Abstract
Several studies in cell cultures and in animal models have demonstrated that cannabinoids have important antitumoral properties. Because many of these effects are mediated through cannabinoid (CB) receptors CB1 and CB2, the study of their expression in human neoplasms has become of great interest in recent years. Fresh and formalin-fixed tissue samples of 20 consecutive clear cell renal cell carcinomas (CCRCCs) were collected prospectively and analyzed for the expression of both CB receptors by using RT-PCR, Western blot (WB), and immunohistochemical techniques. RT-PCR assays demonstrated the expression of mRNA encoding the CB1 in tumor tissue and in adjacent non-neoplastic kidney. Conversely, WB and IHC revealed a marked downregulation of CB1 protein in tumor tissue; CB2 was not expressed. The obtained data suggest a possible implication of the endocannabinoid system in renal carcinogenesis. A posttranscriptional downregulation of CB1 and the absence of expression of CB2 characterize CCRCC. (J Histochem Cytochem 58:1129–1134, 2010)
Keywords: clear cell renal cell carcinoma, cannabinoid receptors, RT-PCR, Western blot, immunohistochemistry
The endocannabinoid system (ECS) consists of cannabinoid (CB) receptors, endocannabinoids, and the enzymes responsible for synthesis and degradation of their endogenous ligands (Mackie 2008). This system is widely distributed in mammalian tissues and regulates nervous, cardiovascular, digestive, reproductive, immune, and metabolic functions by activating CB1 and CB2 receptors (Graham et al. 2009).
There is increasing evidence that endocannabinoids modulate the activity of enzymes and nuclear factors involved in the control of fundamental processes of cell homeostasis and in neoplastic transformation (Flygare and Sander 2008; Pisanti and Bifulco 2009). Actually, numerous pharmacological studies carried out in vitro and in animal models have proposed antitumoral properties to natural and synthetic CBs (Pisanti and Bifulco 2009).
Recent works have reported changes in the expression of the CBs in neoplasms of diverse topographies, such as large bowel (Wang et al. 2008), breast (Caffarel et al. 2006), prostate (Sarfaraz et al. 2005), pancreas (Carracedo et al. 2006; Michalski et al. 2008), and liver (Xu et al. 2006). However, studies in vivo are still scarce in human tissues. In addition, ECS dysregulation needs to be evaluated in other human cancer types (Alpini and DeMorrow 2009).
Very few studies have focused on the ECS in the kidney and its neoplasms. We have found very recently the presence of CB1 and the absence of CB2 receptors in human fetal and adult kidneys (Larrinaga et al. 2010). Other authors (Hart et al. 2004) have studied the effect of CBs on renal cancer cell cultures several years ago.
CB receptors have not been tested to date in renal tumors. They are included in the top ten list of the commonest tumors in males and females and account for ∼4% of adult malignancies (Jemal et al. 2009). Roughly 70% of them are clear cell renal cell carcinomas (CCRCCs) (Lopez Beltran et al. 2009). The 2004 WHO classification of renal tumors in adults links for the first time histology and genetics and attributes the origin of most histological subtypes of renal cancer to distinct parts of the nephron (Lopez Beltran et al. 2006). So, the proximal convoluted tubule cell is considered to be the origin of CCRCC (Lopez Beltran et al. 2006).
Despite the latest advances in genetics, the biology of renal tumors is still far from being completely understood. This study, which includes the analysis of mRNA and protein [Western blot (WB) and IHC] expression of CB in CCRCCs, is an attempt to shed some light on the underlying mechanisms involved in renal neoplasia.
Materials and Methods
All the experiments carried out in this study comply with current Spanish and European Union laws and conform to the principles outlined in the Declaration of Helsinki.
mRNA Expression
An end point PCR assay was used to detect the mRNAs of the CB1 (CNR1) and CB2 (CNR2) receptors and to establish the identity of the amplified products. Fresh renal tissue was obtained from surgical specimens of 20 CCRCCs (16 males, 4 females; mean age: 62 years). Patient consent and Hospital Ethics Committee approval were obtained a priori. Tissue samples were immersed in RNAlater (Ambion; Huntingdon, UK) immediately after dissection and stored at −80C until use.
