Summary
Epstein-Barr virus-induced gene 3 (EBI3) associates with p28 to form IL-27 and with IL-12p35 to form IL-35. IL-27Rα−/− mice studies indicate IL-27 negatively regulates Th17 cell differentiation. However, no EBI3, p28 or p35-deficiency studies that directly address the role of EBI3, p28 or p35 on Th17 cells have been done. Here, we demonstrate that spleen cells derived from EBI3−/− mice produce significantly higher levels of IL-17 as well as IL-22 upon stimulation with OVA. In vitro derived EBI3−/− Th17 cells also produced significantly higher levels of IL-17 and IL-22 than wild type cells. The frequency of IL-17-producing cells was also elevated when EBI3−/− cells were cultured under Th17 conditions. In addition, spleen cells from EBI3−/− mice immunized with Listeria monocytogenes produced significantly elevated levels of IL-17 and IL-22. Furthermore, the Th17 transcription factor RORγt was significantly enhanced in EBI3−/− cells. Finally, EBI3−/− mice exhibited a reduced bacterial load following an acute challenge with L. monocytogenes or a re-challenge of previously immunized mice, suggesting EBI3 negatively regulates both innate and adaptive immunity. Taken together, these data provide direct evidence that EBI3 negatively regulates the expression of IL-17, IL-22 and RORγt as well as protective immunity against L. monocytogenes.
Keywords: Cytokines, Gene regulation, T helper cells
Introduction
Th17 cells comprise a recently identified subset of CD4+ T cells that are distinct from Th1 and Th2 cells. Th17 cells are characterized by the secretion of IL-17 and IL-22, which are key positive regulators of inflammation [1–3]. The family of IL-17-related cytokines includes IL-17A–F. IL-17A, usually referred to as IL-17, shares a similar structure, chromosomal location, and receptor usage with IL-17F [4]. The expression of IL-17A and IL-17F were originally believed to be regulated by IL-23, a cytokine of the IL-12 family that shares a common subunit, p40 with IL-12 and binds to a common receptor, IL-12Rβ1 [5–7]. However, more recently, the role for IL-23 was found to be important for the survival and expansion of Th17 cells. New studies have demonstrated that the combination of IL-6 and TGF-β induces the differentiation of Th17 cells from naïve CD4+ T cells [8–10]. In addition, the orphan nuclear receptor RORγt has been recently found to be the key transcription factor for the differentiation of Th17 cells [11].
EBV-induced gene-3 (EBI3) encodes a 34-kDa glycosylated protein, homologous to the p40 subunit of IL-12. EBI3 and p28, proteins homologous to the p35 subunit of IL-12, combine to form IL-27 [12;13]. EBI3 and IL-12p35 also form a heterodimer which has been named as IL-35 recently [14–16]. It has been suggested that free EBI3, possibly as a homodimer, acts as an antagonist of IL-27 or EBI3/p35 (IL-35) [12;17]. EBI3-deficient mice exhibit disrupted Th2 responses likely due to altered invariant natural killer T cell function [17]. However, impaired Th1 responses to Leishmania major in EBI3-deficient mice were also reported [18]. More recently, EBI3-deficient mice were reported to display enhanced neutrophil migration and oxidative burst capacity in a murine model of peritonitis, resulting in enhanced bacterial clearance and local control of infection [19].
Most studies describing the role of EBI3/p28 (IL-27) have focused on the regulation of T cell and inflammatory responses. IL-27 induces Th1 responses by activating the Jak/STAT pathway of naïve CD4+ T cells to induce expression of the Th1-specific transcription factor T-bet and the IL-12 receptor β2 chain, thereby conferring responsiveness to IL-12 [20;21]. IL-27 synergizes with IL-12 to drive IFN-γ production in naïve but not memory CD4+ T cells. Subsequent studies using mouse models have shown that the function(s) of IL-27 are broader and more complex, and a role for IL-27 as a positive as well as a negative regulator of T cell responses has emerged. Recent studies using IL-27Rα deficient mice indicate that IL-27 negatively regulates the development of Th17 cells [22;23]. Liew and colleagues recently demonstrated that IL-35 inhibits Th17 cell differentiation [15]. To assess more directly the role for IL-27Rα and the IL-27 family of cytokines as well as IL-35, an evaluation of mice deficient in IL-27, p28 or EBI3 is needed to determine the role of IL-27 or IL-35 on the expression of IL-17 and the differentiation of Th17 cells.
Towards this end, mice deficient for EBI3 were generated and evaluated for alterations in expression of IL-17, IL-22, RORγt, differentiation of Th17 cells, protective immunity and expression of Th1 cytokines. In this study, we demonstrate that EBI3 plays a negative role in IL-17, IL-22 and RORγt expression as well as both innate and adaptive immunity to Listeria monocytogenes.
Results
Enhanced IL-17 expression in EBI3−/− mice
EBI3-deficient mice were generated using a retroviral insertional mutagenesis strategy (Figure 1A) as described in Materials and Methods. These mice were fertile and exhibited no gross anatomical abnormalities (data not shown). In assays to assess neutrophil, macrophage, T cell and B cell mediated responses, the EBI3−/− mice were comparable to wild type mice. These assays included T cell independent antibody responses, LPS challenge, anti-CD3 challenge, antigen specific DTH and contact hypersensitivities (data not shown). EBI3 mRNA was not detected in spleen cells of EBI3-deficient mice (Figure 1C). We also found that EBI3 protein was not detected in EBI3-deficient dendritic cells (Figure 1D).
Figure 1.
