Abstract
The transcription factor octamer-binding transforming factor 4 (Oct-4) is central to the gene regulatory network responsible for self-renewal, pluripotency, and lineage commitment in embryonic stem (ES) cells and induced pluripotent stem cells (PSCs). This study was undertaken to evaluate differential localization and expression of two major transcripts of Oct-4, viz. Oct-4A and Oct-4B, in adult human testis. A novel population of 5- to 10-μm PSCs with nuclear Oct-4A was identified by ISH and immunolocalization studies. Besides Oct-4, other pluripotent markers like Nanog and TERT were also detected by RT-PCR. Adark spermatogonial stem cells (SSCs) were visualized in pairs and chains undergoing clonal expansion and stained positive for cytoplasmic Oct-4B. Quantitative PCR and Western blotting revealed both the transcripts, with higher expression of Oct-4B. It is proposed that PSCs undergo asymmetric cell division and give rise to Adark SSCs, which proliferate and initiate lineage-specific differentiation. The darkly stained nuclei in Adark SSCs may represent extensive nuclear reprogramming by epigenetic changes when a PSC becomes committed. Oct-4B eventually disappeared in mature germ cells, viz. spermatocytes, spermatids, and sperm. Besides maintaining normal testicular homeostasis, PSCs may also be implicated in germ cell tumors and ES-like colonies that have recently been derived from adult human testicular tissue. (J Histochem Cytochem 58:1093–1106, 2010)
Keywords: Oct-4, Oct-4A, Oct-4B, testis, spermatogonia, embryonic stem cells, proliferation, differentiation, nuclear reprogramming
Self-renewal and pluripotency are the hallmarks of stem cells. The ability of a cell to give rise to the three germ lineages in an organism is defined as pluripotency. Octamer-binding transforming factor 4 (Oct-4), considered to be the master regulator of these pluripotent stem cell (PSC) properties, has been implicated in cancer stem cell hypothesis and is downregulated during differentiation (Niwa et al. 2000; Pesce and Scholer 2001; Jones et al. 2004; Campbell et al. 2007; Lengner et al. 2008). It belongs to POU family of transcription factor genes located on chromosome 6 and is ∼7 kb in humans (Takeda et al. 1992). It encodes for two major spliced variants, Oct-4A and Oct-4B, derived by alternative splicing and four distinct protein isoforms (Wang and Dai 2010). Oct-4A is a transcription factor that regulates the transcription of various genes and is expressed only in PSCs. Oct-4B, on the other hand, is localized in cytoplasm of many non-pluripotent cell types and has no defined function as yet (Lee et al. 2006; Atlasi et al. 2008). Besides embryonic stem (ES) cells, germ cells, primordial germ cells, and germ cell tumors (Looijenga et al. 2003; Jones et al. 2004; Wang and Dai 2010), Oct-4 has also been reported in very small embryonic-like stem cells (VSELs) observed in various adult somatic tissues/organs in the body (Zuba-Surma et al. 2009).
Recently, much progress has been made in the field of human spermatogonial stem cell (SSC) research with the successful derivation of ES cell-like colonies from adult human testicular tissue (Conrad et al. 2008; Golestaneh et al. 2009; Kossack et al. 2009; Mizrak et al. 2009). However, the cells that give rise to such pluripotent ES-like colonies have still not been detected (Dym et al. 2009). Oct-4 is a marker of mouse SSCs (Ohbo et al. 2003; Ohmura et al. 2004; Hofmann et al. 2005) but has not been detected in adult human testicular tissue (Looijenga et al. 2003; Conrad et al. 2008; Kossack et al. 2009; Mizrak et al. 2009; He et al. 2010). Occasional presence of Oct-4-positive interstitial cells has been reported in human testicular sections (He et al. 2010). Thy1+ cells isolated from adult human testicular tissue were found positive for Oct-4 and Nanog. But these Oct-4+ and Nanog+ cells did not result in tumor formation when injected in nude mice (Kobayashi et al. 2009). One possible explanation for this could be that the antibodies and the primer sets used for the experiments were derived from the domain common to both Oct-4A and Oct-4B rather than from the exon 1 that is specific for Oct-4A. Recent reports indicate that multiple isoforms of Oct-4 contribute to much confusion in the field of stem cell biology and may result in misleading conclusions while studying Oct-4 expression to indicate stemness (Wang and Dai 2010). Use of polyclonal antibodies that identify both Oct-4A and Oct-4B isoforms also may yield false-positive results. Hence, it is essential to be prudent with primer designing and antibody selection for Oct-4 studies. Primer sequence specific for exon 1 and therefore amplifying only Oct-4A should be selected. Similarly, monoclonal antibodies (MAbs) raised against aa 1–134 would be reflective of the pluripotent nature of the cells (Liedtke et al. 2007,2008).
In this study, an attempt has been made to explore the presence of PSCs in adult human testis and further delineate the differential expression of Oct-4A and Oct-4B transcripts using specific antibodies and primers (Liedtke et al. 2007,2008). The data generated may help to provide a better understanding of the stem cell niche and its function during renewal and proliferation or differentiation of spermatogonia and help in identifying the currently elusive cell type that results in ES-like colonies from the adult human testis (Conrad et al. 2008; Golestaneh et al. 2009; Kossack et al. 2009; Mizrak et al. 2009; He et al. 2010).
Materials and Methods
This study was approved by the Institute Ethics Committee of National Institute for Research in Reproductive Health, Tata Memorial Centre, and King Edward Memorial Hospital. Testes were collected from men (age range 58–65 years) undergoing orchidectomy as a part of their management for prostate cancer after appropriate written informed consent.
The testicular tissue was collected in DMEM–F12 (Invitrogen; Carlsbad, CA) with antibiotics (Pen-Strep; Invitrogen) and transported on ice to the lab. Part of the tissue was immediately frozen for protein and RNA studies and a part was fixed in neutral buffered formalin for immunohistochemical studies. In addition, germ cell suspension (devoid of interstitial components) was prepared by sequential enzymatic treatment as described earlier (Golestaneh et al. 2009) for immunocytochemical and ISH studies. Briefly, this involved two-step enzymatic digestion of tissue with 10 mg/ml collagenase (Collagenase type IV; Invitrogen) for 30 min at 37C followed by 0.25% trypsin EDTA (Sigma-Aldrich; St Louis, MO) for 15 min at 37C. The cell suspension was filtered through 100-, 70-, and 40-μm nylon cell strainers (BD Falcon; Bedford, MA) under sterile conditions. The filtrate was pelleted at 1000 rpm for 8 min at room temperature and reconstituted in PBS (Sigma). This cell suspension was used to make smears on aminopropyltriethoxysilane (Sigma)-coated slides and smears were fixed with 4% paraformaldehyde (Sigma) for 15 min, washed with PBS, air-dried, and stored for future use at 4C. All precautions were taken to avoid degradation of mRNA while preparing germ cell suspension.
Primer and Antibodies
Specific primers and probes were used for RT-PCR, quantitative PCR (Q-PCR), and ISH studies for human Oct-4 (common for both Oct-4A and Oct-4B), and those specific only to Oct-4A (NCBI accession number NM_002701), TERT, and Nanog are mentioned in Table 1.
Table 1.
