Abstract
Different navigation procedures (based on 2D-, 3D-fluoroscopy or CT modalities) with their respective limitations are established in orthopedic surgery. The hypothesis is that intraoperative matching of different modalities (fluoro and CT) increases the precision of navigated screw placement and reduces the fluoroscopy time. Vertical unstable pelvic ring fractures of 12 patients were treated with vertebro-pelvic fixations (6 in the standard technique and 6 using the fluoro-CT navigation). An optimal osseous corridor could be determined by the navigation procedure increasing the overall precision of screw placement (no misplacement in the second group as compared to one malplaced pedicle screw in the standard group). The achieved screw lengths were [(mean ± SE) 78 ± 5 vs. 53 ± 4 mm, p < 0.001). Less invasive open approaches and a reduction of fluoroscopy time (time per screw in seconds: 121 vs. 62 s) were observed. CT-fluoro-matched navigation improves the intraoperative visualization of osseous structures and increases the precision of screw placement with less radiation exposure.
Keywords: Computer-assisted surgery, CT-fluoro matching, Navigation, Spino-pelvic dissociation, Pelvic fracture, Vertebro-pelvic fixation
Introduction
Vertical unstable pelvic ring lesions are rare but severe injuries. Different conservative and operative treatment methods are recommended and discussed in the literature [1–6]. Especially in severe comminuted sacral fractures a vertebro-pelvic fixation is the preferred procedure, which provides sufficient fracture stability for postoperative mobilization without loss of alignment and allows consistent fracture union [6–8]. Extensive open approaches with complete exposure of bone surfaces are necessary in cases where radiological landmarks are not confidently visualized, which further increases the common high risk for wound infections [6]. A better intraoperative visualization of bone structures and a less invasive approach is preferable. With the introduction of different navigation systems (CT-based, as well as 2D and 3D fluoroscopic-based) an increased intraoperative visualization and precision for screw placement was reported. Dependent on the indication, each procedure has specific advantages, as well as significant drawbacks and limitations. By fusion of different image modalities, a synergistic effect of these procedures may be achieved.
We describe a new navigation procedure based on preoperative acquired computed tomography (CT) images matched with intraoperative fluoroscopic images (CT-fluoro matching) for the screw placement of vertebro-pelvic fixation devices. The results of the first six patients were compared to a retrospective control group operated in the standard technique, as described by Schildhauer et al. [6, 7] and Tiemann et al. [8, 9].
Materials and methods
Patient selection
Since January 2004, 12 of 134 patients with operatively treated pelvic ring fractures were stabilized with a vertebro-pelvic fixation. Depending on the type of fracture a triangular (Fig. 1a) or bilateral fixation (Fig. 1b) was preferred [8]. Inclusion criteria were comminuted or severe dislocated sacral fractures and spino-pelvic dissociations, where the fracture could not be addressed by an iliosacral screw fixation or in cases of accompanied unstable lumbar fractures.
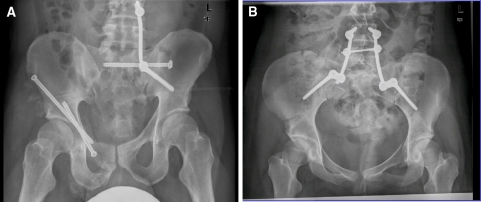
Fig. 1.
Different vertebro-pelvic fixations. aTriangular fixation a 27-year-old male injured in a motorcycle accident; combined pelvic ring fracture 61C1.3.3c2 and acetabular fracture 62 A2.2.1; bbilateral fixation a 32-year-old female injured by a fall off a height; pelvic fracture 61C1.3.2c1
The results of CT-fluoro navigation (group II) were compared to the last six cases performed with the standard technique (group I). Epidemiologic data and fracture characteristics of all 12 patients are displayed in Table 1.
Table 1.