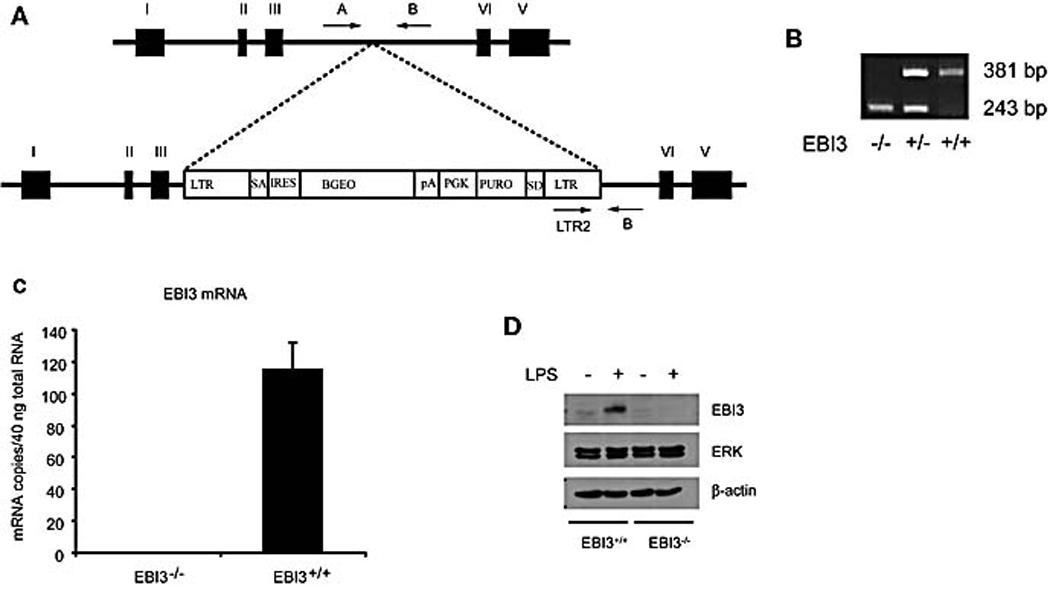
Generation of EBI3 deficient mice. (A) EBI3−/− mice were generated as described in Materials and Methods. (B) For genotyping of EBI3 deficient mice, a set of three PCR primers was used to amplify regions of genomic DNA present in either wild-type or knockout genomic DNA samples as described in Material and Methods. (C) Total RNA was prepared from spleen cells and EBI3 mRNA level was confirmed by real time TaqMan PCR using primers and probe in the region of exon 3 and 4 of EBI3. Data were representative of two experiments. (D) Bone marrow-derived dendritic cells were activated with LPS for 16 hrs and Brefeldin A was added into the culture. Cells were then incubated for another 6 hrs. Western blot for EBI3, ERK and β-actin were performed. Data were representative of two experiments.
IL-27Rα deficient mice studies indicate that IL-27 negatively regulates the development of Th17 cells [22;23]. However, there is no IL-27 (EBI3 or p28) deficiency study to directly prove the role of IL-27 on the regulation of IL-17 and Th17 cells. To analyze further the role of EBI3 in the induction of IL-17, we generated EBI3−/− mice bearing the DO11.10 TCR transgene. Spleen cells from these mice were stimulated with OVA for 48 hours and the supernatants were harvested to measure IL-17. EBI3−/− spleen cells produced much higher levels of IL-17 than wild type cells (Figure 2A). Moreover, a significant increase in the level of IL-17 mRNA was observed after EBI3−/− spleen cells were stimulated with anti-CD3 plus IL-6 and TGFβ for 4 hours (data not shown). To analyze further the characteristics of the IL-17 secreting cells, splenocytes from EBI3−/− mice on the DO11.10 background were cultured under conditions that favor the differentiation to Th0 or Th17 cells for 7 days. IL-17 levels were then measured following a re-stimulation with anti-CD3. After anti-CD3 stimulation EBI3−/− Th17 cells secreted more IL-17 compared to wild type controls (Figure 2B). To determine the effect of EBI3 deficiency on the proportion of cells that express IL-17 intracellular staining was performed on both Th0 and Th17 differentiated cells. No effect of EBI3 deficiency was observed on the proportion of IL-17-producing Th0 cells. However when the cells were polarized under Th17 conditions, 24.8 % of the EBI3 deficient cells was IL-17 positive whereas 15.1 % of the wild type derived cells were IL-17 positive (Figure 2C). Cells were also differentiated under Th1 and Th2 conditions. EBI3-deficiency did not significantly alter the ability of cells to differentiate to a Th1 or Th2 phenotype (data not shown). In summary, EBI3 exhibits a negative effect on the differentiation of Th17 cells and on the expression of IL-17.
Figure 2.

EBI3-deficient T cells on DO11.10 background demonstrate increase in IL-17 expression and the number of IL-17-producing cells in vitro. (A) Wild type or EBI3−/− DO11.10 splenocytes were stimulated with OVA for 48 hours and the supernatants were harvested for detection of IL-17. *, p < 0.01 vs EBI3 deficient cells. (B) Wild type or EBI3−/− DO11.10 splenocytes were differentiated under Th0 or Th17 condition for 7 days. Cells were then harvested and restimulated with anti-CD3 for 24 hours. The supernatants were used to determine the titer of IL-17. *, p < 0.01 vs EBI3 deficient cells. (C) Wild type or EBI3−/− DO11.10 splenocytes were differentiated under Th0 or Th17 conditions for 7 days. Cells were then harvested and restimulated with PMA/ionomycin for 6 hours. Intracellular staining for IL-17 was performed. Data are representative of three to four experiments.
