Abstract
The purpose of this retrospective clinical study was to evaluate the factors that affect recompression of operated vertebrae after percutaneous balloon kyphoplasty (PKP) for osteoporotic vertebral compression fractures (VCFs) and assess their clinical importance. PKP has been used for VCFs with satisfactory results. Several studies about subsequent VCFs adjacent to cemented vertebrae have been reported after PKP. However, the presence and significance of recompression of operated vertebrae have not been adequately described. In total, 80 patients treated with PKP for single thoracolumbar VCFs were reviewed. The follow-up period was at least 1 year. Patients were divided into those without recompression (maintained group, n = 70) and those with recompression (recompressed group, n = 10). Plain roentgenography (preoperative, operative, and last), preoperative BMD, and preoperative MRI were checked. Age, gender, T-score in BMD, duration of symptom, compression rate (CR) of VCF, reduction rate, kyphotic angle (KA), reduction angle, intervertebral cleft (IVC), and non-PMMA-endplate-contact (NPEC) were evaluated. To evaluate the clinical results, we checked the VAS score at each follow-up period. All data were analyzed statistically. The CR for the recompressed group increased significantly after surgery and decreased at the last follow-up (p < 0.05). The last CR was not significantly different from the preoperative CR. The KA showed the same pattern. The preoperative, postoperative, and last VAS scores were significantly different from one another in both groups (p < 0.05). Between the groups, preoperative KA, postoperative KA, last KA, IVC, and NPEC were significantly different (p < 0.05). In particular, last KA, IVC, and NPEC showed highly significant differences (p < 0.001). In a correlation test for the evaluated factors, IVC (r = 0.557) and NPEC (r = 0.496) were the most significant. The presence of IVC and NPEC may play an important role in inducing recompression of treated vertebrae after PKP. Careful observation of patients with these conditions is necessary to prevent deterioration of their clinical course.
Keywords: Osteoporotic vertebral compression fracture, Percutaneous kyphoplasty, Recompression, Intervertebral cleft, Non-PMMA-endplate-contact
Introduction
Percutaneous balloon kyphoplasty (PKP) is a minimally invasive surgical technique for treating osteoporotic vertebral compression fractures (VCFs). It has several advantages, such as reduction of the fracture, restoration of vertebral height, relief of pain, and reduced chance of cement leakage. Numerous authors have reported the advantages of PKP and satisfactory clinical results after the procedure [3, 4, 8, 9, 20, 21, 28, 29].
Several studies have reported newly developed fractures in adjacent vertebrae after PKP and affecting factors [6, 7, 10, 16, 26]. However, there are few reports of further compression of previously treated vertebrae [15, 17, 18]. Lavelle and Cheney studied [17] recurrent fracture after kyphoplasty of a previously operated vertebra. They reported a 10% incidence rate for recurrent fracture and noted that it occurred primarily within the first 90 days after surgery. However, they did not mention the characteristics or significance of this condition. Heo et al. [12] reported recollapse of the same vertebra after percutaneous vertebroplasty (PVP). They suggested that preoperative osteonecrosis might be the most important predisposing factor for recollapse. The authors reported a preliminary study on the recompression of the operated vertebral body after PKP with no additional trauma [15]. In that study, all patients had the intervertebral cleft (IVC) before PKP. The authors suggested that the presence of IVC might be related to recompression of the treated vertebra but did not find a significant relationship with recompression. The purpose of the present study was to determine factors related to the recompression of operated vertebral bodies after PKP and to evaluate their clinical significance.
Materials and methods
Selection of patients
To rule out bias in the selection of patients and extravertebral effects, the inclusion criteria were as follows: (1) single-level VCF in the thoracolumbar or lumbar area; (2) treatment with single-level PKP via bilateral portals; (3) follow-up period of at least 1 year; (4) no additional history of trauma after surgery; (5) no complication after surgery, including anaphylactic shock, leakage of polymethylmethacrylate (PMMA) into the spinal canal, or postoperative neurologic deficit; and (6) regular radiologic studies and osteoporotic medications throughout the follow-up period. Exclusion criteria were (1) non-osteoporotic VCF, including fractures related to malignancy, infection, or other medical conditions; (2) burst fracture with retro-pulsed bony fragment into the spinal canal before surgery; (3) clinically significant neurologic deficit before and after surgery; (4) presence of pathological conditions without osteoporosis at two adjacent vertebrae above and below the treated vertebra; (5) presence of subsequent fracture after PKP at an adjacent vertebra; (6) life-threatening systemic disease, such as malignancy, systemic infection, or serious disorder of a vital organ; and (7) use of steroids.