Patients’ characteristics in both groups
| Age (years) | ISS | PTS | Type of fracture (AO classification) | Spino-pelvic fixation | Hospital stay (days) | |
|---|---|---|---|---|---|---|
| Group I (control) | ||||||
| 1 | 40 | 29 | 26 | 61 C1.3.a2.c5 | Triangular | 28 |
| 2 | 64 | 34 | 32 | 61 C.2.3.2b2.2c1 + 62A3.1a1 | Bilateral | 100 |
| 3 | 32 | 20 | 12 | 61 C1.3.2c1 | Triangular | 18 |
| 4 | 41 | 41 | 38 | 61 C1.3.2.c4 | Triangular | 30 |
| 5 | 54 | 41 | 50 | 61 C3.1.2.b2c5 | Triangular | 48 |
| 6 | 32 | 41 | 40 | 61 C3.3.4.c10 | Bilateral | 104 |
| Mean | 43.8 | 34.3 | 33.0 | 54.7 | ||
| SE | 5.2 | 3.5 | 5.3 | 15.5 | ||
| Group II (CT-fluoro) | ||||||
| 1 | 17 | 41 | 24 | 61 C1.3.2c3 | Bilateral | 54 |
| 2 | 22 | 48 | 45 | 61 C3.2.2b4c3 | Bilateral | 38 |
| 3 | 76 | 41 | 62 | 61 C1.3.2c1 + 62C1.2a2 | Bilateral | 40 |
| 4 | 24 | 41 | 32 | 61 C1.3.3c1 | Triangular | 33 |
| 5 | 27 | 25 | 31 | 61 C1.3.3c2 + 62 A2.2.1 | Triangular | 35 |
| 6 | 37 | 36 | 36 | 61 C1.3.3c7 | Bilateral | 30 |
| Mean | 33.8 | 38.7 | 38.3 | 38.3 | ||
| SE | 8.9 | 3.1 | 5.5 | 3.5 | ||
| p | <0.36 | <0.38 | <0.50 | <0.35 | ||
Group I, using the conventional technique (control group); Group II, using the navigation technique based on CT-fluoro matching
ISS Injury Severity Score, PTS Hannover Polytrauma Score
Treatment procedure
In accordance with the damage control concept, the initial care was focused on closed pelvic ring reduction [10] and stabilization by a supra-acetabular placed external fixator. A pelvic packing procedure followed, if hemodynamic instability persisted. After resuscitation at the intensive care unit, the definitive operative procedures were performed as soon as possible, depending on the patient’s physical condition, by two surgeons. In one case, a definitive operative procedure including an open decompression was indicated on the day of administration after failed closed reduction.
Reconstructions of the anterior pelvic ring (in four cases) and acetabular fractures (in two cases) were addressed in an additional operative step in the supine position. For the lumbar-pelvic fixation the patients were placed on a radiolucent carbon table in prone position with sterile draped free moveable legs to allow intraoperative manipulation for indirect reduction maneuvers.
The two following operative workflows were performed:
Group I: standard technique (control group)
Paramedian skin incisions (from L4 to the posterior–superior iliac spine) were made for triangular vertebro-pelvic fixations (Fig. 1a) and midline incisions (from L4 to S2) for bilateral vertebro-pelvic fixations (Fig. 1b). After subcutaneous tissue retraction, the thoracolumbar fascia was dissected and the paravertebral muscle mass of the erector spinae was elevated from the midline by a sharp dissector to prepare the entry points for the pedicle screws using typical landmarks, as well as for the ilium screws using the posterior–superior iliac spines. The intraosseous pathway of screws was prepared by a pedicle awl under fluoroscopic control (posterior–anterior and true lateral for pedicle screws; obturator inlet and obturator outlet for ilium screws). The length of screws was measured by a reverse ruler. After placing the screws, the rods and cross-connectors were placed via the open approach (Fig. 2).
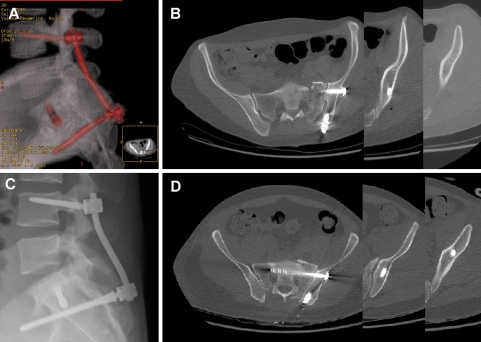
Fig. 2.