IL-17 was shown to be involved in host defense and protective immunity [4]. LFA-1-deficient mice have been shown to be resistant to Listeriosis. Serum levels of IL-17 as well as G-CSF were elevated in LFA-1-deficient mice. The resistance to Listeria infection in LFA-1-deficient mice was suggested to be due to the increased G-CSF and IL-17 [24]. Thus, we asked whether EBI3 deficiency could affect the expression of IL-17 in mice immunized with L. monocytogenes. Mice on the 129/C57BL/6 background were used for the L. monocytogenes studies. Spleen cells were prepared from wild type and EBI3−/− mice immunized intravenously with viable L. monocytogenes (2 × 103 CFU) for 7 days. Cells were then stimulated with heat-killed L. monocytogenes for 4 hours or 24 hours to analyze mRNA and protein expression respectively. Spleen cells derived from EBI3−/− mice exhibited a 6-fold elevation of IL-17 mRNA following stimulation with L. monocytogenes (Figure 3A). Furthermore, EBI3−/− spleen cells produced higher levels of IL-17 protein than wild type cells after restimulation with heat-killed L. monocytogenes (Figure 3B).
Figure 3.
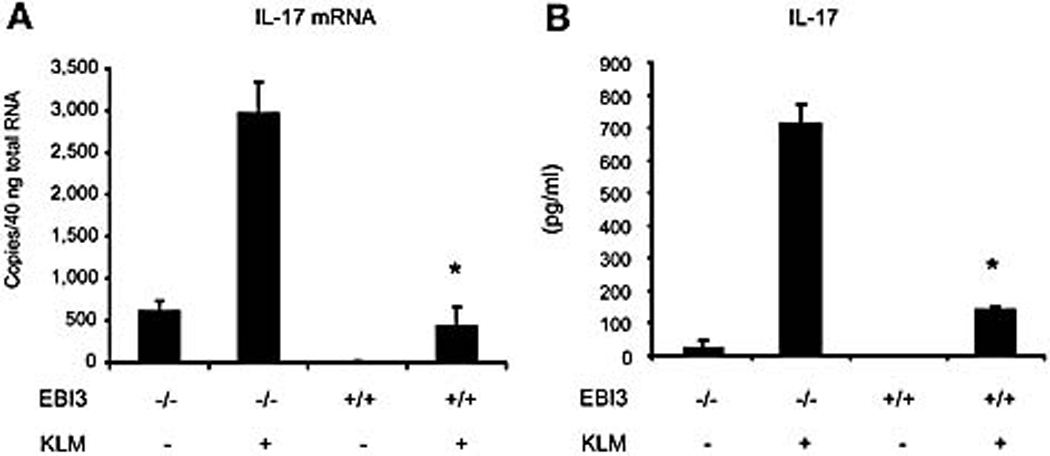
EBI3 deficiency enhanced IL-17 expression by spleen cells from mice immunized with L. monocytogenes. (A) Spleen cells were prepared from wild type and EBI3−/− mice on the 129/ C57BL/6 background immunized intravenously with viable L. monocytogenes (2 × 103 CFU) for one week. Cells were stimulated with KLM for 4 hrs before total RNA extraction. TaqMan RT-PCR was performed for IL-17 mRNA expression. *, p < 0.01 vs EBI3 deficient cells. (B) Cells from Figure 3A were stimulated with KLM for 24 hrs and an IL-17 ELISA was performed. *, p < 0.01 vs EBI3 deficient cells. Data are representative of three experiments.
EBI3 deficiency enhances IL-22 expression
It was reported that both IL-22 and IL-17 are produced by Th17 cells. IL-22 in combination with IL-17A or IL-17F cooperatively enhances the expression of antimicrobial peptides [2]. IL-23 is involved in in vivo Th17 generation [5–7] and IL-22 mediates IL-23-induced dermal inflammation [3]. However, the production of IL-22 and IL-17 from TH17 cells is differentially regulated [3]. Thus, we asked whether EBI3 is involved in IL-22 regulation. Figure 4A shows that EBI3−/− DO11.10 spleen cells stimulated with OVA for 48 hours produced significantly higher levels of IL-22. Cells were then differentiated under Th17 conditions for 1 week and the IL-22 production in the supernatants were determined after the cells were restimulated for 24 hours. EBI3 deficiency enhanced IL-22 production by Th17 cells (Figure 4B). Finally, we determined the IL-22 production from spleen cells of mice immunized with L. monocytogenes. As shown in Figure 4C, there is a significant increase in IL-22 production by EBI3−/− spleen cells from L. monocytogenes immunized mice. Thus, EBI3 also negatively regulates IL-22 production.
Figure 4.

Enhanced IL-22 expression by spleen cells from EBI3−/− mice. (A) Cells and supernatants were prepared as in Figure 2A and IL-22 titers in the supernatants were determined by ELISA. (B) Cells and supernatants were prepared as in Figure 2B and IL-22 titers in the supernatants were determined by ELISA. (C) Cells and supernatants were prepared as in Figure 3B and IL-22 titers in the supernatants were determined by ELISA. *, p < 0.01 vs EBI3 deficient cells. Data are representative of three experiments.
