Abstract
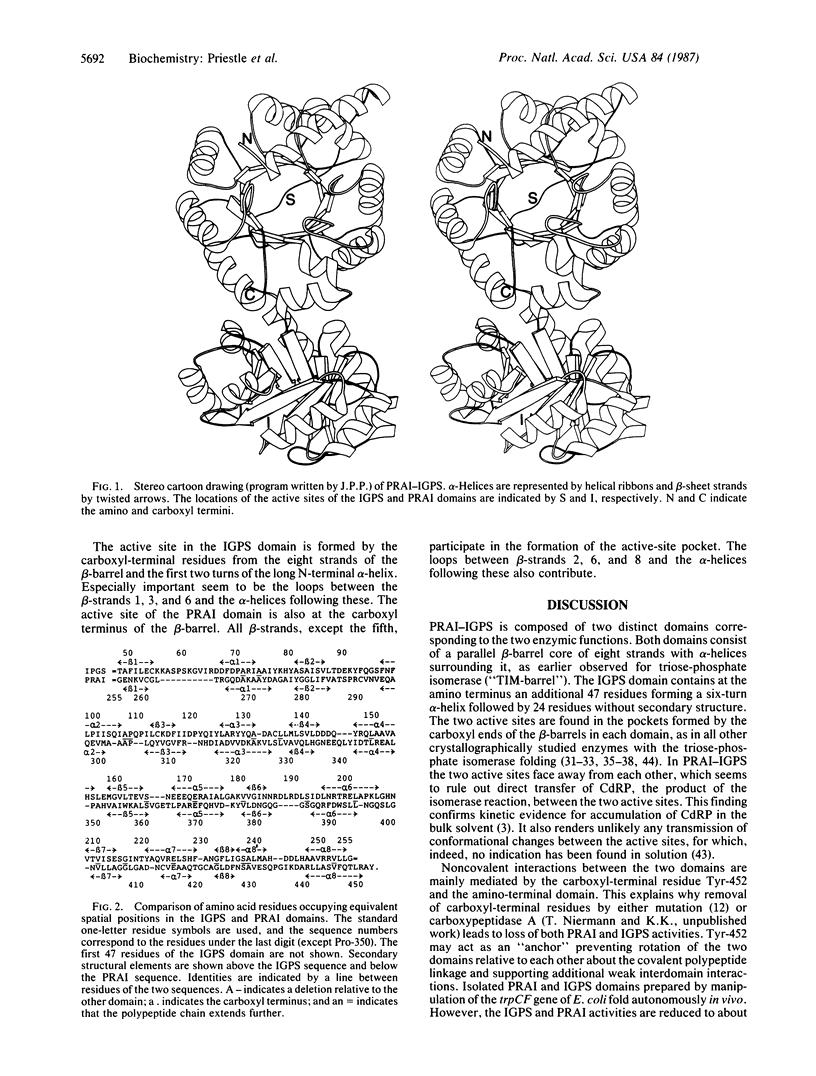
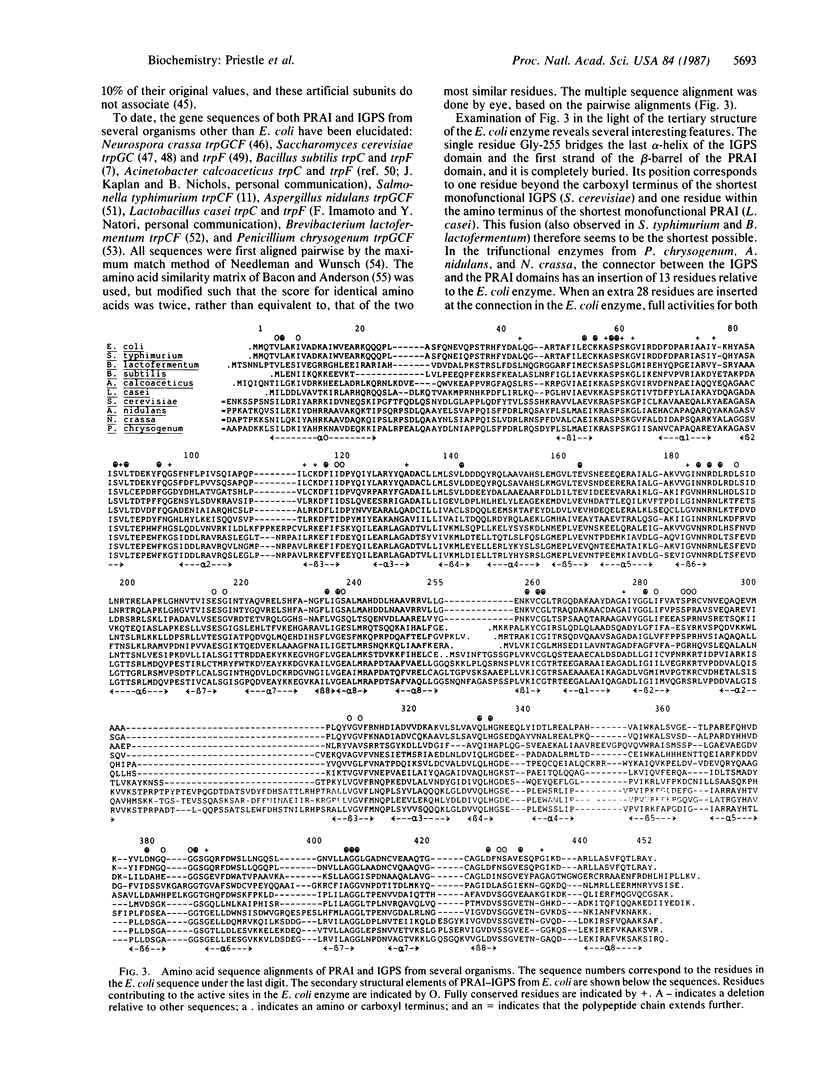
N-(5'-Phosphoribosyl)anthranilate isomerase-indole-3-glycerol-phosphate synthase from Escherichia coli is a monomeric bifunctional enzyme of Mr 49,500 that catalyzes two sequential reactions in the biosynthesis of tryptophan. The three-dimensional structure of the enzyme has been determined at 2.8-A resolution by x-ray crystallography. The two catalytic activities reside on distinct functional domains of similar folding, that of an eightfold parallel beta-barrel with alpha-helices on the outside connecting the beta-strands. Both active sites were located with an iodinated substrate analogue and found to be in depressions on the surface of the domains created by the outward-curving loops between the carboxyl termini of the beta-sheet strands and the subsequent alpha-helices. They do not face each other, making "channeling" of the substrate between active sites virtually impossible. Despite the structural similarity of the two domains, no significant sequence homology was found when topologically equivalent residues were compared.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Banner D. W., Bloomer A. C., Petsko G. A., Phillips D., Rivers P. S., Wilson I. A. On the three-dimensional structure and catalytic mechanism of triose phosphate isomerase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):159–171. doi: 10.1098/rstb.1981.0069. [DOI] [PubMed] [Google Scholar]
- Artymiuk P. J., Blake C. C. Refinement of human lysozyme at 1.5 A resolution analysis of non-bonded and hydrogen-bond interactions. J Mol Biol. 1981 Nov 15;152(4):737–762. doi: 10.1016/0022-2836(81)90125-x. [DOI] [PubMed] [Google Scholar]
- Bacon D. J., Anderson W. F. Multiple sequence alignment. J Mol Biol. 1986 Sep 20;191(2):153–161. doi: 10.1016/0022-2836(86)90252-4. [DOI] [PubMed] [Google Scholar]
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisswanger H., Kirschner K., Cohn W., Hager V., Hansson E. N-(5-Phosphoribosyl)anthranilate isomerase-indoleglycerol-phosphate synthase. 1. A substrate analogue binds to two different binding sites on the bifunctional enzyme from Escherichia coli. Biochemistry. 1979 Dec 25;18(26):5946–5953. doi: 10.1021/bi00593a029. [DOI] [PubMed] [Google Scholar]
- Burnett R. M., Grütter M. G., White J. L. The structure of the adenovirus capsid. I. An envelope model of hexon at 6 A resolution. J Mol Biol. 1985 Sep 5;185(1):105–123. doi: 10.1016/0022-2836(85)90186-x. [DOI] [PubMed] [Google Scholar]