A total of 80 patients were reviewed for this study. The patients were divided into two groups. The maintained group consisted of patients without recompression of operated vertebral bodies, and the recompressed group consisted of patients who experienced recompression at operated vertebral bodies after PKP.
Surgical procedure and postoperative care
The PKP was done using specialized instruments (Kyphon®, Medtronic, Minneapolis, MN, USA) and PMMA cement via bilateral portals according to routine procedures and under local or general anesthesia. For each vertebral body, 6–10 mL PMMA was inserted. Self-ambulation was permitted as soon as possible after surgery. Back braces were applied to all patients after surgery for 1–2 months. Osteoporotic medications, including bisphosphonates, vitamin D, or raloxifen, were used postoperatively. Conservative treatments were followed for all patients in the recompressed group after detection of recompression of previously treated vertebrae.
Radiological assessment
To evaluate the radiological results of PKP, we checked initial, immediate postoperative, and last follow-up period plain roentgenograms. Preoperative T-scores for bone mineral densities were measured using dual-energy X-ray absorptiometry. Preoperative magnetic resonance imaging was used to observe the fractured vertebral body and to reveal the presence of IVC.
Recompression of the operated vertebral body was defined as a final anterior body height more than 1.0 mm less than the postoperative anterior body height, considering the bias in measurement. The rates of vertebral body compression (CRs) at each follow-up period were measured as the rate of the anterior body height of the fractured vertebra to the mean anterior body height of the upper and lower vertebrae. Reduction rates were calculated using the difference between preoperative and postoperative CRs. Kyphotic angles (KAs) were measured using Cobb’s method between the superior endplate of the vertebra directly above and the lower endplate of the vertebra directly below. Reduction angles were calculated using the difference between the preoperative and postoperative KAs. Non-PMMA-endplate-contact (NPEC) was determined when postoperative plain roentgenograms revealed that the inserted PMMA did not come into contact with any portion of the endplate. Two authors measured twice individually and independently to eliminate intra- and inter-observer bias. If there was a noticeable difference in any result, the authors conferred to determine the final result. All radiologic measurements were checked digitally using PACS (M-view™, Marotech, Seoul, Korea).
Factors evaluated
Age, gender, duration of symptom (duration from onset of symptom to time of checking MRI), preoperative T-score in bone mineral density, CR (preoperative, postoperative, and last), reduction rate, KA (preoperative, postoperative, and last), reduction angle, IVC, and NPEC were evaluated.
Assessment of clinical results
Assessments of clinical results were made using VAS scores at the initial, immediate postoperative, and last follow-up periods.
Statistical analysis
All data were analyzed using SPSS Ver. 13.0 for Windows® (SPSS Inc., Chicago, IL, USA). The Mann–Whitney U-test was used to evaluate intergroup differences, and Pearson’s correlation test was used to determine the correlation with recompression.
Results
Patient characteristics
In total, 80 patients were reviewed (maintained group, n = 70; recompressed group, n = 10). The incidence of recompression of treated vertebrae was 12.5%, and recompression developed at 3.4 ± 0.5 months (3–4 months) after surgery. Patient characteristics for both groups are noted in Table 1, and those for patients in the recompressed group only are shown in Table 2 (Fig. 1).
Table 1.