RT-PCR reactions were performed as previously described (Candenas et al. 2001; Varona et al. 2007; Blanco et al. 2008; Larrinaga et al. 2010). Total RNA of ∼30 mg of human renal tissue was isolated in every case according to the method described by Chomczynski and Sacchi (1987). First-strand cDNA was synthesized from 25 μg of total RNA of each human sample using Moloney murine leukemia virus RT and random hexamers according to the manufacturer's instructions (First-strand cDNA Synthesis Kit; Amersham Biosciences, Essex, UK). The resulting cDNA samples were amplified using PCR with specific oligonucleotide primer pairs designed with the analysis software Primer3 (Rozen and Skaletsky 2000). The sequences of the primer pairs used for human CNR1 and CNR2 are expressed in Table 1. On the basis of previous experiments on human renal cell carcinoma (Jung et al. 2007; Varona et al. 2007; Blanco et al. 2008), the human gene succinate dehydrogenase complex, subunit A (SDHA) was chosen as endogenous reference gene and used to assure that similar amounts of cDNA were used in all RT-PCR reactions. Sequences for SDHA are also displayed in Table 1. All primers were synthesized and purified by Sigma-Genosys (Cambridge, UK). A pool of cDNAs from 20 different human tissues (Human Total RNA Master Panel; Clontech, Mountain View, CA) was used as a positive control of amplification.
Table 1.
Primer sequences for CNR1, CNR2, and SDHA
| Forward | Reverse | |
|---|---|---|
| CNR1 | 5′-AGGGGATGCGAAGGGATT-3′ | 5′-AGTGGTGATGGTGCGGAAG-3′ |
| CNR2 | 5′-CACCCACAACACAACCCAAA-3′ | 5′-AGCCATCCTTGGAGCCATT-3′ |
| SDHA | 5′-TCTGCCCACACCAGCACT-3′ | 5′-CCTCTCCACGACATCCTTCC-3′ |
SDHA, succinate dehydrogenase complex subunit A.
PCR mixes contained 0.2 μmol primers, 1.5 U of heat-activated thermostable DNA polymerase (Immolase; Bioline, London, UK), the buffer supplied, 2.5 mmol MgCl2, 200 μmol dNTPs, and 25 μl cDNA. PCR was performed for 35 cycles with cycling parameters of 15 sec at 94C, 20 sec at 60C, and 20 sec at 72C. The PCR products were separated by agarose gel electrophoresis, and the amplicon sizes were verified by comparison with a DNA mass ladder. mRNA levels for the CB receptors and SDHA were analyzed on each tissue, with each RT-PCR assay being performed in triplicate. The identity of each product was established by DNA sequence analysis.
WB Assays
We performed a WB analysis of eight CCRCCs. Membrane proteins (10 μg) were solubilized in sample buffer and were resolved by electrophoresis in 12% SDS-PAGE gel. Then, proteins were transferred to polyvinylidene difluoride membranes. Blots were blocked in milk and incubated overnight with the CB1 (1:250 dilution; catalog ref. PA1-743, ABR Affinity BioReagents, Golden, CO) and CB2 (1:200 dilution; catalog ref. 101550, Cayman Chemical, Ann Arbor, MI) polyclonal antibodies. Later, the blots were washed and incubated for 2 hr at room temperature with a horseradish peroxidase–conjugated goat anti-rabbit IgG secondary antibody (1:2500 dilution; ABR Affinity BioReagents). Membranes incubated without the primary antiserum were used as negative controls. Immunoreactive bands were visualized using the enhanced chemiluminescence system.