- Carrell H. L., Rubin B. H., Hurley T. J., Glusker J. P. X-ray crystal structure of D-xylose isomerase at 4-A resolution. J Biol Chem. 1984 Mar 10;259(5):3230–3236. [PubMed] [Google Scholar]
- Christie G. E., Platt T. Gene structure in the tryptophan operon of Escherichia coli. Nucleotide sequence of trpC and the flanking intercistronic regions. J Mol Biol. 1980 Oct 5;142(4):519–530. doi: 10.1016/0022-2836(80)90261-2. [DOI] [PubMed] [Google Scholar]
- Cohn W., Kirschner K., Paul C. N-(5-Phosphoribosyl)anthranilate isomerase-indoleglycerol-phosphate synthase. 2. Fast-reaction studies show that a fluorescent substrate analogue binds independently to two different sites. Biochemistry. 1979 Dec 25;18(26):5953–5959. doi: 10.1021/bi00593a030. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. N-(5'-phosphoribosyl)anthranilate isomerase-indol-3-ylglycerol phosphate synthetase of tryptophan biosynthesis. Relationship between the two activities of the enzyme from Escherichia coli. Biochem J. 1970 Dec;120(4):699–707. doi: 10.1042/bj1200699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616–4624. [PubMed] [Google Scholar]
- Eichele G., Ford G. C., Glor M., Jansonius J. N., Mavrides C., Christen P. The three-dimensional structure of mitochondrial aspartate aminotransferase at 4.5 A resolution. J Mol Biol. 1979 Sep 5;133(1):161–180. doi: 10.1016/0022-2836(79)90255-9. [DOI] [PubMed] [Google Scholar]
- Enatsu T., Crawford I. P. Enzymes of the tryptophan synthetic pathway in Pseudomonas putida. J Bacteriol. 1968 Jan;95(1):107–112. doi: 10.1128/jb.95.1.107-112.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A., Ollis D. L., Steitz T. A. Crystal structure of muconate lactonizing enzyme at 3 A resolution. J Mol Biol. 1987 Mar 5;194(1):143–153. doi: 10.1016/0022-2836(87)90723-6. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Band L., Shimotsu H. Nucleotide sequence of the Bacillus subtilis tryptophan operon. Gene. 1985;34(2-3):169–177. doi: 10.1016/0378-1119(85)90125-8. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Crawford I. P. Enzymes of the tryptophan pathway in three Bacillus species. J Bacteriol. 1973 Nov;116(2):685–693. doi: 10.1128/jb.116.2.685-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H., Van Arsdell J., Platt T. Nucleotide sequence of the trpD and trpC genes of Salmonella typhimurium. J Mol Biol. 1983 Oct 5;169(4):775–797. doi: 10.1016/s0022-2836(83)80136-3. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Kaplan J. B., Goncharoff P., Seibold A. M., Nichols B. P. Nucleotide sequence of the Acinetobacter calcoaceticus trpGDC gene cluster. Mol Biol Evol. 1984 Nov;1(6):456–472. doi: 10.1093/oxfordjournals.molbev.a040331. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Szadkowski H., Henschen A., Lottspeich F. Limited proteolysis of N-(5'-phosphoribosyl)anthranilate isomerase: indoleglycerol phosphate synthase from Escherichia coli yields two different enzymically active, functional domains. J Mol Biol. 1980 Nov 15;143(4):395–409. doi: 10.1016/0022-2836(80)90219-3. [DOI] [PubMed] [Google Scholar]
- Levine M., Muirhead H., Stammers D. K., Stuart D. I. Structure of pyruvate kinase and similarities with other enzymes: possible implications for protein taxonomy and evolution. Nature. 1978 Feb 16;271(5646):626–630. doi: 10.1038/271626a0. [DOI] [PubMed] [Google Scholar]
- Lindqvist Y., Brändén C. I. Structure of glycolate oxidase from spinach at a resolution of 5.5 A. J Mol Biol. 1980 Oct 25;143(2):201–211. doi: 10.1016/0022-2836(80)90198-9. [DOI] [PubMed] [Google Scholar]
- Matsui K., Sano K., Ohtsubo E. Complete nucleotide and deduced amino acid sequences of the Brevibacterium lactofermentum tryptophan operon. Nucleic Acids Res. 1986 Dec 22;14(24):10113–10114. doi: 10.1093/nar/14.24.10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Kusunoki M., Harada W., Tanaka N., Iga Y., Yasuoka N., Toda H., Narita K., Kakudo M. Molecular structure of taka-amylase A. I. Backbone chain folding at 3 A resolution. J Biochem. 1980 May;87(5):1555–1558. doi: 10.1093/oxfordjournals.jbchem.a132896. [DOI] [PubMed] [Google Scholar]
- Mavridis I. M., Hatada M. H., Tulinsky A., Lebioda L. Structure of 2-keto-3-deoxy-6-phosphogluconate aldolase at 2 . 8 A resolution. J Mol Biol. 1982 Dec 5;162(2):419–444. doi: 10.1016/0022-2836(82)90536-8. [DOI] [PubMed] [Google Scholar]
- McQuade J. F., 3rd, Creighton T. E. Purification and comparison of the N-(5'-phosphoribosyl)anthranilic acid isomerase-indole-3-glycerol phosphate synthetase of tryptophan biosynthesis from three species of Enterobacteriaceae. Eur J Biochem. 1970 Oct;16(2):199–207. doi: 10.1111/j.1432-1033.1970.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Mullaney E. J., Hamer J. E., Roberti K. A., Yelton M. M., Timberlake W. E. Primary structure of the trpC gene from Aspergillus nidulans. Mol Gen Genet. 