Comparison of data between maintained group and recompressed group
| Maintained group (n = 70) | Recompressed group (n = 10) | ||||
|---|---|---|---|---|---|
| Age | 76.0 ± 7.1 | (58–90) | 76.9 ± 6.3 | (66–84) | |
| Sex | Male | 10 | 0 | ||
| Female | 60 | 10 | |||
| Fx. level | D10 | 4 | 0 | ||
| D11 | 5 | 1 | |||
| D12 | 20 | 5 | |||
| L1 | 20 | 3 | |||
| L2 | 21 | 1 | |||
| Sx. duration (days) | 15.4 ± 8.2 | (4–34) | 24.9 ± 29.5 | (4–105) | |
| FU (mos.) | 26.2 ± 9.4 | (12–46) | 23.2 ± 9.9 | (12–46) | |
| Preop. T-score | −4.0 ± 0.5 | (−3.0 to 4.9) | −3.7 ± 0.6 | (−3.0 to −4.9) | |
| Compression rate (%) | Preop. | 45.5 ± 11.3 | (19.7–75.5) | 38.8 ± 13.6 | (14.7–58.3) |
| Postop. | 24.1 ± 11.1 | (2.2–58.3) | 17.4 ± 9.3 | (4.3–33.3) | |
| Last | 25.1 ± 11.2 | (3.7–58.3) | 32.0 ± 10.6 | (13.0–52.9) | |
| Reduced | 21.4 ± 8.8 | (1.8–40.9) | 21.4 ± 7.4 | (10.4–35.3) | |
| Kyphotic angle (°) | Preop. | 17.1 ± 6.3* | (5–34) | 21.4 ± 5.9* | (11–31) |
| Postop. | 11.8 ± 5.3* | (2–29) | 16.3 ± 4.5* | (9–26) | |
| Last | 11.8 ± 5.3* | (2–29) | 22.2 ± 5.8* | (14–33) | |
| Reduced | 6.0 ± 3.4 | (0–14) | 5.1 ± 3.7 | (2–14) | |
| IVC (%) | 20* | (14/70) | 100* | (10/10) | |
| Non-PMMA-endplate- contact (%) | 8.6* | (6/70) | 70* | (7/10) | |
| VAS | Preop. | 8.8 ± 0.6 | (8–10) | 8.6 ± 0.5 | (8–9) |
| Postop. | 2.1 ± 0.6 | (1–3) | 2.2 ± 0.8 | (1–3) | |
| Last | 3.1 ± 0.7 | (2–4) | 3.5 ± 1.2 | (2–6) | |
* p < 0.05
Table 2.
Details of patients in recompressed group
| No. | Age | Sex | Fx. level | Sx. duration (days) | FU (mos.) | Preop. T-score | Compression rate (%) | Kyphotic angle (°) | Onset of recompression (postop. mos.) | Presence of IVC | Non-PMMA-endplate-contact | VAS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop. | Postop. | Last | Reduced | Preop. | Postop. | Last | Reduced | Preop. | Postop. | Last | ||||||||||
| 1 | 73 | F | D12 | 28 | 24 | −3.1 | 14.7 | 4.3 | 13.0 | 10.4 | 11 | 9 | 14 | 2 | 4 | Yes | Yes | 9 | 2 | 3 |
| 2 | 77 | F | L2 | 14 | 25 | −4.9 | 58.3 | 33.3 | 41.7 | 25.0 | 24 | 16 | 23 | 8 | 4 | Yes | No | 8 | 3 | 3 |
| 3 | 82 | F | D11 | 7 | 30 | −4.2 | 39.4 | 18.2 | 23.5 | 21.2 | 18 | 15 | 16 | 3 | 3 | Yes | Yes | 8 | 2 | 6 |
| 4 | 72 | F | L1 | 105 | 46 | −3.8 | 31.0 | 6.9 | 31.0 | 24.1 | 16 | 14 | 23 | 2 | 3 | Yes | Yes | 9 | 3 | 4 |
| 5 | 81 | F | D12 | 6 | 25 | −4.0 | 34.5 | 13.8 | 31.0 | 20.7 | 31 | 26 | 33 | 5 | 3 | Yes | Yes | 9 | 2 | 2 |
| 6 | 70 | F | D12 | 13 | 18 | −4.0 | 48.0 | 20.0 | 28.0 | 28.0 | 22 | 18 | 21 | 4 | 3 | Yes | No | 9 | 2 | 4 |
| 7 | 80 | F | D12 | 21 | 12 | −3.3 | 37.5 | 16.7 | 29.1 | 20.8 | 23 | 20 | 30 | 3 | 3 | Yes | Yes | 8 | 1 | 2 |