Sequence of primers/probes used for detection of Oct-4 and Oct-4A
| Sequence | |
|---|---|
| Primer sets | |
| Oct-4A | |
| F | AGCCCTCATTTCACCAGGCC (nt 9–30) |
| R | TGGGACTCCTCCGGGTTTTG (nt 445–464) |
| Oct-4 | |
| F | CTTGCTGCAGAAGTGGGTGGAGGAA |
| R | CTGCAGTGTGGGTTTCGGGCA |
| Nanog | |
| F | AGTCCCAAAGGCAAACAACCCACTTC |
| R | TGCTGGAGGCTGAGGTATTTCTGTCTC |
| TERT | |
| F | AGCTATGCCCGGACCTCCAT |
| R | GCCTGCAGCAGGAGGATCTT |
| GAPDH | |
| F | AGCCACATCGCTCAGACACC |
| R | GTACTCAGCGGCCAGCATCG |
| Oligoprobe | |
| Oct-4A | CTCCTGCTTCGCCCTCAGGCTGAGAGGTCTCCAAG (nt 321–355) |
Oct-4, octamer-binding transforming factor 4; F, forward; R, reverse; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
For analysis at the protein level by immunolocalization and Western blotting, antibodies that detected Oct-4, i.e., both Oct-4A and Oct-4B (polyclonal antibody raised against residues of aa 300 to C-terminus of human Oct-4; Abcam, Cambridge, UK), and those that specifically detected only Oct-4A (MAb raised against residues of aa 1–134 of human Oct-4; Santa Cruz Biotechnology, Santa Cruz, CA and Millipore–Chemicon, Temecula, CA) were selected from different commercial sources.
RNA Isolation and cDNA Synthesis
Total RNA was extracted using the TRIzol reagent (Invitrogen) and treated with DNase I (Amersham Biosciences; Piscataway, NJ). First-strand cDNA was synthesized using the Omniscript RT Kit according to the manufacturer's instructions (Qiagen; Hilden, Germany). Briefly, 1 mM random hexamer primers, 0.5 mM deoxynucleotide triphosphates (dNTPs), 5 U of Moloney murine leukemia virus RT, and 10 U of RNase inhibitor in a single-strength reaction buffer were used to synthesize the first strand of cDNA in a total volume of 20 μl. The primers and the RNA were initially incubated at 70C for 2 min and then after the addition of other components, reverse transcription reaction was carried out at 37C for 1 hr using GSTORM thermocycler (Gene Technologies; Braintree, UK).
RT-PCR for Pluripotent Markers
Total RNA extracted from the testicular tissue was reverse transcribed as mentioned earlier and was used for detecting pluripotency markers, viz. Oct-4, TERT, and Nanog, using specific primers (Table 1). Total RNA extracted from human ES cells (KIND1) derived and maintained in our lab (Kumar et al. 2009) was used as a positive control, and somatic human endometrial stromal cells were used as a negative control. cDNA mix (2 μl) was amplified using 10 pmol of each primer described in Table 1, 1 U of Taq DNA polymerase (Fermentas Life Sciences; Vilnius, Lithuania), 1.5 mM MgCl2, and 0.4 mM dNTPs in a 25 μl reaction volume in a GSTORM thermocycler. Amplification was carried out for 35 cycles, with each cycle consisting of denaturation at 94C for 1 min, annealing at the specified temperature for each set of primers (Table 1) for 1 min 30 sec, and extension at 72C for 2 min. The products were analyzed on 1.5% agarose gel stained with 0.5 μg/ml ethidium bromide (Bangalore Genei; Bangalore, India) and visualized under ultraviolet transillumination. The product size was approximated using a 100-bp DNA ladder (Bangalore Genei). The negative control did not include cDNA in the reaction mixture.
Q-PCR (Real-Time PCR) to Study Relative Expression of Oct-4 and Oct-4A
The relative levels of Oct-4 (Oct-4A and Oct-4B) and Oct-4A mRNA in relation to 18S rRNA (housekeeping) were estimated by CFX96 real-time PCR system (Bio-Rad Laboratories; Hercules, CA) using SYBR Green chemistry (Bio-Rad Laboratories). For each primer pair, reaction efficiency was estimated by the amplification of serial dilution of human testicular cDNA pool over a 10-fold range. The amplification conditions for Oct-4 were as follows: initial denaturation at 94C for 3 min followed by 40 cycles comprising denaturation at 94C for 30 sec, primer annealing at 55C for 30 sec, and extension at 72C for 30 sec. The final extension was carried out for 5 min at 72C. For Oct-4A and 18S rRNA, the conditions were the same as earlier, except that the annealing temperature was optimized at 57C and 60C, respectively. The fluorescence emitted at each cycle was collected for the entire period of 30 sec during the extension step of each cycle. The homogeneity of the PCR amplicons was verified by running the products on 1.5% agarose gels and also by studying the melt curve. All PCR amplifications were carried out in duplicate, and each experiment was repeated two times to test the reproducibility. Mean Ct values generated in each experiment using the CFX Manager software (Bio-Rad Laboratories) were used to obtain the standard curve, and the cDNA concentrations in the samples were computed and normalized to the housekeeping gene (18S RNA). The relative expression ratios were calculated manually by the ΔCt method.
Western Blotting
Testicular tissue was homogenized in buffer containing 20 mM phosphate buffer, 150 mM NaCl, 50 mM sodium fluoride, 0.5 mM sodium orthovanadate, 2% v/v Triton X-100 (all from Sigma) and Complete mini protease inhibitor (Roche Diagnostics; Mannheim, Germany). The homogenate was centrifuged at 12,000 × g for 30 min, and the supernatant was collected. Aliquots of the preparation were stored at −20C, and the concentration of the total protein was estimated using the Folin–Ciocalteau method. The samples were heated at 95C for 5 min with loading buffer (containing 2 mM dithiothreitol) and chilled immediately. Electrophoresis was carried out on 10% SDS-PAGE gel under reducing conditions (Laemmli 1970). Each lane was loaded with 40 μg of protein along with prestained molecular weight marker (Fermentas Life Sciences). The separated proteins were transferred on a polyvinylidene difluoride membrane (Hybond-C Extra; Amersham Biosciences), followed by blocking with 5% non-fat dry (NFD) milk powder in TBS at room temperature for 2 hr. The blots were further incubated at 4C for 18–20 hr with Oct-4 antibodies (rabbit polyclonal from Abcam, 1:400 dilution; mouse monoclonal from Santa Cruz, 1:50 dilution; and mouse monoclonal from Millipore, 1:500 dilution) and housekeeping gene β-actin (mouse monoclonal from Chemicon, 1:5000 dilution) diluted with 1% NFD milk powder in TBS. The blots were washed three times with TBS for 10 min and then incubated for 2 hr at room temperature with horseradish peroxidase–conjugated goat anti-rabbit secondary antibody (Millipore; dilution 1:5000) and goat anti-mouse secondary antibody (dilution 1:4000; Millipore) washed three times with TBS for 10 min each and detected using the chemiluminescence detection system (Supersignal West Femto Kit; Pierce, Rockford, IL).