Comparison of the ilium screw trajectory and length in postoperative CT (true lateral X-ray re-formation and axial CT-slices) by use of a conventional technique (control group I); showing the shorter ilium screw due to a suboptimal screw trajectory (a, b). Navigation technique based on CT-fluoro matching (group II); showing the longer ilium screw reaching the supra-acetabular region due to an optimal screw trajectory (c, d)
Group II: CT-fluoro-matched navigation
The spinous process of L4 vertebra was identified by one lateral fluoroscopic image and prepared first via a short incision for fixation of the reference base of the navigation system (Fig. 3a). Thereafter, two fluoroscopic images (posterior–anterior and true lateral) were acquired for the matching procedure with the initial data of the trauma screening CT scan. The detailed matching procedure and the navigation technique are described later.
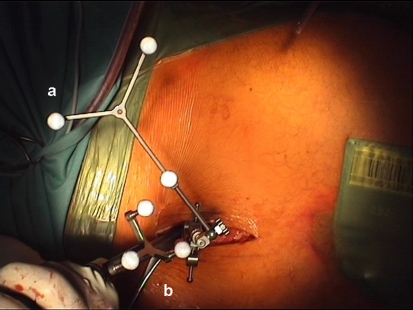
Fig. 3.
Less invasive approach for screw insertion using the navigation technique (group II). a Reference base placed at the spinous process of L4 vertebra. b Navigated pedicle awl for preparation of the pedicle-screw pathway
After visualization of the planned screw trajectory on the navigation screen, the exact further incision could be determined in extension to the osseous corridor. This step facilitates reduced soft tissue dissection and a less invasive pathway to the L4 pedicles. After placing the navigated pedicle screws in L4 vertebra, the reference base was moved to the spinous process of S1 vertebra and a second matching procedure with new fluoroscopic images (pelvis anterior–posterior and lateral) was performed for the navigated placement of ilium screws. The rods and cross-connectors were introduced by tunneled corridors under the muscle mass of the erector spinae.
An exact fracture reduction was preliminary to the tightening of the internal fixator. A screw-head countersink was used to avoid prominent ilium screw heads causing soft tissue irritations [4, 6].
Iliosacral screws were percutaneously placed in the S1 vertebra, according to the previous described techniques [8, 11]. In three cases (two in the control and one in the navigation group), the XIA system (Stryker, Germany) was used; in all other cases, the USS II ilio-sacral system (Synthes, Germany) was preferred.
Matching and navigation procedure
A passive optoelectronic navigation system (Vector Vision, Brainlab, Germany) and the Vector Vision Spine and fluoro to CT registration software (Brainlab, Germany) were used.
The pelvic image data of the initial CT trauma scan (2.5 mm slices, Light Speed, General Electric, Germany) were preserved using digital imaging communications in medicine (DICOM) and transferred to the navigation system, where a three-dimensional re-formation was generated. For the matching procedure a segmentation of L4 or S1 vertebra was done and matched with the two intraoperative acquired (2D)-fluoroscopic images (Ziehm Vario, Ziehm Imaging Inc., USA). The segmented single vertebra from the CT scan was superimposed on its fluoroscopic images (posterior–anterior and true lateral) and displayed on the navigation monitor- automatic CT-fluoro matching (Fig. 4a). This procedure is comparable to pair points or surface registration, known in CT-based navigation to synchronize the CT data with the patient position on the operation table [12].
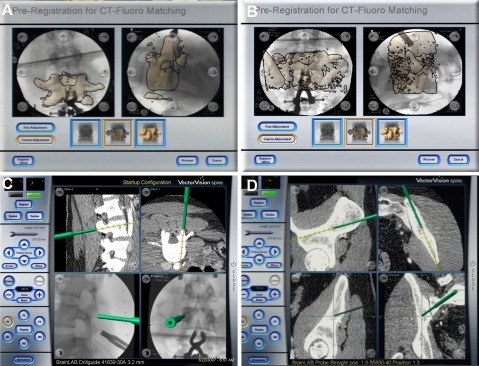
Fig. 4.
