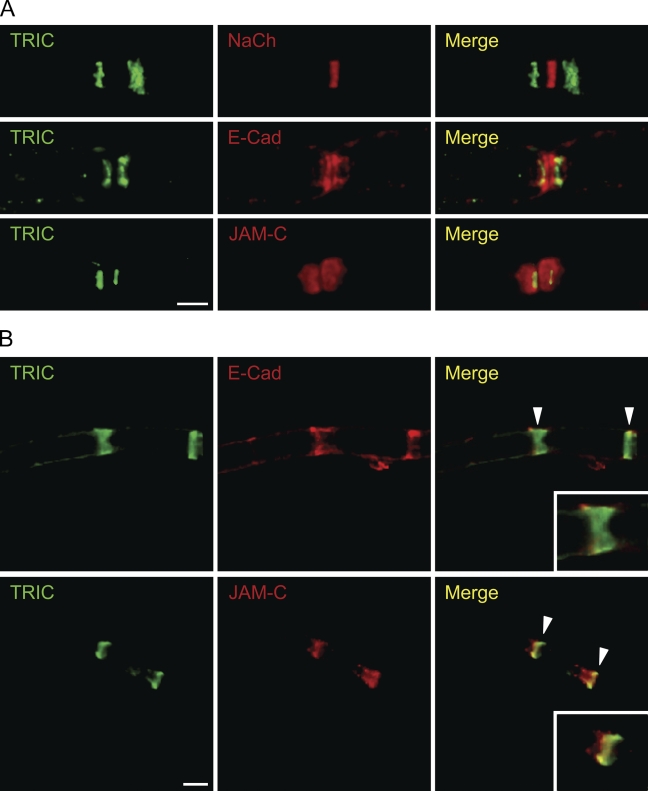
E-cadherin is concentrated at the paranode, Schmidt–Lanterman incisure, and mesaxon and plays a crucial role in maintaining the structural integrity of non-compact myelin regions (Fannon et al. 1995; Tricaud et al. 2005). On the other hand, JAM-C is concentrated at tight junctions and desmosomes (Zen et al. 2004; Betanzos et al. 2009). More recently, it was reported that JAM-C was expressed in the paranode, Schmidt–Lanterman incisure, and mesaxon (Scheiermann et al. 2007). We performed double staining for TRIC and NaCh (as a biomarker protein for nodes of Ranvier), TRIC and E-cadherin, and TRIC and JAM-C and examined the detailed distribution of TRIC in the paranode and Schmidt–Lanterman incisure. In the paranode, TRIC was expressed in the thin region and there was a gap between TRIC and NaCh. The expression of TRIC was observed more distally from the node than E-cadherin and in part of JAM-C immunoreactive (Figure 3A). In the Schmidt–Lanterman incisure, TRIC was also expressed in the thin region and was localized in part of E-cadherin or JAM-C (Figure 3B).
Figure 3.
(A) Paranodal regions of teased mouse sciatic nerves were stained for anti-TRIC (TRIC) and anti-pan Na+ channel (NaCh), anti-E-cadherin (E-cad), or anti-junctional adhesion molecule (JAM)-C antibodies. The right panels represent merged images of the left and center panels in the same row (Merge). (B) Schmidt–Lanterman incisures of teased mouse sciatic nerves were stained for anti-TRIC (TRIC) and anti-E-cadherin (E-cad) or anti-JAM-C (JAM-C) antibodies. The right panels represent merged images of the left and center panels in the same row (Merge). Higher magnification images of merged images are presented in the inset in the right panels. Arrowheads indicate Schmidt–Lanterman incisures. Bar = 5 μm.
Discussion
In this study, TRIC, which is a novel tricellular tight junction protein, was identified in autotypic tight junctions of mouse myelinating Schwann cells (Table 1). TRIC was expressed in the mesaxons, paranodal loops, and Schmidt–Lanterman incisures, like the autotypic tight junction protein Cldn-19 in myelinating Schwann cells.
Table 1.
Localization of tight junction proteins in the non-compact myelin region of myelinating Schwann cells
| Protein | Mesaxons | Schmidt–Lanterman incisure | Paranodal loop | Reference |
|---|---|---|---|---|
| TRIC | + | + | + | Current study |
| JAM-C | + | + | + | Current study and Scheiermann et al. (2007) |
| E-cadherin | + | + | + | Current study and Fannon et al. (1995) |
| Claudin-1 | + | − | + | Poliak et al. (2002) |
| Claudin-2 | − | − | − | Poliak et al. (2002) |
| Claudin-5 | − | + | − | Poliak et al. (2002) |
| Claudin-11 | − | − | − | Morita et al. (1999) |
| Claudin-19 | + | + | + | Miyamoto et al. (2005) |
| Occludin | − | − | − | Nagaoka et al. (1999) |
The autotypic adherens junction protein E-cadherin is concentrated at the paranode, Schmidt–Lanterman incisure, and mesaxon and plays a crucial role in maintaining the structural integrity of non-compact myelin regions (Fannon et al. 1995). In double staining for TRIC and E-cadherin, TRIC was found to localize in part of E-cadherin in the paranode and Schmidt–Lanterman incisure. These results suggested that expression of TRIC might be dependent on adherens junctions and on the regulation of tight junctions in epithelial cells.