Immunolocalization Studies
Immunolocalization studies were performed by both IHC (on paraffin-embedded sections) and ICC (on cell smears). The cell smears were processed for immunolocalization of Oct-4 and Oct-4A using Vectastain Elite ABC kit (Vector Laboratories; Burlingame, CA). In brief, the endogenous peroxide was blocked using 3% hydrogen peroxide for 30 min in dark at room temperature. Antigen retrieval was done by treating the smears with sodium citrate buffer (10 mM sodium citrate, pH 6.0) at high power for 5 min in a microwave oven. This was followed by permeabilization step with 0.3% Triton X-100 for 10 min. Blocking was done with 10% normal blocking serum (goat serum for polyclonal antibody and horse serum for MAb) for 1 hr. Germ cells were then incubated with the primary antibody (dilution 1:200 for polyclonal antibody and dilution 1:50 for MAb) at 4C overnight. Primary antibody was replaced with blocking solution for negative control. After washing with PBS, the cells were incubated with the biotinylated secondary antibody (anti-rabbit IgG for polyclonal antibody and anti-mouse IgG for MAb) for 30 min followed by avidin–biotin complex formation step for 30 min; the cells were then extensively washed with PBS and then detected using DAB (BioGenex; San Ramon, CA) and counterstained with hematoxylin.
For immunohistochemical localization, the paraffin sections were deparaffinized and rehydrated through a graded ethanol series. Endogenous peroxide was blocked using 0.3% hydrogen peroxide for 30 min in dark at room temperature. The sections were further treated in a manner similar to that followed for the cell smears.
Immunostaining of the smears and sections was repeated at least three times on three different samples. Representative areas were photographed under a Nikon 90i microscope (Nikon; Tokyo, Japan), and the data were recorded.
In Situ Hybridization
The cell preparations were used for mRNA localization using specific oligoprobe for Oct-4A (Table 1). Briefly, the smears were incubated with ×2 sodium saline citrate (×1 containing 0.15 M NaCl and 0.015 M sodium citrate) buffer for 10 min at room temperature. This was followed by a prehybridization step wherein the cells were incubated in prehybridization cocktail (containing 50% formamide, 10% dextran sulphate, 0.25% yeast tRNA, 0.25% herring sperm DNA, and ×4 sodium saline citrate) for 1 hr at room temperature in a humid chamber. Digoxigenin tail–labeled Oct-4 and Oct-4A oligoprobes were used at a concentration of 5 pmol/ul (diluted in prehybridization cocktail) for hybridization. Hybridization was carried out at 42C overnight in a humid chamber.
Following overnight hybridization, the slides were stringently washed with ×4, ×2, and ×1 sodium saline citrate and further incubated with Genius buffer 1 [0.01 M Tris–HCl (pH 7.4) with 20 mM NaCl] for 15 min. Blocking was done using 3% normal goat serum containing 0.3% Triton X-100 for 2 hr at room temperature. Alkaline phosphatase–conjugated anti-Dig antibody (1:500 dilution; Roche Diagnostics) was applied to the smears at 4C and left overnight. Final substrate detection was done using Nitroblue tetrazolium salt/5-bromo-4-chloro-2-indoyl phosphate according to manufacturer's instructions (Roche Diagnostics). Representative areas were photographed under a Nikon 90i microscope, and the data were recorded.
Results
Detection of Pluripotent Markers in the Adult Human Testis
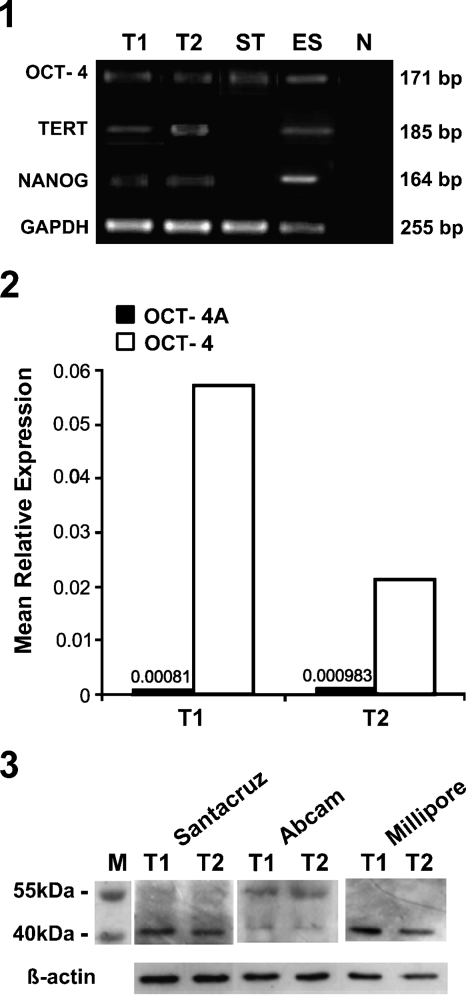
The presence of pluripotent gene transcripts Oct-4, Nanog, and TERT was studied in adult human testes, ES cells, and endometrial stromal cells. Bands of expected size corresponding to Nanog (164 bp) and TERT (185 bp) were detected only in the testicular and human ES cells, whereas Oct-4 (171 bp) expression was also detected in stromal cells (Figure 1). This could be because we used Oct-4 primers that fail to discriminate between Oct-4A and Oct-4B. These results prompted us to study the relative levels of Oct-4 and Oct-4A isoforms in the human testis by real-time PCR using primers specific for both the transcripts (Table 1) and Western blotting.
Figure 1.
RT-PCR amplification of pluripotent markers in adult human testicular tissue. Bands of expected sizes (164, 171, 185, and 255 bps) corresponding to NANOG, octamer-binding transforming factor 4 (Oct-4), TERT, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were detected in both the testicular samples T1 and T2, in positive control embryonic stem (ES) cells, and also in adult somatic stromal cells (ST) because primers used were common for Oct-4A and Oct-4B. No bands were detected in the negative control (N). However, NANOG and TERT did not amplify in adult somatic stromal cells.
Levels of Oct-4 and Oct-4A Transcripts in the Adult Human Testis
Figure 2 represents the mean relative expression of Oct-4 and Oct-4A mRNA in two independent samples in comparison to an internal reference gene by Q-PCR analysis. In comparison with the expression levels of Oct-4 (0.17–5.7), the expression of Oct-4A isoform was found to be very low (3.5 × 10−5 to 9.8 × 10−4). Tissue-to-tissue variation in the levels of both the transcripts was observed; however, a similar trend of high expression of Oct-4 and low expression of Oct-4A transcripts was noted in all samples analyzed.
Figure 2.
Relative expression of Oct-4 and Oct-4A mRNA by quantitative PCR (Q-PCR). Values are of mean relative expression of two adult human testicular samples T1 and T2 normalized to 18S rRNA by Q-PCR. Expression of Oct-4A isoform transcript was very low compared with that of the total Oct-4 (Oct-4A and Oct-4B) transcript in both the samples analyzed.
Human Oct-4A and Oct-4B show identity in exons 2–5, and exon 1 is absent in Oct-4B (Liedtke et al. 2008); thus, primers for Oct-4A were designed from exon 1 to distinguish between the two at the transcript level. Besides the splice variants, at least six pseudogenes with high homology to parental gene have been ascribed to Oct-4 (Liedtke et al. 2008; Wang and Dai 2010). To ensure complete removal of genomic DNA (gDNA), DNAseI-treated RNA sample was used for PCR as a control during initial standardizations (data not shown). Absence of bands indicated complete removal of gDNA. Also, the RT-PCR products revealed the presence of a single band of expected size (171 bp). No additional bands above the expected size were observed. These strategies ensured that we were able to analyze specific Oct-4A and Oct-4B without any contamination of pseudogenes.