Increased RORγt expression in EBI3−/− cells
Recently, Littman and colleagues demonstrated that the orphan nuclear receptor RORγt is the key transcription factor for the differentiation of Th17 cells [11]. RORγt induces transcription of the genes encoding IL-17 and the related cytokine IL-17F in naïve CD4+ T cells. RORγt-deficient mice display attenuated autoimmune disease and lack tissue-infiltrating Th17 cells. Given the negative effect of EBI3 on IL-17 expression, we asked if EBI3 deficiency could enhance the expression of RORγt. Indeed, RORγt mRNA expression was significantly higher in spleen cells from EBI3−/− mice immunized with L. monocytogenes (Figure 5A). In addition, EBI3−/− spleen cells on a DO11.10 background also expressed significantly increased levels of RORγt mRNA (Figure 5B). Thus, EBI3 is a negative regulator of RORγt.
Figure 5.

EBI3−/− cells demonstrate enhanced RORγt mRNA expression. (A) Wild type or EBI3−/− mice on the 129/C57BL/6 background were immunized with L. monocytogenes for 1 week and the spleen cells were stimulated with KLM for 4 hours. Cells were then harvested for RNA extraction and TaqMan RT-PCR. (B) Wild type or EBI3−/− mice on DO11.10 background were stimulated with anti-CD3 for 4 hours and TaqMan RT-PCR was performed. *, p < 0.01 vs EBI3 deficient cells. Data are representative of three experiments.
IL-27 inhibits the expression of IL-17, IL-22 and RORγt
EBI3 and p28 forms the cytokine IL-27. IL-27 was shown to inhibit the expression of IL-17. It is interesting to know whether IL-27 could also inhibit the expression of IL-22 and RORγt . Thus, the effects of IL-17, IL-22 and RORγt by IL-27 were studied. Naïve CD4+ T cells were differentiated under Th17 conditions with or without addition of recombinant IL-27 for 5 days. Cells were then stimulated with PMA and Inomycin for 5 hours before RT-PCR was performed. We found that IL-27 inhibited the expression of IL-17, IL-22 and RORγt (Figure 6).
Figure 6.
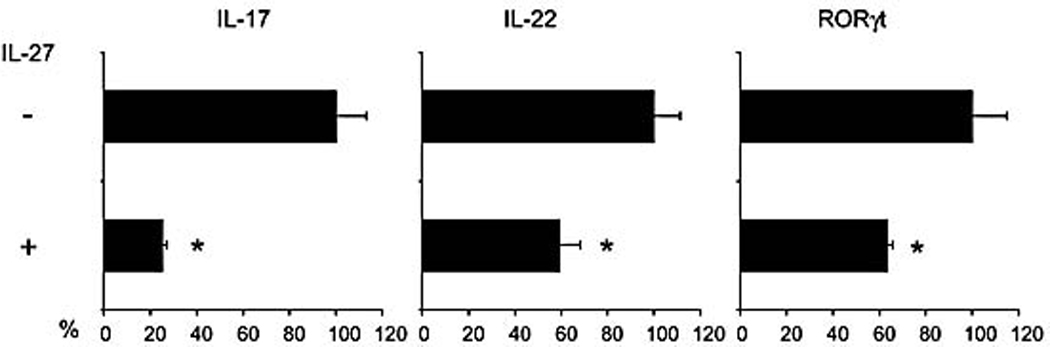
IL-27 inhibits the expression of IL-17, IL-22 and RORγt. Naïve CD4+ T cells were differentiated under Th17 conditions with or without addition of recombinant IL-27 (10 ng/ml) for 5 days. Cells were then restimulated with PMA/Inomycin for 5 hours and RT-PCR was performed. Data were the percentage of inhibition by IL-27. *, p < 0.05 vs none IL-27 treated Th17 cells. Data are representative of two experiments.
EBI3−/− mice are resistant to L. monocytogenes infection
Since EBI3 deficiency enhanced IL-17 expression and IL-17 was shown to be involved in host defense and protective immunity [4], EBI3 may play an important negative role in protective immunity against L. monocytogenes. We examined the course of L. monocytogenes infection in EBI3−/− and wild type mice. EBI3−/− and wild type mice were infected intravenously with 2 × 103 CFU of L. monocytogenes and the splenic bacterial load was determined 48 hours post injection. Unexpectedly, EBI3−/− mice exhibited approximately 45-fold less bacteria in spleen than wild type mice (Figure 7A), suggesting that EBI3 plays a negative role in innate immunity to L. monocytogenes. To address the role of EBI3 in adaptive immunity to L. monocytogenes infection, mice were immunized with 2 × 103 CFU of L. monocytogenes and then challenged with 2 × 106 CFU of L. monocytogenes seven days later. EBI3-deficient mice again showed a 150-fold less bacterial load in livers after re-challenge (Figure 7B), suggesting that EBI3 also plays a negative role in adaptive immunity.
Figure 7.

EBI3−/− mice are resistant to L. monocytogenes infection. (A) EBI3−/− and wild type mice were intravenously infected with 2 ×103 CFU of L. monocytogenes for two days and the bacterial load in spleens was determined. *, p < 0.004 vs wild type mice. (B)EBI3−/− and wild type mice were intravenously immunized with 2 ×103 CFU of L. monocytogenes for 7 days. The mice were intravenously challenged with 2 ×106 CFU of L. monocytogenes for 2 days and the bacterial load in livers was determined. *, p < 0.0003 vs wild type mice. Data are representative of three experiments.