The Cldn family, consisting of 24 members, is solely responsible for forming tight junction strands and shows tissue- and cell-specific expression of individual members (Gow et al. 1999; Tsukita et al. 2001). Some Cldns were observed as a component of myelinating glial cells in the PNS and CNS. Cldn-11, which was first detected as an oligodendrocyte-specific protein, was identified as the so-called interlamellar strands in CNS myelin sheathes, but was not observed in the PNS (Gow et al. 1999; Morita et al. 1999; Tsukita et al. 2001). Interestingly, distinct Cldns form different autotypic tight junctions in myelinating Schwann cells (Poliak et al. 2002). For example, Cldn-1 was detected in mesaxon and paranodal loops. Cldn-5 was localized to Schmidt–Lanterman incisures. In contrast to Cldn-1 and Cldn-5, Cldn-19 is localized to the mesaxon, Schmidt–Lanterman incisures, and paranodal loops (Miyamoto et al. 2005; Furuse 2009). In this study, TRIC and Cldn-11 were completely restricted to the PNS and CNS, respectively. TRIC was expressed in the mesaxons, paranodal loops, and Schmidt–Lanterman incisures, like Cldn-19. Autotypic tight junctions are proposed to function as a mechanical link and as a permeability barrier separating the extracellular space outside the myelin sheath from the intramyelinic space between the lamellae (Mugnaini and Schnapp 1974; Fannon et al. 1995; Balice-Gordon et al. 1998; Poliak et al. 2002; Spiegel and Peles 2002). This suggests that TRIC expression is important to keep the integral function and morphology of all autotypic tight junctions in myelinating Schwann cells, but the function of TRIC in myelinating Schwann cells is unknown.
JAM-C is a member of the immunoglobulin subfamily of JAMs, composed of JAM-A, JAM-B, JAM-C, JAM4, ESAM, and CAR, which are specifically enriched at tight junctions of cell–cell contacts (Martin-Padura et al. 1998; Ebnet et al. 2003). In addition, JAM-C plays a crucial role in establishing cell polarity and the formation of endothelial tight junctions (Ebnet et al. 2003). Recently, it was reported that JAM-C was restricted to the PNS, concentrated at the paranodal loops and Schmidt–Lanterman incisures, and played an important role in maintaining the integrity and function of myelinated peripheral nerves (Scheiermann et al. 2007). In this study, TRIC was expressed in thin regions more than JAM-C and was localized with part of JAM-C in the paranode and Schmidt–Lanterman incisure, but not the mesaxon. These findings indicated that TRIC was expressed only in tight junctional areas, whereas JAM-C might be concentrated at both tight junctions and desmosomes.
More interestingly, in this study, TRIC was expressed in the thin region of the paranode and there was a gap between TRIC and NaCh. Furthermore, the TRIC was more distally located from the node than E-cadherin and localized in part of JAM-C. The difference of localization may have an important functional meaning, but our results are insufficient to discuss the function. It is safe to say that tight junction molecules, including TRIC, are a component to maintain the integrity for PNS myelin function and morphology.
On the other hand, two multi-PDZ domain proteins among the other tight junction proteins, PATJ and MUPP1, are colocalized with Cldn-1 in the paranodal loops and with claudin-5 in the Schmidt–Lanterman incisures, respectively (Poliak et al. 2002). Although much more work will be necessary to clarify the molecular relationship between TRIC and the other tight junction proteins, including scaffold proteins and cell polarity molecules, studies on the regulation of TRIC in autotypic tight junctions provide a useful framework for considering the specific functions of autotypic tight junctions in myelinating Schwann cells.