Oct-4 Protein Expression in the Adult Human Testis
A distinct band of ∼43 kDa was observed using both monoclonal (Millipore and Santa Cruz) and polyclonal (Abcam) antibodies for Oct-4A (Figure 3). In addition, another band was observed with polyclonal antibody having molecular mass between 55 and 70 kDa, similar to that reported earlier in mice (www.abcam.com/ab19857). This may represent Oct-4B isoform (supported by our real-time PCR data), which has molecular mass ∼33 kDa but gets blotted between 55 and 70 kDa, possibly because of posttranslational modifications. To identify the specific cell types that expressed Oct-4 protein/transcripts, immunolocalization studies followed by ISH were undertaken.
Figure 3.
Western blot analysis of Oct-4 in adult human testis. An expected band of 43 kDa was detected in both the samples (T1 and T2) using antibodies directed against the N-terminal, specific to Oct-4A isoform (Millipore and Santa Cruz) and antibody directed against the common C-terminus (Abcam). An additional band ∼55 kDa was also observed with the Abcam antibody, as reported earlier in mice (www.abcam.com/ab19857). β-actin was used as the loading control.
Cellular Localization of Oct-4 in the Adult Human Testis
Immunocytochemistry
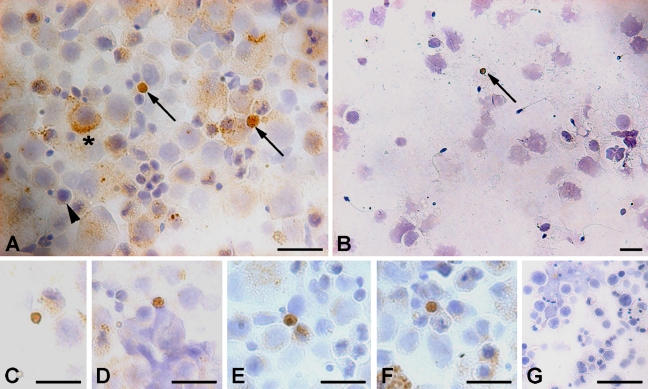
The cell smears showed an interesting immunolocalization pattern using polyclonal Oct-4 antibody (Abcam; Figures 4A and 4C–4F). A distinct population of very small cells with a distinct nuclear staining and a thin rim of unstained cytoplasm was observed. These cells varied in size between 5 and 10 μm in diameter and were very few in number. Besides these small cells with nuclear staining, large cells with cytoplasmic staining were also evident. These cells showed different staining intensity and also there were many cells of different shapes and size, which were devoid of Oct-4 staining. Using MAb against Oct-4A (Santa Cruz; Figure 4B), specific staining was observed only in the nucleus of very small-sized cells (Figure 4B). No cytoplasmic staining was observed in large cells using this antibody. These results prompted us to further study these cells in their in vivo niche in testicular sections.
Figure 4.
Immunocytochemical localization of Oct-4A and Oct-4B in testicular germ cell smears. A distinct cell population stained positive using polyclonal antibody (Abcam) with nuclear Oct-4A and a characteristic unstained rim of cytoplasm (arrow, A). These cells were few in number, thus different fields were captured to demonstrate their presence (C–F). Another population comprised large-sized cells with cytoplasmic Oct-4B (asterisk, A). A distinct gradation of staining intensity for Oct-4B was observed in the cytoplasm of these cells, ranging from dark brown to no stain (arrowhead). Only nuclear Oct-4-positive cells (arrow) were detected using MAb from Santa Cruz (B), and no stain was observed in negative control (G). Bar = 20 μm.
Immunohistochemistry
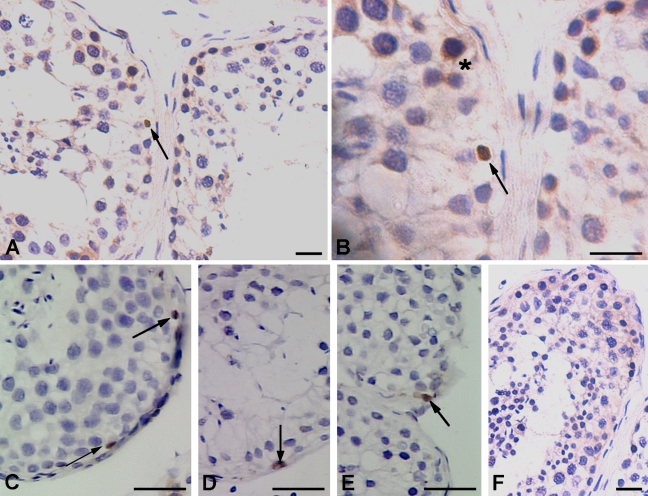
The majority of tubules in testicular tissue section stained positive using polyclonal antibody (Abcam); however, the staining pattern was restricted to small patches and was not uniform throughout the tubules (Figure 5A). Using polyclonal antibody, Oct-4 was localized in the cytoplasm of spermatogonial cells located adjacent to the basement membrane of the tubules. These spermatogonial cells were visualized both in chains (Figures 5B–5D) and pairs (Figures 5E and 5F) with distinct cytoplasmic bridges, which also stained positive for Oct-4. All these spermatogonial cells in pairs and chains appeared to have densely hematoxylin-stained nucleus and probably represent the Adark spermatogonia. Few cells with lightly stained nucleus also revealed cytoplasmic staining and were probably the Apale spermatogonia (Figures 5C and 5D). Discrimination between Apale and B-type spermatogonia was not possible in this study. The more differentiated germ cell types, viz. spermatocytes, spermatids, sperm, and also the somatic Sertoli cells were negative for Oct-4 (Figure 5).
Figure 5.
Immunohistochemical localization of Oct-4 pluripotency network in adult human testis. (A,B) Using polyclonal antibody from Abcam, two distinct staining patterns were observed in seminiferous tubules, including cells with nuclear Oct-4A (arrow) and spermatogonial Adark cells with cytoplasmic Oct-4B (asterisk). Three different representative fields of seminiferous tubules show the presence of Oct-4A-positive cells (arrow) using MAb from Santa Cruz (C–E) and negative control showed no staining (F). Bar = 20 μm.
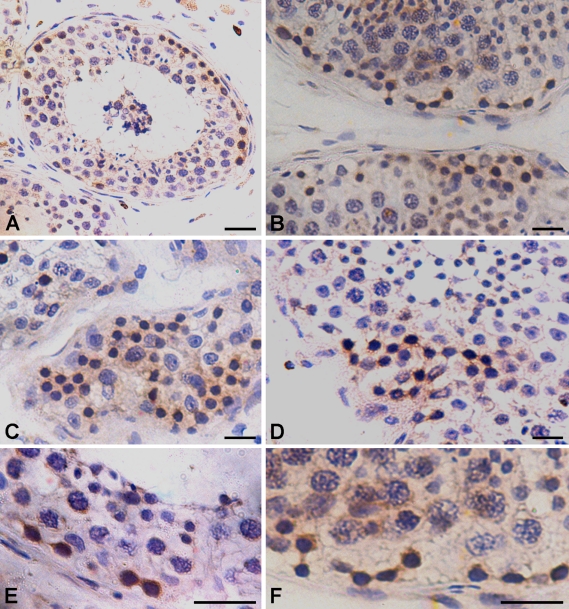
In addition to Adark spermatogonia with cytoplasmic Oct-4, few 5- to 10-μm cells with distinct nuclear Oct-4 staining (Figures 6A and 6B) were observed at places. These cells were localized next to the basement membrane of the tubules. A distinct rim of unstained cytoplasm was clearly visible around these cells.