Screen shots of the navigation system (Vector Vision, Brainlab) displaying the CT-2D-fluoro matching. a segmented L4 vertebra of the preoperative acquired CT data superimposed on intraoperative acquired fluoroscopic images; b segmented S1 vertebra from the preoperative CT data superimposed on intraoperative acquired fluoroscopic images; c, d results of the matching procedures and virtual visualization of instrument movement (left drill guide, right pedicle awl) based on CT-fluoro-matched images
After calibration of instruments and verification of the precision of the matching procedure by placing the pointer to prominent bony landmarks (spinous process of L4 and posterior–superior iliac spine):
the drill sleeve was navigated to visualize the planned screw trajectory and determine the exact soft tissue pathway, as well as the osseous entry point, and
the pedicle awl was navigated to prepare the exact screw pathway.
The navigation system facilitates real-time tracking of instruments and visualization of their virtual movement in up to four images, displayed on a sterile covered touch screen (Fig. 4c, d). The two previously acquired 2D-fluoroscopic images: anterior–posterior and lateral (known from the standard technique) and additional axial CT-slices and re-formations of the CT data (Fig. 4d) were used for orientation. The final position of the awl and of the screws was controlled in additional fluoroscopic images.
For the placement of ilium screws, we performed a second matching procedure in the same manner as described above, with the S1 vertebral segmentation and CT-fluoro matching (Fig. 4b). Two new fluoroscopic images were mandatory after attaching the reference base to the spinous process of S1.
Postoperative treatment and evaluation of implant positioning
Postoperative computed tomography scans (2.5 mm slices, Light Speed, General Electric, Germany) were performed to evaluate fracture reduction and implant position. The screw position was assessed according to: secure position in the bone, and penetration of the cortical bone.
Depending on the type of fracture and associated upper or lower limb injuries all patients were mobilized by wheelchair transfer for 6 weeks or by pain-adapted weight bearing.
Statistics
Results are expressed as mean ± standard error. Differences were analyzed by the Student’s t test. p values of <0.05 were considered as significant. The study was approved by the institutional ethics committee (processing number: 2418-11/08).
Results
The patient distribution concerning age, fracture characteristics and treatment strategy was comparable in both groups (Table 1).
Causes of injury included eight falls from a significant height (six in a suicide attempt), three traffic accidents (two on a motorbike, one in a car) and one industrial accident with crush injury.
Forty-two percent of patients were directly administered in our department and 58% were first treated at other hospitals and thereafter transferred for definitive pelvic surgery.
Eleven pelvic lesions were initially stabilized by an external fixator as an emergency procedure (two of them including a laparotomy and pelvic packing). In one patient with a combined fracture of the pelvic ring and acetabulum, additional limb traction was applied. The definitive vertebro-pelvic fixations (in group I vs. group II) were performed at the 8.8 ± 1.3 versus 8.7 ± 1.8 days, with a mean operating time of 157 ± 26 versus 190 ± 17 min (n.s.).
In total, we inserted eight pedicle and eight ilium screws using the conventional technique and ten screws each, using the navigation system. Patients’ mean fluoroscopy time was 342 ± 55 versus 186 ± 20 s (p < 0.06). The fluoroscopy time per screw was 121 versus 62 s.
In postoperative computed tomography scans the quality of reduction was categorized by the principles of Majeed and all fractures had a residual displacement ≤5 mm independent of the used technique [13]. Only one asymptomatic malplaced pedicle screw was observed in the conventional group, whereas all navigated screws were placed exactly. In 9 of 12 patients an accompanying preoperative neurological deficit was found, but no patient had a postoperative neurological deterioration.
In each group, one patient developed a wound infection with need for operative revision. In the conventional group one additional postoperative lower limb thrombosis was observed.
Discussion
Navigation procedures were introduced in spine surgery to enhance the precision of pedicle screw placement and to reduce fluoroscopy time for the patient and the operating team [14–17]. Based on the used image modalities (2D-, 3D-fluoroscopy and computed tomography) different advantages and fields of applications are reported [12]:
The CT-based imaging enables the best visualization with the largest region of interest and highest resolution, but requires time-consuming intraoperative registration procedures or a CT in the operation room [18].