Acknowledgments
This work was supported by Grants-in-Aid from the National Project “Knowledge Cluster Initiative” (2nd stage, “Sapporo Biocluster Bio-S”) and Program for developing the supporting system for upgrading the education and research; the Ministry of Education, Culture, Sports, Science and Technology; and the Ministry of Health, Labour and Welfare of Japan.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL (2002) Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci 22:6458–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Bone LJ, Scherer SS (1998) Functional gap junctions in the Schwann cell myelin sheath. J Cell Biol 142:1095–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos A, Schnoor M, Severson EA, Liang TW, Parkos CA (2009) Evidence for cross-reactivity of JAM-C antibodies: implications for cellular localization studies. Biol Cell 101:441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer zu Brickwedde MK, et al. (2003) The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci 116:3879–3891 [DOI] [PubMed] [Google Scholar]
- Fannon AM, Sherman DL, Ilyina-Gragerova G, Brophy PJ, Friedrich VL Jr, Colman DR (1995) Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J Cell Biol 129:189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M (2009) Knockout animals and natural mutations as experimental and diagnostic tool for studying tight junction functions in vivo. Biochim Biophys Acta 1788:813–819 [DOI] [PubMed] [Google Scholar]
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, et al. (1999) CNS myelin and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 99:649–659 [DOI] [PubMed] [Google Scholar]
- Gumbiner BM (2000) Regulation of cadherin adhesive activity. J Cell Biol 148:399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Williams PL (1969) Some observations on the Schmidt-Lanterman incisures in peripheral mammalian nerve. J Anat 105:215. [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S (2005) Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171:939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, Kikuchi S, et al. (In Press) c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol. Published online June 7, 2010 (DOI: 10.1002/jcp.22273) [DOI] [PubMed]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- MacKenzie ML, Ghabriel MN, Allt G (1984) Nodes of Ranvier and Schmidt-Lanterman incisures: an in vivo lanthanum tracer study. J Neurocytol 13:1043–1055 [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, et al. (1998) Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, Nakayama K, et al. (2005) Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. J Cell Biol 169:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S (1999) Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol 145:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E, Schnapp B (1974) Possible role of zonula occludens of the myelin sheath in demyelinating conditions. Nature 251:725–727 [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Oyamada M, Okajima S, Takamatsu T (1999) Differential expression of gap junction proteins connexin26, 32, and 43 in normal and crush-injured rat sciatic nerves. Close relationship between connexin43 and occludin in the perineurium. J Histochem Cytochem 47:937–948 [DOI] [PubMed] [Google Scholar]
- Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E (2002) Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J Cell Biol 159:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel JP, Hamilton DW (1969) The double nature of the intermediate dense line in peripheral nerve myelin. Anat Rec 163:7–15 [DOI] [PubMed] [Google Scholar]
- Ryu EJ, Yang M, Gustin JA, Chang LW, Freimuth RR, Nagarajan R, Milbrandt J (2008) Analysis of peripheral nerve expression profiles identifies a novel myelin glycoprotein, MP11. J Neurosci 28:7563–7573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri C, Van Buren JM, Akert K (1977) Membrane morphology of the vertebrate nervous system. A study with freeze-etch technique. Prog Brain Res 46:1–384 [PubMed] [Google Scholar]
- Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H (2003) Tight junctions and human diseases. Med Electron Microsc 36:147–156 [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Meda P, Aurrand-Lions M, Madani R, Yiangou Y, Coffey P, Salt TE, et al. (2007) Expression and function of junctional adhesion molecule-C in myelinated peripheral nerves. Science 318:1472–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Deschenes SM, Xu YT, Grinspan JB, Fischbeck KH, Paul DL (1995) Connexin32 is a myelin-related protein in the PNS and CNS. J Neurosci 15:8281–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD (2004) The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286:C1213–1228 [DOI] [PubMed] [Google Scholar]
- Spiegel I, Peles E (2002) Cellular junctions of myelinated nerves (Review). Mol Membr Biol 19:95–101 [DOI] [PubMed] [Google Scholar]
- Tabira T, Cullen MJ, Reier PJ, Webster H deF (1978) An experimental analysis of interlamellar tight junctions in amphibian and mammalian C.N.S. myelin. J Neurocytol 7:489–503 [DOI] [PubMed] [Google Scholar]
- Tetzlaff W (1978) The development of a zonula occludens in peripheral myelin of the chick embryo. A freeze-fracture study. Cell Tissue Res 189:187–201 [DOI] [PubMed] [Google Scholar]
- Tetzlaff W (1982) Tight junction contact events and temporary gap junctions in the sciatic nerve fibres of the chicken during Wallerian degeneration and subsequent regeneration. J Neurocytol 11:839–858 [DOI] [PubMed] [Google Scholar]
- Trapp BD, Andrews SB, Wong A, O'Connell M, Griffin JW (1989) Co-localization of the myelin-associated glycoprotein and the microfilament components, F-actin and spectrin, in Schwann cells of myelinated nerve fibres. J Neurocytol 18:47–60 [DOI] [PubMed] [Google Scholar]
- Tricaud N, Perrin-Tricaud C, Bruses JL, Rutishauser U (2005) Adherens junctions in myelinating Schwann cells stabilizes Schmidt-Lanterman incisures via recruitment of p120 catenin to E-cadherin. J Neurosci 25:3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293 [DOI] [PubMed] [Google Scholar]
- Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA (2004) JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell 15:3926–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]