Figure 6.
Immunohistochemical localization of cytoplasmic Oct-4B in spermatogonial Adark cells showing extensive proliferation. The cytoplasmic Oct-4B-positive spermatogonial Adark cells are present in patches next to the basement membrane of the seminiferous tubule (A). These cells show proliferation and are easily visualized in pairs (B,E,F) and also in chains (C,D). They undergo clonal expansion and cytoplasmic bridges between dividing cells are easily visualized as they also stain positive for Oct-4B. The cells appear dark and stand out in the section because of brown cytoplasmic Oct-4B and hematoxylin-stained dark nuclei, characteristic of Adark spermatogonial cells. Bar = 20 μm.
Using MAb specific to Oct-4A (Santa Cruz), very few cells were found positive for nuclear staining. Interestingly, the whole nucleus was not stained brown as observed with polyclonal antibody (Figures 6A and 6B), but the staining appeared as a horizontal streak across the nuclei (Figures 6C–6E). A distinct rim of unstained cytoplasm was clearly visible around the cells. Interstitial cells showed some cytoplasmic staining with both the polyclonal antibody (Figure 5A) and theMAb.
mRNA Localization of Oct-4 in the Adult Human Testis
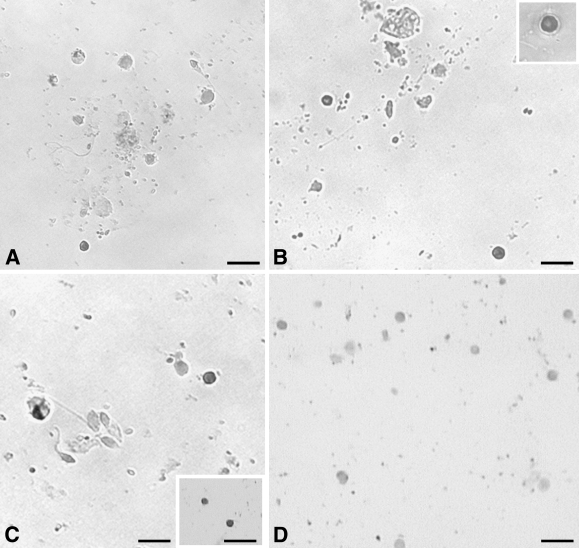
These experiments were carried out on cell smears using Oct-4A-specific oligoprobe. Small cells appeared to show nuclear staining (Figures 7A–7C) and were possibly the same cell type that showed nuclear localization of Oct-4A. The stained cells had a high nucleocytoplasmic ratio, and a distinctive rim of unstained cytoplasm was visible. A distinct population of unstained cells was also observed. Staining was absent in the negative control hybridized using the sense probe (Figure 7D).
Figure 7.
Oct-4A mRNA expression on adult human testicular germ cell smears. Different fields show the presence of distinct nuclear staining for Oct-4A mRNA (A–C) in small-sized cells with a distinct unstained cytoplasm. Many different cell types were observed but were distinctly negative for Oct-4A expression. Insets: B, high magnification image of a positively stained cell; C, positively stained cells. The negative control (D) hybridized with sense oligoprobe showed no expression of Oct-4A. Bar = 20 μm.
Discussion
Our study provides evidence for the presence of a distinct population of 5- to 10-μm-sized embryonic-like cells positive for nuclear Oct-4A for the first time in adult human testis. The presence of pluripotent gene transcripts like Oct-4, Nanog, and TERT by RT-PCR studies (Figure 1) suggested the presence of ES-like cells. Earlier studies have also reported the presence of Oct-4 gene transcripts (Conrad et al. 2008; Kossack et al. 2009; Mizrak et al. 2009) and UTF-1 and REX-1 (Kristensen et al. 2008) in adult human testis by RT-PCR. Q-PCR (Figure 2) and Western blot (Figure 3) results of the present study provided further evidence for the presence of Oct-4A-positive cells in the testis. Results of immunolocalization (Figures 4 and 5) and ISH (Figure 7) studies provided direct evidence for the presence of embryonic-like stem cells in the adult human testis. Initial ICC studies enabled us to view a large number of germ cells, and we observed occasional presence of very small cells with nuclear Oct-4A and characteristic unstained rim of cytoplasm (Figures 4A and 4C–4F). IHC studies, carried out using both polyclonal (Figures 5A and 5B) and monoclonal antibodies (Figures 5C–5E), revealed the presence of this rare cell population in its in vivo niche. These cells were present in very small numbers, next to the basement membrane of seminiferous tubules in adult human testis. It is probably because they are present in very small numbers that they have been missed to date. Had we carried out only IHC studies using MAb, we might have missed this novel cell population similar to earlier reports by He et al. (2010).
Oct-4 is a marker for SSCs and progenitor cells in rodents (Ohbo et al. 2003; Ohmura et al. 2004; Hofmann et al. 2005); however, these Oct-4-positive cells have not been detected earlier in adult human testis (Looijenga et al. 2003; Conrad et al. 2008; Golestaneh et al. 2009; Kossack et al. 2009; Mizrak et al. 2009; He et al. 2010). This could be due to the choice of fixative, antigen retrieval protocol, antibody source, etc. (Table 2), which are important factors to obtain successful immunolocalization results (Berod et al. 1981). We preferred immunoperoxidase staining method over immunofluorescence because staining was easily studied in the morphological context of a cell. Earlier, we have reported stage-specific localization of c-KIT in spermatogonial cells in adult human testicular sections (Unni et al. 2009), which is in contrast to the recent report by He et al. (2010) that KIT is present in late spermatocytes and round spermatids and not in spermatogonial cells. This may be due to the different epitopes recognized by the antibodies used.
Table 2.
Studies carried out to date to study Oct-4 pluripotency network in adult human testis elucidating reasons for earlier negative results
| Serial no. | Reference | Results by RT-PCR | Immunolocalization (IHC/ICC) | Fixative used | Antigen retrieval | Antibody source | Additional comments |
|---|---|---|---|---|---|---|---|
| 1 | Looijenga et al. 2003 | ND | Prepubertal testis negative for Oct-4 | 10% Formalin | Antigen retrieval not done | Polyclonal antibody from Santa Cruz | – |
| 2 | Jones et al. 2004 | ND | Negative Oct-4 IHC on archived sections | Fixative not mentioned | Tris–EDTA in water bath | Polyclonal antibody from Santa Cruz | – |
| 3 | Rajpert-De Meyts et al. 2004 | ND | IHC on prepubertal testis negative for Oct-4 | Cleland, buffered formalin, Steve | Microwave method for Cleland fixed tissue only. No antigen retrieval in other fixatives | Polyclonal antibody from Santa Cruz | – |
| 4 | Conrad et al. 2008 | Faint band for Oct-4, Stat3, Sox2 in testis sample studied | Negative results by IHC | Not mentioned | Not done | Polyclonal antibody from Abcam | – |
| 5 | He et al. 2010 | ND | Negative for Oct-4 by IHC. Few interstitial cells showed positive stain for Oct-4 | 4% Paraformaldehyde or Bouin's fixative | Citra Plus solution from BioGenex | Mouse monoclonal antibody (MAb) from Chemicon | – |
| 6 | Kossack et al. 2009 | Oct-4 and SOX2 detected in commercially available testis sample | ND | – | – | – | – |
| 7 | Mizrak et al. 2009 | Oct-4 and Sox2 detected in four samples studied | ND | – | – | – | – |
| 8 | Bhartiya et al., this study | Oct-4, Nanog, and TERT detected in two testicular samples studied | Both ICC and IHC showed presence of nuclear Oct-4A-positive ES-like cells and cytoplasmic Oct-4B-positive Adark and Apale spermatogonia | Neutral buffered formalin | Sodium citrate buffer in microwave | Polyclonal antibody from Abcam and MAb from Santa Cruz | Oct-4A- and Oct-4B-positive cells confirmed by ISH, Q-PCR, and Western blot analysis |
ES, embryonic stem; Q-PCR, quantitative PCR.