Immunohistochemistry
Archival material obtained from the files of the Department of Anatomic Pathology, University Hospital of Cruces, University of the Basque Country, Barakaldo, Spain, was used for this study. Representative formalin-fixed and paraffin-embedded material obtained from 20 consecutive CCRCCs including cases with conventional histology and Furhman's grades 1–3 (Fuhrman et al. 1982) was analyzed.
Consecutive slides of the selected blocks in every case were immunostained with CB receptors CB1 and CB2 following routine methods in an automatized immunostainer (Dako Autostainer Plus; Dako, Glostrup, Denmark). CB1 (working dilution 1:1000; ABR Affinity BioReagents) and CB2 (working dilution 1:200; Cayman Chemical) polyclonal antibodies were used. The specificity of these antibodies in human cells was previously assessed by our group (Agirregoitia et al. 2010; Larrinaga et al. 2010). Negative control slides were not exposed to the primary antibody and were incubated in PBS and then processed in the same conditions as the test slides. Immunohistochemical slides were studied using a Nikon Eclipse 80i microscope (Tokyo, Japan).
Results
mRNA Expression of CB Receptors
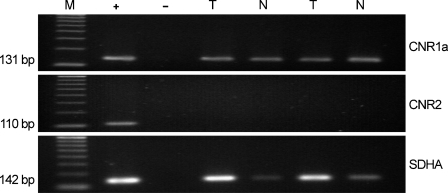
RT-PCR revealed the presence of a single transcript, corresponding to the expected product size encoding the cannabinoid CB1 receptor (131 bp). The identity of the amplified fragment was confirmed by DNA sequence analysis. The CNR1 transcript could be observed in both tumor and surrounding normal tissues assayed after amplification of cDNA for 35 cycles. Conversely, the mRNA encoding the 110-bp product expected for the cannabinoid CB2 receptor was undetectable in cDNA from tumor or normal renal tissues. The mRNAs of CNR1, CNR2, and SDHA genes were all detected in the cDNA pool used as positive control (Figure 1).
Figure 1.
mRNA expression of CB1 receptors in kidney tumor (T) and surrounding normal tissue (N). Agarose gel showing the presence of a single transcript, corresponding to the expected product size encoding the cannabinoid CB1 receptor (131 bp, CNR1). The mRNA encoding the 110-bp product expected for the cannabinoid CB2 receptor (CNR2) was undetectable. The expression of SHDA, used as an internal control, is also shown. M, molecular size standards; +, positive control showing the expression of all genes in a pool of cDNAs from 20 different human tissues; −, negative control with no RNA in the reverse transcriptase reaction.
No PCR product was detectable when the samples were amplified without the RT step, suggesting that genomic DNA contamination was eliminated by DNase treatment. Similarly, no products were detected when the RT-PCR steps were carried out with no added RNA, indicating that all reagents were free of target sequence contamination. Figure 1 shows the negative control with no RNA in the RT reaction.
Protein Expression of CB Receptors
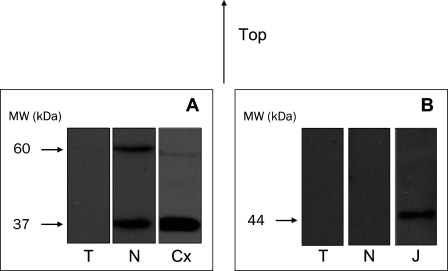
WB assays showed that CB1 receptor expression was practically nonexistent in CCRCC (Figure 2). Two immunoreactive bands were observed in each lane corresponding to normal tissue, one at around 60 kDa, corresponding in size to the putative CB1 monomer, and a lower molecular mass form at around 37 kDa (Figure 2A). The lower band could correspond to a deglycosylation and/or proteolysis product of the 60-kDa-band receptor (De Jesús et al. 2006). The lane on the right shows the presence of similar bands in samples of cerebral cortex, which is used as a positive control for the CB1 receptor (Figure 2A). The CB2 protein was undetectable both in normal and tumor tissues. Positive staining was, however, observed in Jurkat cells, used as a positive control for the CB2 receptor, with the appearance of the expected band at 44 kDa (Figure 2B).