EBI3 has no effect on the expression of IFNγ and TNFα as well as the population of Foxp3+CD25+ regulatory T cells
IFN-γ and TNF-α are Th1 cytokines critical for protective immunity against L. monocytogenes [25]. The decreased bacterial load observed in EBI3-deficient mice could be due to the enhanced production of IFN-γ and TNFα. To test this possibility, spleen cells from mice immunized with L. monocytogenes were re-stimulated with heat-killed L. monocytogenes and analyze for IFN-γ and TNF-α mRNA expression and cytokine production. No significant changes in either IFN-γ or TNF-α production were found in cells from EBI3−/− mice compared to wild type mice (Figure 8A, B). Analysis of IFN-γ and TNF-α mRNA corroborated these findings (data not shown). The unaltered expression of IFN-γ and TNF-α was also reported in a study of EBI3−/− mice subjected to cecal ligation and puncture surgery [19]. We also determined the titers of IFN-γ and IL-17 in sera after wild type and EBI3-deficient mice were intravenously infected with 2 × 104 CFU of L. monocytogenes for 24 hours. Similar levels of IFN-γ were found from sera of wild type and EBI3-deficient mice. However, IL-17 titers were higher in sera of EBI3-deficient mice (data not shown). These results suggest that IFN-γ and TNF-α do not contribute to the enhanced protective immunity in EBI3−/− mice.
Figure 8.
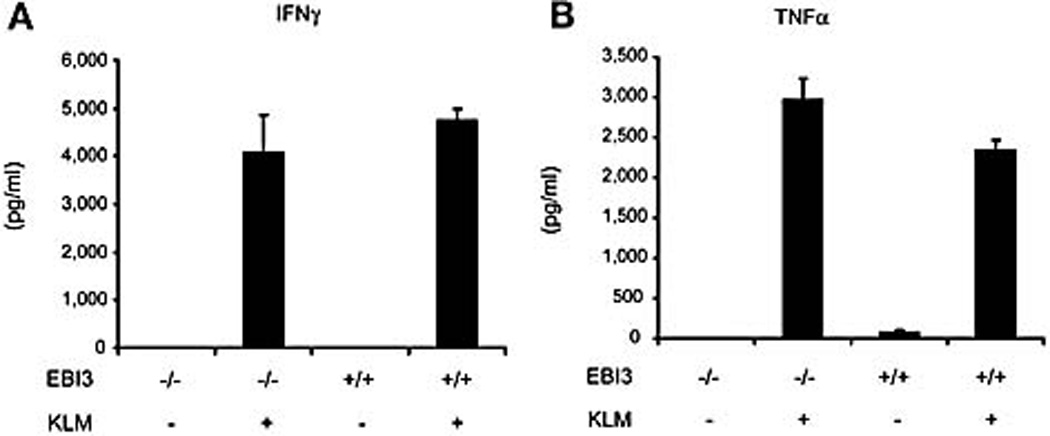
Unimpaired IFNγ and TNFα production in EBI3−/− mice. Spleen cells were prepared from wild type and EBI3−/− mice immunized intravenously with viable L. monocytogenes (2 × 103 CFU) for one week. Cells were stimulated with heat-killed L. monocytogenes (KLM) for 24 hours and supernatants were harvested. The titers of IFN-γ and TNFα in the supernatants were determined by CBA. Data are representative of two experiments.
In addition to effector subsets, CD4+ T cells can differentiate into distinct regulatory subsets characterized by their ability to suppress adaptive T cell responses and to regulate autoimmunity [26]. The above data show that the IL-17 expression was enhanced in EBI3−/− mice. To determine if the enhanced IL-17 expression in EBI3−/− mice was due to impaired differentiation of regulatory T cells, activated spleen cells from L. monocytogenes immunized EBI3−/− mice were evaluated for regulatory T cell marker expression by flow cytometry. Foxp3 is a master transcription factor of CD4+CD25+ regulatory T cells [26]. The percentage of Foxp3+CD25+ T cells from wild type and EBI3−/− mice was unchanged (Figure 9A). In addition, we also measured the mRNA expression of Foxp3. Spleen cells from EBI3−/− and wild type mice immunized with L. monocytogenes were restimulated with heat-killed L. monocytogenes and Foxp3 mRNA was analyzed by quantitative RT-PCR. Cells from EBI3-deficient mice expressed equivalent levels of Foxp3 relative to those from wild type mice (Figure 9B). Cell activation might enhance the expression of many transcription factors including Foxp3. Here, the Foxp3 mRNA and protein level were increased after cells were stimulated with heat-killed L. monocytogenes (Figure 9A and 9B). Consistent with these data, Ghilardi and colleagues reported that IL-27 specifically suppressed IL-6-induced proliferation of CD4+CD25− effector but not regulatory T cells [22]. Thus, the enhanced IL-17 expression in EBI3−/− mice is unlikely due to an inhibition of the population of Foxp3+CD25+ regulatory T cells.
Figure 9.

EBI3 deficiency does not affect the Foxp3+CD25+ regulatory T cell population nor Foxp3 mRNA expression. (A) Splenocytes were prepared as in Fig. 3 and stimulated with KLM for 24 hours. The Foxp3+ and CD25+ population was then analyzed by FACS. (B) Cells and RNA were prepared as in Fig. 3. TaqMan RT-PCR was performed for the expression of Foxp3 mRNA. Data are representative of two experiments.