The small size of Oct-4A-positive cells and their “spore-like” appearance (Figures 4 and 5) seem to indicate that these may be the “missing pearls” or VSELs recently reported in various body tissues/organs (Zuba-Surma et al. 2009) and probably similar to the putative stem cells that reside in the human ovarian surface epithelium (Virant-Klun and Skutella 2010; unpublished data).
Using polyclonal antibody, two distinct cell populations were found to stain positive for Oct-4 (Figure 6). Besides the small ES-like cells with nuclear Oct-4A, the other population comprised a large number of Adark-type spermatogonia with cytoplasmic localization of Oct-4 and dark hematoxylin-stained nuclei. This cytoplasmic Oct-4 is probably the spliced Oct-4B isoform that is generated by alternate splicing of Oct-4 (Lee et al. 2006; Wang et al. 2009). Almost 3- to 10-fold greater expression of Oct-4B mRNA as compared with Oct-4A by real-time PCR results (Figure 2) correlated well with the abundance of cells staining positive for cytoplasmic Oct-4B and is in agreement with the fact that stem cells represent only a small proportion of spermatogonia in the adult testis (de Rooij and Russell 2000). These cells were present in pairs (Figures 6F–6G) and also in chains (Figures 6A–6D), depicting extensive proliferation, “spermatogenic wave,” or “clonal expansion” as described earlier (Russell et al. 1990). They were easily identified by cytoplasmic Oct-4B staining, which also stained the intercellular cytoplasmic connections. Few cells with pale nuclei, possibly Apale spermatogonia, also stained positive (Figures 4 and 6), but Oct-4B eventually disappeared in mature germ cells, viz. spermatocytes, spermatids, and sperm. Thus, Oct-4 appears to be a good early marker to visualize events during initial premeiotic development of germ cells. Compared with Oct-4, c-KIT appears to be a better marker for more mature germ cells, possibly Apale spermatogonia (Unni et al. 2009), which also showed proliferation but not clonal expansion as observed with Oct-4 in this study.
The Oct-4A-positive cells are possibly the elusive PSCs that undergo asymmetric cell division to initiate spermatogenesis and give rise to Adark-type spermatogonial cells. Most probably, Oct-4A functions as a transcription factor during self-renewal (analogous to continued proliferation) of pluripotent ES cells in vitro. On becoming committed, the cell enters the differentiation pathway. Oct-4A being no longer required is alternatively spliced to produce Oct-4B, which translocates to the cytoplasm of differentiating spermatogonia and is eventually lost. The cytoplasmic Oct-4B has no reported biological function to date and possibly undergoes modifications after being transported to the cytoplasm, as indicated by the Western blot results (Figure 3). It is possible that this cytoplasmic Oct-4B present in differentiated germ cells may have been detected by Kobayashi et al. (2009) in their study on Thy1+ cells because they used a polyclonal antibody (R&D; Minneapolis, MN), which detects both Oct-4A and Oct-4B epitopes and explains why they did not get any teratoma (a property only of PSCs) on injection. Similar confusion existed when adult peripheral blood mononuclear cells were reported positive for Oct-4; it was later clarified that the positive results were due to Oct-4B and not due to Oct-4A isoform (Zangrossi et al. 2007; Atlasi et al. 2008; Kotoula et al. 2008).
Self-renewal and proliferation or differentiation of SSCs are the unique attributes of germ cells in the testis, and despite the scheme for spermatogonial renewal involving dark and pale spermatogonia described nearly 40 years ago by Clermont (1972) and recently revised (Ehmcke and Schlatt 2006), the identity of true SSCs still remains elusive (Dym et al. 2009; Golestaneh et al. 2009). To the best of our knowledge, this study is the first report of a cross talk between Oct-4A and Oct-4B in a normal non-tumorigenic adult human tissue, where self-renewal, proliferation, and differentiation events occur in a highly orchestrated manner. Gonadal tissue may be an exception but it has been suggested earlier that the Oct-4 pluripotency network active in ES cells and reestablished in induced PSCs may not exist in any somatic tissue (Lengner et al. 2008). The embryonic-like stem cells possibly represent the residual primordial germ cells that survive into adulthood, and not a subpopulation of Adark and/or Apale spermatogonia as speculated earlier (Dym et al. 2009).
On the basis of the results of this study, we propose a new scheme explaining initial proliferation of stem cells (Figure 8) during spermatogenesis. Oct-4A-positive cells are the PSCs that persist into adulthood and result in spermatogenesis throughout the life of an individual. These cells undergo self-renewal and produce progenitor cells, viz. Adark spermatogonia, which further undergo clonal expansion and differentiate into Apale and B-type spermatogonia, resulting in sperm production by undergoing meiosis to maintain tissue homeostasis. It is intriguing that the nomenclature for proliferation pattern of A-type SSCs is different among different species; in rodents they are termed as As, Apr, and Aal, whereas in higher primates they are classified as Adark and Apale (recently reviewed by Ehmcke et al. 2006; Hermann et al. 2010). Human A-type spermatogonia are understood to undergo minimum clonal expansion when compared with those of rodents and monkeys before production of spermatocytes (Johnson et al. 2001). In contrast to the existing paradigm, our results show the presence of Adark spermatogonia in pairs and also in chains in adult human testis (Figure 6), showing a high degree of clonal expansion (indirectly suggesting that they do not undergo asymmetric division as suggested by few groups), unlike the current thinking that they are mitotically inactive or quiescent in nature and are being termed “reserve stem cells.” Our results are in agreement with the model proposed by Clermont (1966a,b) that Adark cells show a high proliferation rate and give rise to Apale cells rather than their relative mitotic inactivity recently proposed by Ehmcke and Schlatt (2006). Even after many years of research, one is struck by the fact that we understand so little about spermatogenesis. Our results indicate that it is probably the same biology active in ES cells, involving Oct-4 pluripotency network, that nature has conserved in testis in a well-regulated manner even in 50- to 65-year-old men. It will be interesting to study this phenomenon in testicular tissue of young men.
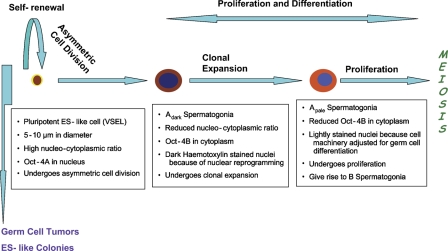
Figure 8.