Figure 2.
(A) Western blot analysis of CB1 receptor in human kidney tumor (T) and surrounding normal tissue (N). Lower signal of CB1 in tumor tissue. Cerebral cortex (Cx) is shown as positive control. (B) Western blot of CB2 receptor in tumor and normal tissues. Absence of CB2 in both tumor and normal tissues. Jurkat cells (J) are shown as positive control. Molecular masses (kDa) are indicated on the left.
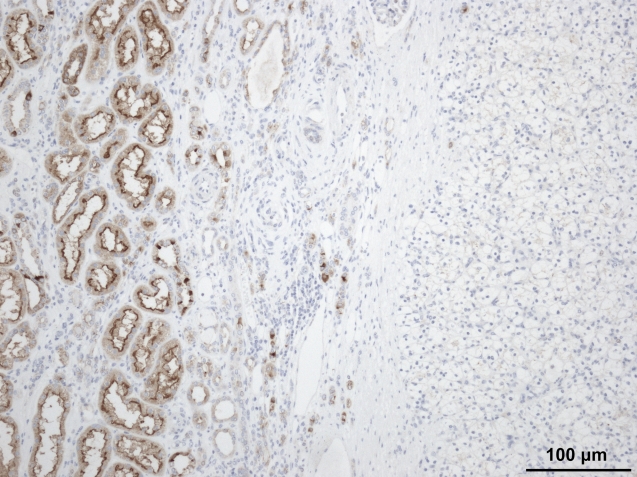
IHC studies corroborated these data. CB1 immunostaining was negative in CCRCC and positive in adjacent normal proximal convoluted tubules (Figure 3), whereas CB2 was absent both in CCRCC and in normal tissue.
Figure 3.
Negative immunostaining for CB1 receptor in clear cell renal cell carcinomas (right side). Note that the surrounding non-tumor tissue shows positive immunoreaction in proximal tubules.
Discussion
In the last decade, several studies in cell cultures and in animal models have demonstrated that CBs have proapoptotic, antiproliferative, antimetastatic, and antiangiogenic effects in various cancer types (Alexander et al. 2009). Although no clinical basis exists to recommend the use of natural/synthetic CBs in patients with renal cancer, this knowledge has spurred a clinical trial examining the efficacy and safety of giving Δ9-tetrahydrocannabinol locally to patients with other neoplasias, such as recurrent glioblastoma multiforme (Guzmán et al. 2006).
Because many of these antitumoral effects of cannabinoids are mediated through CB receptors, the analysis of the expression of CB1 and CB2 receptors in a wide variety of human neoplasms has become of great interest, with some promising results appearing in the recent literature (Flygare and Sander 2008; Alpini and DeMorrow 2009). Although CCRCC is a paradigm of multi–antitumoral-drug-resistant neoplasia, a study on the role of ECS on this tumor is lacking.
As we previously observed in human normal kidneys (Larrinaga et al. 2010), tumor tissues expressed mRNA of the CB1, whereas the mRNA of CB2 was absent. Conversely, there was no correlation between CB1 mRNA and its protein expression because both WB and IHC failed to demonstrate any trait of CB1 protein in tumor tissue. This finding was constant in all the CCRCCs studied, irrespective of the tumor stage and grade. This result suggests that the protein modification of CB1 could occur in these tumors at a posttranscriptional level, thus illustrating the importance of not relying only on mRNA level for the evaluation of any protein change. This assertion has also been indicated by several authors in other kidney diseases (Kasinath et al. 2006).