Discussion
This study describes a role of EBI3 as a negative regulator of IL-17 expression. We demonstrated that spleen cells from EBI3-deficient mice on a DO11.10 background produced significantly higher levels of IL-17 upon stimulation with OVA. In addition, spleen cells from EBI3-deficient mice immunized with L. monocytogenes also produced significantly elevated levels of IL-17 upon restimulation with heat-killed L. monocytogenes. Furthermore, in vitro derived EBI3-deficient Th17 cells produced significantly higher levels of IL-17 expression than wild type cells. The frequency of IL-17-producing cells was also elevated when EBI3-deficient cells were cultured under conditions which favor the development of Th17 cells. Furthermore, EBI3 deficiency significantly enhanced the Th17 transcription factor RORγt mRNA expression. IL-22 is believed to be another Th17 cytokine [2]. We showed that IL-22 expression is enhanced in EBI3−/− spleen cells. IFN-γ, which has been shown to inhibit Th17 cell development [5–7], is induced by L. monocytogenes both in vivo and in vitro. Therefore, the enhanced IL-17 production by cells from EBI3−/− mice could be due to the decreased production of IFN-γ. However, no decrease in IFN-γ expression was observed (Figure 8A). To further analyze the role of IFN-γ in EBI3−/− mice, we treated spleen cells from L. monocytogenes immunized EBI3−/− mice with an IFN-γ neutralizing antibody. We found that neutralizing IFN-γ did not affect IL-17 production (data not shown), suggesting that IFN-γ does not affect the enhanced IL-17 expression observed in EBI3−/− mice. Therefore, IFN-γ does not play an important role in EBI3-induced inhibition of IL-17 expression. We determined the expression of IL-12/IL23 p40 and IL-23 p19 in wild type and EBI3-deficient peritoneal macrophages. The expressions of p40 and p19 were similar between wild type and EBI3-deficient peritoneal macrophages (data not shown). Thus, EBI3 do not affect p40 and p19 expression. The enhanced IL-17 expression in EBI3−/− mice is unlikely dependent on regulatory T cells since EBI3 deficiency does not alter the population of Foxp3+CD25+ T regulatory cells as well as the mRNA level of Foxp3 (Figure 9). Upon challenge with L. monocytogenes we observed that EBI3-deficient mice display a 45-fold less bacterial load in spleens compared to wild type mice. We also discovered that EBI3-deficient mice have 150-fold less bacterial load in livers after L. monocytogenes re-challenge. Together, these data suggest that EBI3 negatively regulates the expression of IL-17, IL-22 and RORγt as well as innate and adaptive immunity to L. monocytogenes infection.
Previous studies indicate that the protective immunity against Leishmania major and L. monocytogenes are dependent on Th1 immune response. Our current study indicates that EBI3 may play different role in the immunity to L. major and L. monocytogenes. Zahn et al reported that EBI3-deficient mice showed impaired Th1 response and impaired protection to L. major at the early stage when mice were challenged with low doses of L. major. However, at the late stage of infection, enhanced Th1 cytokine IFN-γ and reduced Th2 cytokine IL-4 production were observed. Lesion in wild type and EBI3-deficient mice resolved at late stage [18]. This phenomenon may be due to the involvement of IL-27 on the production of naïve CD4+ T cells at the early stage of Th1 differentiation [12]. Rosas et al reported that IL-27R−/− mice display enhanced resistance to Leishmania donovani [27]. Thus, the mechanism of EBI3 and IL-27 in the immunity to Leishmania and Listeria should be further clarified.
Th17 cells are a distinct subset of T cells linked to the production of IL-17 [1] and IL-22 [2]. It has been established that aberrant responses of these Th17 cells are associated with autoimmunity. The Th17 response has been associated with the development of disease in animals models of multiple sclerosis, inflammatory bowel diseases and rheumatoid arthritis [1]. For example, the number of Th17 cells needed to induce experimental autoimmune encephalomyelitis (EAE) is much lower than that of Th1 cells [6]. The role of Th17 in controlling intracellular bacteria infection has not been clearly established, however Th17 cells are reported to be involved in acute resistance to Toxoplasma gondii [28]. L. monocytogenes is a facultative bacterium which induces a characteristic Th1 immune response in vivo. In the present study we found L. monocytogenes infection induced IL-17 expression and EBI3-deficiency significantly enhanced IL-17 production. Our results indicate that IL-17 is involved in the immune defense against L. monocytogenes.
IL-27 has been demonstrated to play a regulatory role in other inflammatory scenarios. Karin and colleagues demonstrated that neutralizing the function of the p28 subunit of IL-27 suppressed ongoing EAE as well as adjuvant-induced arthritis, suggesting a pro-inflammatory role for IL-27 in these models [29;30]. Recently, two papers demonstrated an involvement of IL-27 in the generation of Th17 cells by using IL-27Rα-deficient mice. The IL-27 receptor consists of a unique chain IL-27Rα (also called TCCR and WSX-1) and gp130, a common receptor chain also used by several other cytokines including IL-6 [13]. Hunter and colleagues found that IL-27Rα-deficient mice developed severe neuroinflammation after the mice were chronically infected with Toxoplasma gondii. The inflammation was dependent on CD4+ T cells and associated with a prominent IL-17 response [23]. They also found that the p28 subunit of IL-27 alone suppressed IL-17 production, although not as efficiently as IL-27[23]. Ghilardi and colleagues discovered that IL-27Rα-deficient mice exhibited increased susceptibility to EAE and generated more IL-17-producing T cells than wild type mice. In addition, they found that IL-27 in vitro acted directly on effector T cells to suppress the development of IL-17-producing T helper cells driven by IL-6 and TGF-β [22]. These results indicate but not directly prove a negative regulatory role of IL-27 in controlling Th17 immune responses in vivo. IL-27-deficient mice are required to fully understand the mechanism. Here we report the generation of mice deficient for EBI3 and demonstrate that this component of IL-27 is a negative regulator of IL-17. It is also important that we, to our knowledge, are the first to demonstrate that EBI3 also negatively regulates the expression of IL-22 and RORγt.