| 8 | 66 | F | L1 | 4 | 15 | −3.0 | 26.7 | 10.0 | 36.7 | 16.7 | 23 | 16 | 21 | 7 | 3 | Yes | Yes | 9 | 1 | 3 |
| 9 | 84 | F | L1 | 21 | 14 | −3.1 | 56.9 | 21.6 | 33.3 | 35.3 | 28 | 14 | 23 | 14 | 4 | Yes | Yes | 9 | 3 | 4 |
| 10 | 84 | F | D12 | 30 | 23 | −3.2 | 41.2 | 29.4 | 52.9 | 11.8 | 18 | 15 | 18 | 3 | 4 | Yes | No | 8 | 3 | 4 |
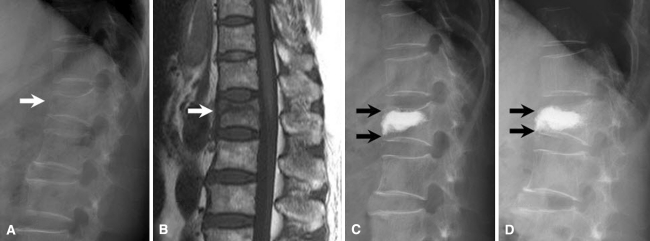
Fig. 1.
Case no. 8, female, 66 years old. a Osteoporotic VCF in L1 vertebra (white arrow). T-score in bone mineral density was −3.0. Preoperative CR, KA, and VAS scores were 26.7%, 23º, and 9, respectively. b T1-weighted magnetic resonance imaging showed IVC in the central area of fractured vertebral body (white arrow). c After PKP, postoperative CR, KA, and VAS scores were 10.0%, 16º, and 1, respectively. NPECs were seen above and below the PMMA mass (black arrows). d After 15 months of follow-up, last CR, KA, and VAS scores were 36.7%, 21º, and 3, respectively. The normal portions of treated vertebra had disappeared, and recompression had developed (black arrows)
Statistical results
The CR for the recompressed group increased significantly after surgery and decreased at last follow-up (p < 0.05). The last CR was not significantly different than the preoperative CR. The KA showed the same pattern. The preoperative, postoperative, and last VAS scores were significantly different from one another (p < 0.05) in both groups.
The Mann–Whitney test revealed that preoperative KA, postoperative KA, last KA, IVC, and NPEC differed significantly between the groups (p < 0.05). In particular, last KA, IVC, and NPEC showed highly significant differences (p < 0.001). Pearson’s correlation test revealed r values of 0.542, 0.577, and 0.496, respectively, for last KA, IVC, and NPEC (p < 0.001). Finally, preoperative conditions with IVC and NPEC appeared to be the most important factors related to the recompression of treated vertebrae after KPK.
Discussion
Researchers to date have not uniformly described the further compression of previously cemented vertebrae. Lavelle and Cheney [17] described ‘recurrent fracture’ of previously treated vertebrae, although they found no episodes of additional trauma; this term could be easily misunderstood as fracture after trauma. Heo et al. [12] reported recollapse of the same vertebrae after PVP. The term ‘recollapse’ might be confused with additional collapse or crushing of a treated vertebra, including breakage of the cement mass. In the present study, there was no additional trauma, and further compression developed only in the bony vertebra, not the cement mass. For these reasons, we suggest that the term ‘recompression’ should be used to describe this condition.