The 2D-fluoroscopic-based imaging is limited by the standard summation images in anterior–posterior and lateral projections. The advantage compared to the conventional technique is the possibility to plan the screw position in these images and to control the virtual instrument movement in all projections simultaneously without further radiation exposure. An additional autopilot, using a bull’s-eye mode, ameliorates the precise instrument orientation.
The 3D-fluoroscopic-based imaging is limited by minor resolution quality and a small region of interest (12 × 12 cm) [12] as compared to the CT-based navigation. For vertebro-pelvic fixations at least two 3D-scans are mandatory to visualize the L4, as well as both iliac wings, extending the operative time and increasing the radiation exposure for the patient.
Matching of different image modalities can increase the overall intraoperative visualization of anatomic structures and the orientation of the surgeon, as shown in two recent case reports:
retrograde drilling of an osteochondral lesion by matching MRT and computed tomography images [19];
fixation of a spondylolisthesis of L4/L5 by matching computed tomography and fluoroscopy images [20].
In contrast to the L4/5 fusion procedure described by Sakai et al. [20], we performed two separate matching algorithms for each vertebra (L4 and S1), as they are in relative different positions to each other not only in prone and supine patient position, but also during breathing excursions.
Compared to the 2D-fluoroscopic-based navigation, the fluoro-CT matching enables the visualization of osseous structures in additional CT images (axial slices and sagittal re-formation). Further “inline” reconstruction images, orthogonal to the drill trajectory, facilitate a view ahead on the structures in front of the awl or drill tip (Fig. 4d).
As compared to the CT-based navigation, the registration procedure for the CT-fluoro matching is less invasive by fusion of the CT and fluoroscopic images. Especially in cases of highly degenerated and pathologically altered vertebrae, the definition of landmarks for the surface registration of the CT-based navigation procedure can be difficult, with need for larger soft tissue dissection [20]. Whereas, the recently available 3D-fluoroscopy image intensifiers had a limited scan volume of 12 cm3, newer flat panel generation devices cover a larger scan volume with an increased resolution. In the future, for navigated vertebro-pelvic fixations, just one scan could be sufficient for the whole procedure.
Following reduction—in two navigated cases—the position of the reduced injured ilium as compared to the preoperative CT images was changed. The solution, in these cases, was the acquisition of two additional 2D-fluoroscopic images of the changed ilium position and screw navigation on the basis of only these two images.
As common for most navigation procedures, our approach enables a higher precision in screw placement, due to a planned trajectory in the CT-fluoro-matched images, and therefore exact orientation of the ilium screws in the osseous corridors (Fig. 4d). The statistical power for this statement is limited due to the small number of inserted screws. Nevertheless, in the navigated group no screw malposition was observed. Furthermore, longer iliac screws could be placed due to an exact visualization of the osseous corridor by CT-fluoro matching. The potential clinical relevance for placement of screws—with maximum length—is shown in two biomechanical studies where increased fixation strength was reported as compared to shorter screws used for vertebro-pelvic fixations [21, 22].
An additional clinical advantage of CT-fluoro navigation is the less invasive approach (Fig. 3). Schildhauer et al. [6] discussed a reduction of wound-related complications for unilateral triangular stabilization, compared to bilateral fixation due to the less invasive approach. Nevertheless, in cases of insufficient intraoperative fluoroscopic visualization the authors recommended the direct digital palpation of the sciatic notch for safe guidance of ilium screw placement [6]. If necessary the dissection of the gluteus muscles from the outer table of ilium would help in orientation but would increase the invasiveness of the approach and the risk for postoperative infections.
Wound infections after vertebro-pelvic fixations via a posterior open approach are reported in up to 14–25% of the operated patients, with need of operative revision in most cases [4, 6, 23, 24]. Furthermore, these injuries are sometimes associated with decollement lesions [24], predisposing postoperative wound infection.