Revised scheme for premeiotic development of germ cells in adult human testis. It is hypothesized that small and rare ES-like cells detected for the first time in testis undergo asymmetric cell division to give rise to Adark spermatogonial cells with cytoplasmic Oct-4B. These cells undergo extensive nuclear reprogramming by epigenetic changes because pluripotent cell machinery is shut OFF and specific genes necessary for committed cell fate are turned ON. This may result in the dark appearance of their nuclei and later transform into Apale spermatogonial stem cells (SSCs) with normal pale nucleus and decreased expression of Oct-4B. Apale SSCs also proliferate as indicated by studying c-KIT staining, reported earlier by our group (Unni et al. 2009), undergo further differentiation and meiosis to complete the spermatogenesis cycle.
The presence of dark hematoxylin-stained nuclei in Adark SSCs with cytoplasmic Oct-4B (Figure 6) further supports the view that pluripotent ES-like cells, with unlimited potential for self-renewal and capacity to differentiate into any lineage, are indeed the testicular reserve cell population responsible for asymmetric cell division. The Adark SSCs produced by asymmetric cell division of these cells undergo extensive proliferation “spermatogenic wave” (Figure 6) and enter differentiation, whereby the plasticity of the cell is lost as it becomes more committed for lineage-specific function. This is understood to be associated with downregulation of embryonic genes and upregulation of cell-specific genes (Keenen and de La Serna 2008). All this occurs by epigenetic modifications and is termed “nuclear reprogramming,” during which a dramatic change in facultative heterochromatin occurs (Sha and Boyer 2009). Cells with pluripotent properties, i.e., the nuclear Oct-4A-positive cells, probably have abundant transcription permissive euchromatin, which becomes compacted due to stable association of histones with the chromatin in Adark spermatogonia, similar to what is reported during ES cell differentiation (Phanstiel et al. 2008). Thus, because of intense “nuclear reprogramming,” we speculate that the early progenitor cells, viz. Adark spermatogonia, appear dark and, once the cell machinery committed to germ cells becomes active, cell nuclei exhibit the normal pale appearance.
Recent technological advances in the field of SSC biology and success in deriving pluripotent embryonic-like stem cells from adult mice (Guan et al. 2006; Seandel et al. 2007) and human testicular tissue (Conrad et al. 2008; Golestaneh et al. 2009; Kossack et al. 2009; Mizrak et al. 2009) have several exciting and important applications. However, the precise origin of cells that give rise to ES-like pluripotent colonies remains to be established because in all four studies where ES-like cells have been successfully cultured from adult human testis, a mixed germ cell system was used to initiate cultures that led to ES-like colonies (Golestaneh et al. 2009). The ES-like colonies are thought to arise by “dedifferentiation” of a subpopulation of SSCs and/or progenitors to a state of pluripotency (Kanatsu-Shinohara et al. 2008; Golestaneh et al. 2009). It is believed that possibly the interaction with Sertoli cells directs germ cells to spermatogenesis and inhibits multilineage differentiation in testis. On depriving the somatic niche in vitro and being exposed to conducive culture conditions, germ cells convert to pluripotent/multipotent stage. However, definitive evidence as to the germ cell type that gives rise to ES-like colonies is still lacking (Dym et al. 2009). According to the second and less-accepted school of thought, these ES-like colonies may arise from residual pluripotent cells and the main argument against this is that ES-like colonies have never been established directly from primary cultures but appear after several weeks in SSC cultures. The results of the present study support the second school of thought that a novel population of nuclear Oct-4A-positive putative stem cells in adult human testicular tissue may give rise to ES-like colonies. The rarity of this cell type may explain the low frequency of success in deriving ES-like colonies. The differences in methylation pattern of SSC and ES-like cells observed by various investigators is also best explained by the presence of Oct-4A-positive cells. Otherwise, it is difficult to explain why, even after culturing SSCs for more than 6 months, more cells do not get reprogrammed and acquire embryonic-like methylation pattern, and conversely, why only a few cells get reprogrammed. A large number of SSCs are exposed to the culture conditions and have been deprived of the inhibitory effect of Sertoli cells.
Furthermore, these PSCs present in very small numbers in normal testicular tissue may become severely perturbed and mutated to give rise to germ cell tumors, which are understood to have their origin in primordial germ cells (Bosl and Motzer 1997) and are rich in Oct-4-positive cells (Looijenga et al. 2003). We believe there is an urgent need to revisit the concept of carcinoma in situ cell origin of germ cell tumors (recently reviewed by Hoei-Hansen et al. 2005). The observations made in this study of a novel and rare population of Oct-4-positive cells in normal adult testicular tissue and increased number of Oct-4-positive cells in germ cell tumors (Looijenga et al. 2003; Jones et al. 2004; Rajpert-De Meyts et al. 2004) together perhaps lend support to the “embryonal-rest hypothesis,” put forth by Rudolf Virchow and Julius Cohnheim in the 19th century (Maulitz 1978), that adult tissues contain embryonic remnants that normally lie dormant, but can be activated to become cancerous, the fact that ES cells and embryonic carcinoma cells are opposite faces of the same coin (Andrews et al. 2005) and that cancer develops from a population of quiescent PSCs (Ratajczak 2005; Hendrix et al. 2007; Ratajczak et al. 2010).
In conclusion, this study provides new insight into the premeiotic development of germ cells in testis. Pluripotent embryonic-like stem cells with nuclear Oct-4A may be the elusive cell source for (a) asymmetric division resulting in spermatogenesis throughout life, (b) germ cell tumors, and (c) recent success of obtaining ES-like colonies from adult human testicular tissue. The findings of this study are just the tip of the iceberg and have thrown open a realm of exciting possibilities that will undoubtedly require further exploration. One needs to keep this novel cell population in mind while addressing infertility, to achieve fertility control, and may be an interesting target to manipulate while addressing issues related to oncofertility.
Acknowledgments
This study was financially supported by institutional core support provided by Indian Council of Medical Research, Government of India.