The CB1 is highly expressed in proximal convoluted tubules of the nephron in both normal adult kidney and mature tubules of fetal kidney (Larrinaga et al. 2010). However, this receptor is not immunohistochemically expressed in the nephrogenic zone of the fetal kidney, where tubular cells are still poorly differentiated (Larrinaga et al. 2010). Interestingly, a parallelism between poorly differentiated tubule cells of fetal kidney and CCRCCs can be raised because CB1 appears downregulated in a renal tumor that supposedly originates in the proximal nephron (Lam et al. 2005; Lopez Beltran et al. 2006). For this reason, we consider that this CB1-related tumor dedifferentiation observed in CCRCCs approaches the tumor to its cell of origin and confirms the current belief on the distinct tumor origin of renal neoplasms expressed in the current WHO classification. Additionally, this immunoprofile may eventually be used as an additional tool with practical interest in the routine diagnosis of CCRCCs.
The precise role of the CB1 in renal cancer still remains to be clarified. It should be noted that a similar CB receptor expression profile has been reported recently in human colorectal cancer by Wang et al. (2008). The authors have suggested that CB1 loss would make cancer cells resistant to the antitumoral effect of endocannabinoids, thus accelerating intestinal tumor growth and biological aggressiveness (Wang et al. 2008). This evidence favors the hypothesis that CB1 downregulation is a general mechanism in some different cancer types. In this sense, Caffarel et al. (2006) also observed downregulation of CB1 in human breast cancer cells. However, CB1 upregulation has also been reported in other human neoplasms, such as prostatic adenocarcinoma (Sarfaraz et al. 2005), hepatocellular carcinoma (Xu et al. 2006), and pancreatic ductal adenocarcinoma (Carracedo et al. 2006; Michalski et al. 2008). Therefore, further studies are needed to clarify the precise mechanisms underlying these apparently divergent results.
Acknowledgments
This work was supported by grants from the Jesús Gangoiti-Barrera Foundation, Gobierno Vasco (GIC07/84), Ministerio de Educación y Ciencia (CTQ2007-61024/BQU), and SAIOTEK (SA-2008/00046).
The authors thank Ana Abascal, Alicia Esteve, and Mar Gonzalez, lab technicians at the Department of Anatomic Pathology, University Hospital of Cruces, University of the Basque Country, Barakaldo, Spain, for their excellent immunohistochemical work.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Agirregoitia E, Carracedo A, Subirán N, Valdivia A, Agirregoitia N, Peralta L, Velasco G, et al. (2010) The CB(2) cannabinoid receptor regulates human sperm cell motility. Fertil Steril 93:1378–1387 [DOI] [PubMed] [Google Scholar]
- Alexander A, Smith PF, Rosengren RJ (2009) Cannabinoids in the treatment of cancer. Cancer Lett 285:6–12 [DOI] [PubMed] [Google Scholar]
- Alpini G, Demorrow S (2009) Changes in the endocannabinoid system may give insight into new and effective treatments for cancer. Vitam Horm 81:469–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L, Larrinaga G, Pérez I, López JI, Gil J, Agirregoitia E, Varona A (2008) Acid, basic and neutral peptidases present different profiles in chromophobe renal cell carcinoma and in oncocytoma. Am J Physiol Renal Physiol 294:F850–858 [DOI] [PubMed] [Google Scholar]
- Caffarel MM, Sarrió D, Palacios J, Guzmán M, Sánchez C (2006) Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res 66:6615–6621 [DOI] [PubMed] [Google Scholar]
- Candenas ML, Magraner J, Armesto CP, Anselmi E, Nieto PM, Martín JD, Advenier C, et al. (2001) Changes in the expression of tachykinin receptors in the rat uterus during the course of pregnancy. Biol Reprod 65:538–543 [DOI] [PubMed] [Google Scholar]