No critical factor related to recompression of previously treated vertebrae after PKP has yet been clearly described. Kim et al. [14] conducted biomechanical studies using cadaver vertebrae treated with vertebroplasty and kyphoplasty with PMMA. They applied cyclic motions to the cemented vertebrae and found that the treated vertebrae showed subsidence in vertebral height. The degree of subsidence was more prominent in kyphoplasty than in vertebroplasty. In PVP, the PMMA fills the cancellous portion of the vertebral body with an interdigitated pattern. The load should be evenly transmitted through the upper endplate, filled PMMA, and lower endplate, in that order. However, in PKP, the PMMA mass is not interdigitated but consists of one or two solid masses, and there are non-cemented bony areas between the PMMA mass and the two endplates. They found that the load did not transfer through the non-cemented bony area and that a stress-shielding effect could occur. The stress-shielding effect made the bony area too weak, resulting in greater height loss in PKP than in PVP. Wilke et al. [30] reported a similar study in which they found that subsidence at the center of the upper endplate was greater in kyphoplasty than in vertebroplasty. In the present study, recompressions were noted primarily in patients with NPEC (7 recompressions out of 15 NPECs).
Heo et al. [12] reported that preoperative osteonecrosis of a treated vertebral body was the most important predisposing factor. They explained that osteonecrosis frequently made a cyst-like defect area and that the injected cement of PVP was primarily in a solid lump rather than contiguously interdigitated. This solid lump led to reexpansion of the compressed vertebra by a volume effect, and recollapse occurred mainly in the ‘PMMA-nonsupported area’. In the present study, we found that IVC (10 recompressions out of 24 IVCs) was one of the factors most strongly related to recompression. IVC provides radiological evidence of osteonecrosis [5, 19, 22, 24, 27], or nonunion and pseudoarthrosis after vertebral fracture [1, 2, 11, 13, 23]. Whatever the reasons, IVC might be made in chronic period of fracture healing process after initial trauma. The authors found statistical difference in duration of symptom neither between two groups nor in the aspect of presence of IVC. The durations of symptom in recompressed group were similar to those of maintained group except one case (case no. 4). Unfortunately, we could not confirm the reason of ICV and its relationship to the duration of symptom in the present study. Regardless, normal bony consistency is lost in the area of IVC, and the vertebral body is weakened and prone to ballooning of the tamp or cement infiltration [25].
In PKP, an inflatable bone tamp is inserted into the vertebral body and ballooned to reduce the fracture. The balloon compresses the adjacent bone to enable the PMMA to fill the space, and the bone density in the compressed area can be elevated in a fractured vertebra without IVC. After the PMMA fills the space, pressure can cause a small amount of PMMA to leak into the bone marrow space and make an interdigitated pattern. If the interdigitated PMMA comes into contact with the endplate, it can support the endplate against loading. However, in vertebrae with IVC, the tamp is readily expanded in the IVC area during ballooning at high pressure. The inflated balloon does not compress the bone but instead pushes the normal bony portion up and down, like a jack. The PMMA mass fills the limited volume made by the ballooned tamp, but no interdigitation of PMMA occurs. We suggest that the PMMA just pushes the endplate because the IVC area still has mobility after the ballooning of the tamp. NPEC is made by the pushed bony portion, and this bone is already weak because of its osteoporotic nature. The stress-shielding effect is applied to this weak bone, and the weakened bone leads to recompression.
In this study, the incidence of recompression in treated vertebrae was 12.5%, and recompression developed 3.4 ± 0.5 months after surgery. These results are similar to those found by Lavelle and Cheney [17]. In the recompression group, CR and KA improved significantly after surgery and worsened by last follow-up. The last CR and KA were not significantly different from preoperative values. VAS scores changed significantly at each follow-up period. PKP can dramatically improve pain and reduce fracture immediately after surgery. However, the patients suffered from varying degrees of pain at the last follow-up, even though their VAS scores were lower than their preoperative scores. The reasons for this cannot be clearly determined. It may have resulted from the recompression itself, aggravation of deformity by recompression, or still osteoporotic and degenerated vertebrae. The VAS score of a patient in the recompressed group changed from 2 to 6 during follow-up. This suggests that recompression of treated vertebrae could result in aggravation of clinical symptoms, with not only pain but also deformity.
A major limitation of this study is that the number of cases was not large. We used strict criteria for patient selection. We sought to evaluate results associated with the bony condition itself and to minimize extravertebral factors. Our efforts resulted in well-selected but small patient groups, and thus it is difficult to generalize our results to the general population. The follow-up period may also be a limitation. The distribution of the follow-up period was wide, which could have resulted in many other factors affecting patients’ final clinical conditions. We also did not examine any effect due to the filling material, because only PMMA was used. Well-designed and prospective studies using various filling materials would be helpful in terms of further evaluating the recompression of treated vertebrae after PKP.