The authors thank Dr. Stefanie Liedtke (University Hospital of Duesseldorf, Germany) for useful discussions on Oct-4 biology and Mr. H. Karekar for all the art work.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Andrews PW, Matin MM, Bahrami AR, Damjanov J, Gokhale P, Draper JS (2005) Embryonic stem cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem Soc Trans 33:1526–1530 [DOI] [PubMed] [Google Scholar]
- Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ, Andrews PW (2008) OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells 26:3068–3074 [DOI] [PubMed] [Google Scholar]
- Berod A, Hartman BK, Pujol JF (1981) Importance of fixation in immunocytochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem 29:844–850 [DOI] [PubMed] [Google Scholar]
- Bosl GJ, Motzer RJ (1997) Testicular germ-cell cancer. N Engl J Med 337:242–253 [DOI] [PubMed] [Google Scholar]
- Campbell PA, Perez-Iratxeta C, Andrade-Navarro MA, Rudnicki MA (2007) Oct4 targets regulatory nodes to modulate stem cell function. PLoS One 2:e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont Y (1966a) Spermatogenesis in man. A study of the spermatogonial population. Fertil Steril 17:705–721 [PubMed] [Google Scholar]
- Clermont Y (1966b) Renewal of spermatogonia in man. Am J Anat 118:509–524 [DOI] [PubMed] [Google Scholar]
- Clermont Y (1972) Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52:198–236 [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, et al. (2008) Generation of pluripotent stem cells from adult human testis. Nature 456:344–349 [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD (2000) All you wanted to know about spermatogonia but were afraid to ask. J Androl 21:776–798 [PubMed] [Google Scholar]
- Dym M, Kokkinaki M, He Z (2009) Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today 87:27–34 [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S (2006) A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction 132:673–680 [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Wistuba J, Schlatt S (2006) Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update 12:275–282 [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, et al. (2009) Pluripotent stem cells derived from adult human testes. Stem Cells Dev 18:1115–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, et al. (2006) Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440:1199–1203 [DOI] [PubMed] [Google Scholar]
- He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M (2010) Isolation, characterization and culture of human spermatogonia. Biol Reprod 82:363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM (2007) Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer 7:246–255 [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Hansel MC, Orwig KE (2010) Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction 139:479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Rajpert-De Meyts E, Daugaard G, Skakkebaek NE (2005) Carcinoma in situ testis, the progenitor of testicular germ cell tumors: a clinical review. Ann Oncol 16:863–868 [DOI] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dym M (2005) Isolation of male germ-line stem cells: influence of GDNF. Dev Biol 279:114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Staub C, Neaves WB, Yanagimachi R (2001) Live human germ cells in the context of their spermatogenic stages. Hum Reprod 16:1575–1582 [DOI] [PubMed] [Google Scholar]
- Jones TD, Ulbright TM, Eble JN, Cheng L (2004) Oct4: a sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res 10:8544–8547 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Shinohara T (2008) Brief history, pitfalls and prospects of mammalian spermatogonial stem cell research. Cold Spring Harb Symp Quant Biol 73:17–23 [DOI] [PubMed] [Google Scholar]
- Keenen B, de la Serna IL (2008) Chromatin remodelling in embryonic stem cells: regulating the balance between pluripotency and differentiation. J Cell Physiol 219:1–7 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Nagoa K, Nakajima K, Miura K, Ishii N (2009) Thy-1+ cells isolated from adult human testicular tissues express human embryonic stem cell genes OCT3/4 and NANOG and may include spermatogonial stem cells. Reprod Med Biol 8:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, et al. (2009) Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells 27:138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoula V, Papamichos SI, Lambropoulos AF (2008) Revisiting OCT4 expression in peripheral blood mononuclear cells. Stem Cells 26:290–291 [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Nielsen JE, Skakkebaek NE, Graem N, Jacobsen GK, Rajpert-De Meyts E, Leffers H (2008) Presumed pluripotency markers UTF-1 and REX-1 are expressed in human adult testes and germ cell neoplasms. Hum Reprod 23:775–782 [DOI] [PubMed] [Google Scholar]
- Kumar N, Hinduja I, Nagvenkar P, Pillai L, Zaveri K, Mukadam L, Telang J, et al. (2009) Derivation and characterization of two genetically unique human embryonic stem cell lines on in-house-derived human feeders. Stem Cells Dev 18:435–445 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Lee J, Kim HK, Rho JY, Han YM, Kim J (2006) The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem 281:33554–33565 [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Welstead GG, Jaenisch R (2008) The pluripotency regulator Oct4: a role in somatic stem cells? Cell Cycle 7:725–728 [DOI] [PubMed] [Google Scholar]
- Liedtke S, Enczmann J, Waclawczyk S, Wernet P, Kogler G (2007) Oct4 and its pseudogenes confuse stem cell research. Cell Stem Cell 1:364–366 [DOI] [PubMed] [Google Scholar]
- Liedtke S, Stephan M, Kogler G (2008) Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem 389:845–850 [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, et al. (2003) POU5F1 (OCT 3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res 63:2244–2250 [PubMed] [Google Scholar]
- Maulitz RC (1978) Rudolf Virchow, Julius Cohnheim and the program of pathology. Bull Hist Med 52:162–182 [PubMed] [Google Scholar]
- Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, et al. (2009) Embryonic stem cell-like cells derived from adult human testis. Hum Reprod 25:158–167 [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24:372–376 [DOI] [PubMed] [Google Scholar]
- Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, Kuwana T, et al. (2003) Identification and characterization of stem cells in prepubertal spermatogenesis in mice small star, filled. Dev Biol 258:209–225 [DOI] [PubMed] [Google Scholar]
- Ohmura M, Yoshida S, Ide Y, Nagamatsu G, Suda T, Ohbo K (2004) Spatial analysis of germ stem cell development in Oct-4/EGFP transgenic mice. Arch Histol Cytol 67:285–296 [DOI] [PubMed] [Google Scholar]
- Pesce M, Scholer HR (2001) Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells 19:271–278 [DOI] [PubMed] [Google Scholar]
- Phanstiel D, Brumbaugh J, Berggren T, Conard K, Feng X, Levenstein ME, McAlister GC, et al. (2008) Mass spectrometry identifies and quantifies 74 unique histone H4 isoforms in differentiating human embryonic stem cells. Proc Natl Acad Sci USA 105:4093–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Hanstein R, Jorgensen N, Graem N, Vogt PH, Skakkebaek NE (2004) Developmental expression of POU5F1 (OCT 3/4) in normal and dysgenetic human gonads. Hum Reprod 19:1338–1344 [DOI] [PubMed] [Google Scholar]
- Ratajczak M (2005) Cancer stem cells—normal stem cells “Jedi” that went over to the “dark side”. Folia Histochem Cytobiol 43:175–181 [PubMed] [Google Scholar]
- Ratajczak MZ, Shin DM, Liu R, Marlicz W, Tarnowski M, Ratajczak J, Kucia M (2010) Epiblast/germ line hypothesis of cancer development revisited: lesson from the presence of Oct-4(+) cells in adult tissues. Stem Cell Rev 6:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L, Ettlin R, Sinha HA, Clegg E (1990) Histological and Histopathological Evaluation of the Testis. Clearwater, FL, Cache River Press
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, et al. (2007) Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature 449:346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha K, Boyer LA (2009) The chromatin signature of pluripotent cells. In StemBook [Internet]. Cambridge, MA, Harvard Stem Cell Institute. doi/10.3824/stembook.1.45.1, http://www.stembook.org [PubMed]
- Takeda J, Seino S, Bell GI (1992) Human Oct3 gene family: cDNA sequences, alternative splicing, gene organization, chromosomal location, and expression at low levels in adult tissues. Nucleic Acids Res 20:4613–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni SK, Modi DN, Pathak SG, Dhabalia JV, Bhartiya D (2009) Stage-specific localization and expression of c-kit in the adult human testis. J Histochem Cytochem 57:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virant-Klun I, Skutella T (2010) Stem cells in aged mammalian ovaries. Aging 2:3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dai J (2010) Isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells 28:885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao Y, Xiao Z, Chen B, Wei Z, Wang B, Zhang J, et al. (2009) Alternative translation of Oct4 by an internal ribosome entry site and its novel function in stress response. Stem Cells 27:1265–1275 [DOI] [PubMed] [Google Scholar]
- Zangrossi S, Marabese M, Broggini M, Giordano R, D'Erasmo M, Montelatici E, Intini D, et al. (2007) Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells 25:1675–1680 [DOI] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ (2009) “Small stem cells” in adult tissues: very small embryonic-like stem cells stand up! Cytometry A 75:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]