Limitations of the navigated procedure are the high acquisition and maintenance costs of a navigation system [12, 25], as well as the long learning curve, when using navigation systems for the first time.
Additionally, an anatomic reduction and open decompression of spinal structures in selected cases is mandatory and should not be disregarded, in favor of a less invasive approach.
A potential bias in this study is the small number of patients per group. In total only 12 vertebro-pelvic fixations in more than 4 years reflect the rare indication for this operative procedure. Additional intraoperative visualizations and instrument orientations offered by the navigation system can compensate for a lack of routine for the surgeon, especially for the ilium screw placement.
Conclusion
Matching of preoperatively acquired computed tomography and intraoperatively generated fluoroscopic images improves visualization of osseous structures and orientation of instruments during surgery and increases the precision of screw insertion for vertebro-pelvic fixations. Additionally, a reduced open approach and decreased fluoroscopy time can be expected, compared to the conventional technique.
Conflict of interest statement
The devices are FDA-approved or approved by the corresponding national agency for this indication. No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Contributor Information
Ivan Marintschev, Phone: +49-3641-9322801, FAX: +49-3641-9322803, Email: ivan.marintschev@med.uni-jena.de.
Kajetan Klos, Email: kajetan.klos@med.uni-jena.de.
Arne Wilharm, Email: arne.wilharm@med.uni-jena.de.
Thomas Mückley, Email: thomas.mueckley@med.uni-jena.de.
Gunther O. Hofmann, Email: gunther.hofmann@med.uni-jena.de
References
- 1.Hunt N, Jennings A, Smith M. Current management of U-shaped sacral fractures or spino-pelvic dissociation. Injury. 2002;33:123–126. doi: 10.1016/S0020-1383(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 2.Kach K, Trentz O. Distraction spondylodesis of the sacrum in “vertical shear lesions” of the pelvis. Unfallchirurg. 1994;97:28–38. [PubMed] [Google Scholar]
- 3.Markel DC, Raskas DS, Graziano GP. A case of traumatic spino-pelvic dissociation. J Orthop Trauma. 1993;7:562–566. doi: 10.1097/00005131-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Mouhsine E, Wettstein M, Schizas C, Borens O, Blanc CH, Leyvraz PF, Theumann N, Garofalo R. Modified triangular posterior osteosynthesis of unstable sacrum fracture. Eur Spine J. 2006;15:857–863. doi: 10.1007/s00586-004-0858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy-Camille R, Saillant G, Gagna G, Mazel C. Transverse fracture of the upper sacrum. Suicidal jumper’s fracture. Spine. 1985;10:838–845. doi: 10.1097/00007632-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Schildhauer TA, Bellabarba C, Nork SE, Barei DP, Routt ML, Jr, Chapman JR. Decompression and lumbopelvic fixation for sacral fracture-dislocations with spino-pelvic dissociation. J Orthop Trauma. 2006;20:447–457. doi: 10.1097/00005131-200608000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Schildhauer TA, Josten C, Muhr G. Triangular osteosynthesis of vertically unstable sacrum fractures: a new concept allowing early weight-bearing. J Orthop Trauma. 1998;12:307–314. doi: 10.1097/00005131-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Tiemann AH, Hofmann GO. Triangular vertebropelvic bracing. Weight-bearing osteosynthesis of unstable C-type fractures of the posterior pelvic ring. Trauma Berufskrankh. 2008;10:123–130. doi: 10.1007/s10039-008-1387-8. [DOI] [Google Scholar]