The results of this study suggest that IVC and NPEC may be important factors related to inducing recompression of previously treated vertebrae after PKP. This recompression may cause operated vertebrae to return to their preoperative condition and may worsen clinical symptoms due to pain or the development of spinal deformities. IVC and NPEC may each be of clinical importance, and careful observation and follow-up is necessary to determine whether this is so.
References
- 1.Baba H, Maezawa Y, Kamitani K, Furusawa N, Imura S, Tomita K. Osteoporotic vertebral collapse with late neurological complications. Paraplegia. 1995;33:281–289. doi: 10.1038/sc.1995.64. [DOI] [PubMed] [Google Scholar]
- 2.Baur A, Stäbler A, Arbogast S, Duerr HR, Bartl R, Reiser M. Acute osteoporotic and neoplastic vertebral compression fractures; fluid sign at MR imaging. Radiology. 2002;225:730–735. doi: 10.1148/radiol.2253011413. [DOI] [PubMed] [Google Scholar]
- 3.Belkoff SM, Mathis JM, Fenton DC, Scribner RM, Reiley ME, Talmadge K. An ex vivo biomechanical evaluation of an inflatable bone tamp used in the treatment of compression fracture. Spine (Phila Pa 1976) 2001;26:151–156. doi: 10.1097/00007632-200101150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bouza C, López T, Magro A, Navalpotro L, Amate JM. Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review. Eur Spine J. 2007;15:1050–1067. doi: 10.1007/s00586-005-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupuy DE, Palmer WE, Rosenthal DI. Vertebral fluid collection associated with vertebral collapse. Am J Roentgenol. 1996;167:1535–1538. doi: 10.2214/ajr.167.6.8956592. [DOI] [PubMed] [Google Scholar]
- 6.Fribourg D, Tang C, Sra P, Delamarter R, Bae H. Incidence of subsequent vertebral fracture after kyphoplasty. Spine (Phila Pa 1976) 2004;29:2270–2276. doi: 10.1097/01.brs.0000142469.41565.2a. [DOI] [PubMed] [Google Scholar]
- 7.Frankel BM, Monroe T, Wang C. Percutaneous vertebral augmentation: an elevation in adjacent-level fracture risk in kyphoplasty as compared with vertebroplasty. Spine J. 2007;7:575–582. doi: 10.1016/j.spinee.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Garfin SR, Buckley RA, Ledlie J, Balloon Kyphoplasty Outcomes Group Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (Phila Pa 1976) 2006;31:2213–2220. doi: 10.1097/01.brs.0000232803.71640.ba. [DOI] [PubMed] [Google Scholar]
- 9.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976) 2001;26:1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Harrop J, Prpa B, Reinhardt MK, Lieberman I. Primary and secondary osteoporosis incidence of subsequent vertebral compression fractures after kyphoplasty. Spine (Phila Pa 1976) 2004;29:2120–2125. doi: 10.1097/01.brs.0000141176.63158.8e. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa K, Homma T, Uchiyama S, Takahashi H. Vertebral pseudarthrosis in the osteoporotic spine. Spine (Phila Pa 1976) 1998;23:2201–2206. doi: 10.1097/00007632-199810150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Heo DH, Chin DK, Yoon YS, Kuh SU. Recollapse of previous vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int. 2009;20:473–480. doi: 10.1007/s00198-008-0682-3. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Hasegawa Y, Toda K, Nakahara S. Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J. 2002;2:101–106. doi: 10.1016/S1529-9430(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 14.Kim MJ, Lindsey DP, Hannibal M, Alamin TF. Vertebroplasty versus kyphoplasty: biomechanical behavior under repetitive loading conditions. Spine (Phila Pa 1976) 2006;31:2079–2084. doi: 10.1097/01.brs.0000231714.15876.76. [DOI] [PubMed] [Google Scholar]