Several functions of IL-35 (EBI3/p35) have been recently uncovered [15;16]. Liew and colleagues demonstrated that IL-35 suppresses the differentiation of Th17 cell as well as the proliferation of CD4+CD25− effector T cells [15]. Our current EBI3-deficient findings are in consistent with the role of IL-35 on Th17 differentiation. They also demonstrated that the in vitro IL-35-expanded CD4+CD25+ Treg cells retained their suppressive functions against CD4+CD25− effector cells [15]. Vignali and colleagues demonstrated that Treg cells produce IL-35 and FACS-purified EBI3-deficient Treg cells are functionally defective [16]. Here, we showed that EBI3-deficiency did not affect the population of the Foxp3+CD25+ Treg cell from spleens of mice immunized with L. monocytogenes. Thus, the detail mechanism regarding the role of EBI3 in the regulation of the population and function of Treg cells in different disease models needs to be further evaluated.
Finally, the role EBI3 homodimer in protective immunity, IL-17, IL-22 and RORγt expression and Th17 differentiation remain unclear. A better understanding of the biology of individual subunits of EBI3, p28 and p35 may provide insight into IL-17-, IL-22- and Th17-mediated regulation of the immune system in vivo. Here we take the first step and demonstrate that EBI3 is an important negative regulator of IL-17, IL-22 and RORγt expression as well as protective immunity against L. monocytogenes.
Materials and Methods
Mice
EBI3+/− founder mice were created at Lexicon Genetics, Inc. (The Woodlands, TX) by gene trapping using random insertional mutagenesis with retroviral vector VICTR23 as described by Zambrowicz et al [31]. The integration site of the targeting cassette is located in the intronic sequence between exon 3 and exon 4 (Figure 1A). EBI3−/− mice were mated to establish our colony in the C57BL/6 albino × 129Sv/Ev mixed genetic background in Boehringer Ingelheim Pharmaceuticals, Inc. The EBI3−/− mice were also maintained in Mount Sinai Medical School. Wild type 129/B6 F2 mice were purchased from Jackson Laboratory (Bar Harbor, Maine). EBI3+/− mice were also backcrossed to DO11.10 TCR transgenic mice [32] for ten generations before obtaining EBI3−/− mice on DO11.10 background. For genotyping of EBI3 deficient mice, a set of three PCR primers was used to amplify regions of genomic DNA present in either wild-type or knockout genomic DNA samples. The following primers were used. A single antisense primer (EBI3’(B): 5’ CTCATTCATTCACATGTTCGC-TTT 3’) corresponding to intron sequence downstream of the insertion site; Two sense primers, one corresponding to intron sequence upstream of the insertion (EBI5’(A): 5’ TACATGAAGGCCATGAGTAAATGA 3’) and the other (LTR2: 5’ AAATGGC-GTTACTTAAGCTAGCTTGC 3’) within the 3′ LTR sequence of the vector VICTR23). The primer pair EBI5’ (A)/EBI3’(B) amplifies a wild type-specific product of 381 base pairs, while the primer pair LTR2/ EBI3’(B) amplifies a mutant-specific fragment of 243 base pairs (Figure 1A and 1B). EBI3 mRNA level was confirmed by real time TaqMan RT-PCR. The animal study protocols were approved by Boehringer Ingelheim Pharmaceutical Animal Care Committee and Mount Sinai Institutional Animal Care and Use Committee.
L. monocyogenes and Immunization of mice
L. monocyogenes 104035, a virulent strain, was used throughout the study. The bacteria were grown in brain heart infusion at 37°C for 16 hrs, washed repeatedly, suspended in phosphate-buffered saline, stored at –80°C until used. Killed cells of L. monocytogenes were prepared by heating the viable bacterial suspension of a known concentration at 74°C for 90 min. Mice were immunized by an intravenous injection with 2 × 103 viable L. monocytogenes cells. One week after the immunization, spleens were removed and single cell suspensions were prepared for further studies.
Cell culture
Spleen cells from wild type or EBI3−/− spleen cells were suspended at 5 × 106 cells per ml in 10% FBS IMDM and stimulated with OVA (500 µg/ml) or heat-killed L. monocytogenes as described in the text. Differentiation of wild type or EBI3−/− DO11.10 T cells to Th17 phenotypes were carried out as follows. Splenic T cells were activated by OVA (500 µg/ml) in the presence of recombinant IL-2 (2 ng/ml). IL-6 (20 ng/ml) and human TGFβ (3ng/ml) (all from R&D systems, Minneapolis, MN) were added into the culture for Th17 development, no additional cytokines were added for Th0 development. One week after the stimulation, cells were harvested and resupended at 1 × 106 cells /ml. Cell were then restimulated with plate-bound anti-CD3 stimulation for 24 hours or PMA/ionomycin for 6 hours.
Cytometric Bead Array and ELISA
The cytokine titers in the supernatants were analyzed using the BD cytometric bead array (CBA) as described previously [33]. The mouse Th1/Th2 Cytokine CBA kits were used (BD Biosciences, San Diego, CA). Briefly, 25 µl of each sample or standard dilutions was mixed with 25 µl of mixed capture beads and 25 µl of the mouse Th1/Th2 PE detection reagent. After the samples were incubated at room temperature for 2 hours in the dark, they were washed once and resuspended in 200 µl of wash buffer before acquisition on a FACScan. Data was analyzed using the CBA software. The concentration of each cytokine in the supernatants was calculated with the corresponding standard curve. IL-17 titers in the supernatants were determined by ELISA according to the manufacture’s procedures (R&D systems).