1985;199(1):37–45. doi: 10.1007/BF00327506. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Ollis D. L., Brick P., Hamlin R., Xuong N. G., Steitz T. A. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. 1985 Feb 28-Mar 6Nature. 313(6005):762–766. doi: 10.1038/313762a0. [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., Sánchez F. The complete nucleotide sequence of the trpC gene from Penicillium chrysogenum. Nucleic Acids Res. 1987 Feb 25;15(4):1874–1874. doi: 10.1093/nar/15.4.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechtman M. G., Yanofsky C. Structure of the trifunctional trp-1 gene from Neurospora crassa and its aberrant expression in Escherichia coli. J Mol Appl Genet. 1983;2(1):83–99. [PubMed] [Google Scholar]
- Schneider G., Lindqvist Y., Brändén C. I., Lorimer G. Three-dimensional structure of ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum at 2.9 A resolution. EMBO J. 1986 Dec 20;5(13):3409–3415. doi: 10.1002/j.1460-2075.1986.tb04662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. I., Levine M., Muirhead H., Stammers D. K. Crystal structure of cat muscle pyruvate kinase at a resolution of 2.6 A. J Mol Biol. 1979 Oct 15;134(1):109–142. doi: 10.1016/0022-2836(79)90416-9. [DOI] [PubMed] [Google Scholar]
- Sugimoto S., Shiio I. Enzymes of the tryptophan synthetic pathway in Brevibacterium flavum. J Biochem. 1977 Apr;81(4):823–833. doi: 10.1093/oxfordjournals.jbchem.a131546. [DOI] [PubMed] [Google Scholar]
- Thaller C., Weaver L. H., Eichele G., Wilson E., Karlsson R., Jansonius J. N. Repeated seeding technique for growing large single crystals of proteins. J Mol Biol. 1981 Apr 15;147(3):465–469. doi: 10.1016/0022-2836(81)90496-4. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Sequence of a yeast DNA fragment containing a chromosomal replicator and the TRP1 gene. Gene. 1980 Jul;10(2):157–166. doi: 10.1016/0378-1119(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Platt T., Crawford I. P., Nichols B. P., Christie G. E., Horowitz H., VanCleemput M., Wu A. M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981 Dec 21;9(24):6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Paluh J. L., van Cleemput M., Moye W. S., Yanofsky C. Nucleotide sequence of Saccharomyces cerevisiae genes TRP2 and TRP3 encoding bifunctional anthranilate synthase: indole-3-glycerol phosphate synthase. J Biol Chem. 1984 Mar 25;259(6):3985–3992. [PubMed] [Google Scholar]