- 15.Kim YY, Park CG, Rhyu KW. Recompression of vertebral bodies after balloon kyphoplasty for vertebral compression fractures—preliminary report. J Korean Spine Surg. 2009;16:89–94. doi: 10.4184/jkss.2009.16.2.89. [DOI] [Google Scholar]
- 16.Korovessis P, Zacharatos S, Repantis T, Michael A, Karachalios D. Evolution of bone mineral density after percutaneous kyphoplasty in fresh osteoporotic vertebral body fractures and adjacent vertebrae along with sagittal spine alignment. J Spinal Disord Tech. 2008;21:293–298. doi: 10.1097/BSD.0b013e31812e6295. [DOI] [PubMed] [Google Scholar]
- 17.Lavelle WF, Cheney R. Recurrent fracture after vertebral kyphoplasty. Spine J. 2006;6:488–493. doi: 10.1016/j.spinee.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Leslie-Mazwi T, Deen HG. Repeated fracture of a vertebral body after treatment with balloon kyphoplasty: case illustration. J Neurosurg Spine. 2006;4:270. doi: 10.3171/spi.2006.4.3.270. [DOI] [PubMed] [Google Scholar]
- 19.Libicher M, Appelt A, Berger I, Baier M, Meeder PJ, Grafe I, DaFonseca K, Nöldge G, Kasperk C. The intravertebral vacuum phenomenon as specific sign of osteonecrosis in vertebral compression fractures: results from a radiological and histological study. Eur Radiol. 2007;17:2248–2252. doi: 10.1007/s00330-007-0684-0. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman I, Reinhardt MK. Vertebroplasty and kyphoplasty for osteolytic vertebral collapse. Clin Orthop Relat Res. 2003;415:S176–S186. doi: 10.1097/01.blo.0000093841.72468.a8. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 2001;26:1631–1638. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 22.Maldague BE, Noel HM, Malghem JJ. The intravertebral vacuum cleft: a sign of ischemic vertebral collapse. Radiology. 1978;129:23–29. doi: 10.1148/129.1.23. [DOI] [PubMed] [Google Scholar]
- 23.Mochida J, Toh E, Chiba M, Nishimura K. Treatment of osteoporotic late collapse of a vertebral body of thoracic and lumbar spine. J Spinal Disord. 2001;14:393–398. doi: 10.1097/00002517-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Naul LG, Peet GJ, Maupin WB. Avascular necrosis of the vertebral body: MR imaging. Radiology. 1989;172:219–222. doi: 10.1148/radiology.172.1.2740507. [DOI] [PubMed] [Google Scholar]
- 25.Oka M, Matsusako M, Kobayashi N, Uemura A, Numaguchi Y. Intravertebral cleft sign on fat-suppressed contrast-enhanced MR: correlation with cement distribution pattern on percutaneous vertebroplasty. Acad Radiol. 2005;12:992–999. doi: 10.1016/j.acra.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Pflugmacher R, Schroeder RJ, Klostermann CK. Incidence of adjacent vertebral fractures in patients treated with balloon kyphoplasty: two years’ prospective follow-up. Acta Radiol. 2006;8:830–840. doi: 10.1080/02841850600854928. [DOI] [PubMed] [Google Scholar]
- 27.Stäbler A, Schneider P, Link TM, Schöps P, Springer OS, Dürr HR, Reiser M. Intravertebral vacuum phenomenon following fractures: CT study on frequency and etiology. J Comput Assist Tomogr. 1999;23:976–980. doi: 10.1097/00004728-199911000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16:1085–1100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voggenreiter G. Balloon kyphoplasty is effective in deformity correction of osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 2005;30:2806–2812. doi: 10.1097/01.brs.0000190885.85675.a0. [DOI] [PubMed] [Google Scholar]
- 30.Wilke HJ, Mehnert U, Claes LE, Bierschneider MM, Jaksche H, Boszczyk BM. Biomechanical evaluation of vertebroplasty and kyphoplasty with polymethylmethacrylate or calcium phosphate cement under cyclic loading. Spine (Phila Pa 1976) 2006;31:2934–2941. doi: 10.1097/01.brs.0000248423.28511.44. [DOI] [PubMed] [Google Scholar]