RNA extraction and quantitative RT-PCR
Total RNA was extracted using the RNeasy Mini Kit (QIAGEN, Valencia, CA). The possible remaining DNA was digested with the RNase-Free DNase I (QIAGEN) during the extraction of total RNA, cDNA synthesis, TaqMan quantitative RT-PCR and GeneChip analysis were described previously [33]. TaqMan RT-PCRs were performed using ABI 7700 to measure mRNA expression level. The TaqMan copy numbers are calculated as the copy numbers generated by using standard curve and normalized to endogenous GAPDH [34]. TaqMan primers and probes are as follows. Murine GAPDH: mGAPDH-864F: GCTACACTGAGGA-CCAGGTTGTCT, mGAPDH-980R: ACCAGGAAATGAGCTTGACAAAGT and mGAPDH-898T FAM MGB probe: CAACAGCAACTCCCACTCTTCCACCTTC. Murine EBI3 (exon 3 and 4), FOXP3, IFNγ, TNFα, and IL-4 TaqMan primers and probes were purchased from Applied Biosystems (Foster City, CA). Murine RORγt TaqMan primers and probes were described by Littman and colleagues [11] and were purchased from Applied Biosystems.
Western blot
Mouse bone marrow derived dendritic cells were generated from bone marrow stem cells obtained from the femurs of the mice as described previously [35]. After lysis of the red blood cells, 1 × 106 bone marrow cells were inoculated in each well of 24-well plates with complete DMEM in the presence of GM-CSF (10 ng/ml) for 7 days. Bone marrow-derived dendritic cells were activated with LPS for 16 hrs and Brededin A was added and cells were incubated for another 6 hrs. The whole cell lysates were collected. Cell lysates and prestained molecular weight markers were subjected to SDS-PAGE and transferred to nitrocellulose. The membranes were blocked with 5% nonfat milk in TBST (Triton X-100 0.5%, Tris-buffered saline), incubated with antibodies to EBI3, ERK and β-acting (Santa Cruz) for 2 hrs, washed with TBST, and stained with peroxidase-conjugated IgG second antibody (1:5000). Immunoreactivity was visualized by enhanced chemiluminescence (ECL kit, Santa Cruz). All antibodies were purchased from Cell Signaling (Santa Cruz).
Intracellular staining and flow cytometry
Cells were stimulated with PMA and ionomycin overnight, Brefeldin A was added into the culture for an additional 2 hours prior to harvesting for intracellular staining. Cells were fixed with IC Fixation Buffer (eBioscience), permeabilized using Permeabilization buffer (eBioscience) and stained with PE-anti-mouse IL-17 and Cy5-anti-mouse CD4 antibodies (eBioscience). Flow cytometry was performed on a FACScan.
Statistical analysis
Statistical analysis was performed using Student's t-Test by Microsoft Excel. p values < 0.05 were considered significant.
Acknowledgements
We thank Dr. Jeanne Magram for critical review of the manuscript. We also thank Lynn Pantages-Torok and Mary McFarland at the Department of Translational Science in Boehringer Ingelheim for animal breeding and genotyping. Dr. Huabao Xiong was supported by NIH grant P01 DK072201, Crohn’s and Colitis Foundation of America, and the Eli and Edythe L. Broad Foundation.
Footnotes
Conflict of interest: The authors declare no financial interest
References
- 1.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J.Exp.Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 4.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal MR, Rennick D, Kastelein RA. IL-27 a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 13.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal MR, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 14.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U.S A. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur.J.Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 16.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S, Glickman J, Bailey D, Yoshida M, Galle PR, Kronenberg M, Birkenbach M, Blumberg RS. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc.Natl.Acad.Sci.U.S.A. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zahn S, Wirtz S, Birkenbach M, Blumberg RS, Neurath MF, von Stebut E. Impaired Th1 responses in mice deficient in Epstein-Barr virus-induced gene 3 and challenged with physiological doses of Leishmania major. Eur.J.Immunol. 2005;35:1106–1112. doi: 10.1002/eji.200425926. [DOI] [PubMed] [Google Scholar]
- 19.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J.Exp.Med. 2006;203:1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, Saris CJ, Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 21.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U.S.A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 23.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto M, Emoto M, Emoto Y, Brinkmann V, Yoshizawa I, Seiler P, Aichele P, Kita E, Kaufmann SH. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J.Immunol. 2003;170:5228–5234. doi: 10.4049/jimmunol.170.10.5228. [DOI] [PubMed] [Google Scholar]
- 25.Freeman MM, Ziegler HK. Simultaneous Th1-type cytokine expression is a signature of peritoneal CD4+ lymphocytes responding to infection with Listeria monocytogenes. J.Immunol. 2005;175:394–403. doi: 10.4049/jimmunol.175.1.394. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu.Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 27.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am.J.Pathol. 2006;168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:6465–6471. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:1171–1178. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 31.Zambrowicz BP, Friedrich GA, Buxton EC, Lilleberg SL, Person C, Sands AT. Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells. Nature. 1998;392:608–611. doi: 10.1038/33423. [DOI] [PubMed] [Google Scholar]
- 32.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Castle BE, Hanidu A, Stevens L, Yu Y, Li X, Stearns C, Papov V, Rajotte D, Li J. Sphingosine Kinase 1 Is a Negative Regulator of CD4+ Th1 Cells. J.Immunol. 2005;175:6580–6588. doi: 10.4049/jimmunol.175.10.6580. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Wang X. Application of real-time polymerase chain reaction for the quantitation of interleukin-1beta mRNA upregulation in brain ischemic tolerance. Brain Res.Brain Res.Protoc. 2000;5:211–217. doi: 10.1016/s1385-299x(00)00015-5. [DOI] [PubMed] [Google Scholar]
- 35.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J.Exp.Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]