- Carracedo A, Gironella M, Lorente M, Garcia S, Guzmán M, Velasco G, Iovanna JL (2006) Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res 66:6748–6755 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- De Jesús ML, Sallés J, Meana JJ, Callado LF (2006) Characterization of cannabinoid receptor immunoreactivity in postmortem human brain homogenates. Neuroscience 140:635–643 [DOI] [PubMed] [Google Scholar]
- Flygare J, Sander B (2008) The endocannabinoid system in cancer-potential therapeutic target? Semin Cancer Biol 18:176–189 [DOI] [PubMed] [Google Scholar]
- Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6:655–663 [DOI] [PubMed] [Google Scholar]
- Graham ES, Ashton JC, Glass M (2009) Cannabinoid receptors: a brief history and “what's hot”. Front Biosci 14:944–957 [DOI] [PubMed] [Google Scholar]
- Guzmán M, Duarte MJ, Blázquez C, Ravina J, Rosa MC, Galve-Roperh I, Sánchez C, et al. (2006) A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer 95:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart S, Fischer OM, Ullrich A (2004) Cannabinoids induce cancer cell proliferation via tumor necrosis factor alfa-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res 64:1943–1950 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249 [DOI] [PubMed] [Google Scholar]
- Jung M, Ramankulov A, Roigas J, Johannsen M, Ringsdorf M, Kristiansen G, Jung K (2007) In search of suitable reference genes for gene expression studies of human renal cell carcinoma by real-time PCR. BMC Mol Biol 8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinath BS, Mariappan MM, Sataranatarajan K, Lee MJ, Feliers D (2006) mRNA translation: unexplored territory in renal science. J Am Soc Nephrol 17:3281–3292 [DOI] [PubMed] [Google Scholar]
- Lam JS, Leppert JT, Figlin RA, Belldegrun AS (2005) Role of molecular markers in the diagnosis and therapy of renal cell carcinoma. Urology 66:1–9 [DOI] [PubMed] [Google Scholar]
- Larrinaga G, Varona A, Pérez I, Sanz B, Ugalde A, Cándenas ML, Pinto FM, et al. (2010) Expression of cannabinoid receptors in human kidney. Histol Histopathol 25:1133–1138 [DOI] [PubMed] [Google Scholar]
- Lopez Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R (2009) 2009 update on the classification of renal epithelial tumors in adults. Int J Urol 16:432–443 [DOI] [PubMed] [Google Scholar]
- Lopez Beltran A, Scarpelli M, Montironi R, Kirkali Z (2006) 2004 WHO classification of the renal tumors of the adults. Eur Urol 49:798–805 [DOI] [PubMed] [Google Scholar]
- Mackie K (2008) Cannabinoid receptors: where they are and what they do. J Neuroendocrinol 20:10–14 [DOI] [PubMed] [Google Scholar]
- Michalski CW, Oti FE, Erkan M, Sauliunaite D, Bergmann F, Pacher P, Batkai S, et al. (2008) Cannabinoids in pancreatic cancer: correlation with survival and pain. Int J Cancer 122:742–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanti S, Bifulco M (2009) Endocannabinoid system modulation in cancer biology and therapy. Pharmacol Res 60:107–116 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386 [DOI] [PubMed] [Google Scholar]
- Sarfaraz S, Afaq F, Adhami VM, Mukhtar H (2005) Cannabinoid receptor as novel target for the treatment of prostate cancer. Cancer Res 65:1635–1641 [DOI] [PubMed] [Google Scholar]
- Varona A, Blanco L, López JI, Gil J, Agirregoitia E, Irazusta J, Larrinaga G (2007) Altered levels of acid, basic, and neutral peptidase activity and expression in human clear cell renal cell carcinoma. Am J Physiol Renal Physiol 292:F780–788 [DOI] [PubMed] [Google Scholar]
- Wang D, Wang H, Ning W, Backlund MG, Dey SK, DuBois RN (2008) Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res 68:6468–6476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Liu Y, Huang S, Liu G, Xie C, Zhou J, Fan W, et al. (2006) Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet 171:31–38 [DOI] [PubMed] [Google Scholar]