- 9.Tiemann AH, Schmidt C, Josten C. Triangular vertebropelvine stabilisation of unstable posterior pelvic ring fractures. Zentralbl Chir. 2003;128:202–208. doi: 10.1055/s-2003-38533. [DOI] [PubMed] [Google Scholar]
- 10.Josten C, Schildhauer TA, Muhr G. Therapy of unstable sacrum fractures in pelvic ring. Results of of osteosynthesis with early mobilization. Chirurg. 1994;65:970–975. [PubMed] [Google Scholar]
- 11.Marintschev I, Gras F, Mückley T, Hofmann GO. Significance of navigation in pelvic surgery. Trauma Berufskrankh. 2008;10:131–135. doi: 10.1007/s10039-008-1401-1. [DOI] [Google Scholar]
- 12.Hufner T, Gebhard F, Grutzner PA, Messmer P, Stockle U, Krettek C (2004) Which navigation when? Injury 35(Suppl 1):S-A30–34 [DOI] [PubMed]
- 13.Majeed SA. External fixation of the injured pelvis. The functional outcome. J Bone Joint Surg Br. 1990;72:612–614. doi: 10.1302/0301-620X.72B4.2380212. [DOI] [PubMed] [Google Scholar]
- 14.Amiot LP, Labelle H, DeGuise JA, Sati M, Brodeur P, Rivard CH. Computer-assisted pedicle screw fixation. A feasibility study. Spine. 1995;20:1208–1212. doi: 10.1097/00007632-199505150-00019. [DOI] [PubMed] [Google Scholar]
- 15.Gebhard F, Weidner A, Liener UC, Stockle U, Arand M (2004) Navigation at the spine. Injury 35(Suppl 1):S-A35–45 [DOI] [PubMed]
- 16.Laine T, Schlenzka D, Makitalo K, Tallroth K, Nolte LP, Visarius H. Improved accuracy of pedicle screw insertion with computer-assisted surgery. A prospective clinical trial of 30 patients. Spine. 1997;22:1254–1258. doi: 10.1097/00007632-199706010-00018. [DOI] [PubMed] [Google Scholar]
- 17.Nolte LP, Slomczykowski MA, Berlemann U, Strauss MJ, Hofstetter R, Schlenzka D, Laine T, Lund T. A new approach to computer-aided spine surgery: fluoroscopy based surgical navigation. Eur Spine J. 2000;9(Suppl 1):S78–S88. doi: 10.1007/PL00010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockle U, Schaser K, Konig B. Image guidance in pelvic and acetabular surgery—expectations, success and limitations. Injury. 2007;38:450–462. doi: 10.1016/j.injury.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Citak M, Kendoff D, Stubig T, Krettek C, Hufner T. Drilling with 3D fluoroscopic navigation in osteonecrosis of the femoral condyle. Unfallchirurg. 2008;111:344–349. doi: 10.1007/s00113-007-1353-0. [DOI] [PubMed] [Google Scholar]
- 20.Sakai Y, Matsuyama Y, Yoshihara H, Nakamura H, Nakashima S, Ishiguro N. Simultaneous registration with ct-fluoro matching for spinal navigation surgery. A case report. Nagoya J Med Sci. 2006;68:45–52. [PubMed] [Google Scholar]
- 21.Akesen B, Wu C, Mehbod AA, Sokolowski M, Transfeldt EE. Revision of loosened iliac screws: a biomechanical study of longer and bigger screws. Spine. 2008;33:1423–1428. doi: 10.1097/BRS.0b013e3181753c04. [DOI] [PubMed] [Google Scholar]
- 22.McCord DH, Cunningham BW, Shono Y, Myers JJ, McAfee PC. Biomechanical analysis of lumbosacral fixation. Spine. 1992;17:S235–S243. doi: 10.1097/00007632-199208001-00004. [DOI] [PubMed] [Google Scholar]
- 23.Eid K, Keel M, Keller A, Ertel W, Trentz O (2005) Influence of sacral fracture on the long-term outcome of pelvic ring injuries. Unfallchirurg 108:35-36, 38-42 [DOI] [PubMed]
- 24.Nothofer W, Thonke N, Neugebauer R. Therapy of unstable sacrum fractures in pelvic ring fractures with dorsal sacrum distraction osteosynthesis. Unfallchirurg. 2004;107:118–127. doi: 10.1007/s00113-004-0725-y. [DOI] [PubMed] [Google Scholar]
- 25.Citak M, Kendoff D, Wanich T, Pearle A, Wubben H, Krettek C, Hufner T. Percutaneous bone biopsy. A new application for 3D navigation: a pilot study. Technol Health Care. 2007;15:231–236. [PubMed] [Google Scholar]