Abstract
The objective of this study was to assess the accuracy of blind placement of caudal epidural needles and the usefulness of the radio-contrast epidurogram. The study involves a prospective case series of 147 consecutive patients with radiological assessment of blind needle placement and epidurogram assessing the accuracy of blind needle placement in caudal epidurals. When the surgical miss rate (26%) and failure of flow of the therapeutic agents (6%) are combined, it can be deduced that up to 32% of non-radiologically guided caudal epidurals may fail to deliver the therapeutic agents to the site of pathology. There was no significant difference in the accuracy of needle placement in adequately trained and experienced middle grade surgeons when compared with consultant surgeons performing these procedures regularly. In conclusion, we recommend radiological guidance and use of epidurogram as the gold standard for the administration of caudal epidurals to increase the likelihood of successful delivery of the therapeutic agents to the site of pathology during the procedure.
Keywords: Caudal epidural, Epidurogram, Miss rates, Sciatica, Complications
Introduction
Epidural injections, via the inter-laminar, trans-foraminal or caudal routes, of local anaesthetic and corticosteroid are accepted treatments in the management of sciatica and spinal stenosis [1]. They offer the patient therapeutic benefit and offer the physician diagnostic information that can help guide future management.
Caudal epidural injections are safe and simple procedures that are frequently performed in outpatient departments or in primary care. Caudal epidurals are easier to administer in the outpatient department than lumbar epidurals.
We noticed a cohort of patients presenting to our spinal clinics who had no short-term (local anaesthetic response) or long-term (steroid response) benefit from non-radiologically confirmed caudal epidural injections. These had been administered by referring physicians and in the majority of cases at least some response to the injection could have been expected. We therefore suspected that there was a small but significant miss rate. We also knew from our own caudal epidural practice that even when we were highly confident that we were within the epidural space, radiological imaging occasionally showed our needle to be outside the spinal canal. We wanted to establish whether fluoroscopy and the use of an epidurogram to assess the cephalad limit of the injected therapeutic agents were necessary or useful in our continuing caudal epidural practice, particularly as important decisions about the subsequent management of the patients spinal pathology can be influenced by the patients response to the local anaesthetic and steroid components of the epidural injection.
Method
We prospectively studied a consecutive case series of 147 patients listed for caudal epidural injections. Radicular leg pain from spinal pathology including prolapsed intervertebral disc and spinal stenosis were the principle indications for the injection. There was no change in our usual practice of performing the caudal epidurals under light sedation with radiological confirmation of needle position. We routinely use a radio-opaque dye epidurogram to assess the delivery of the therapeutic agents.
After obtaining informed consent, the patients were positioned prone on a Montreal mattress and given a small amount of intravenous sedation by an anaesthetist. The skin of the buttocks and natal cleft cleaned with anti-septic chlorhexidine and the anatomical landmarks of the sacral cornua and the soft spot of the sacral hiatus identified.
Using strict aseptic technique, a 22-gauge 7-cm spinal needle was introduced through the sacral hiatus. Placement of the needle involved consideration of the anatomical landmarks and sensory feedback to the operator’s hand. The “give” of passing through the membrane of the sacral hiatus and the slight resistance to advancement of the spinal needle is usually characteristic of entering the epidural space. If the surgeon was not happy with the position of the needle then it was removed and reinserted.
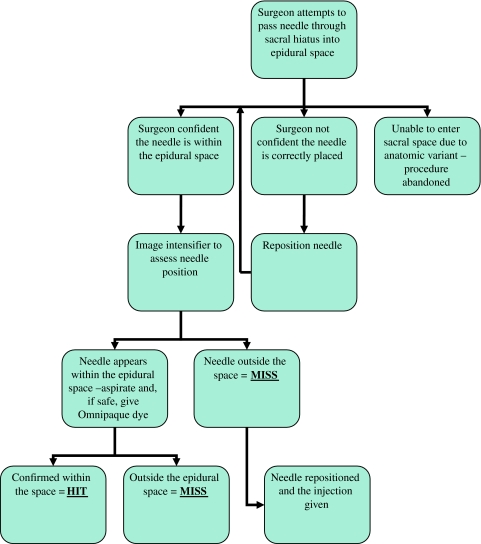
Only when the surgeon was confident that he has correctly entered the epidural space was a lateral radiograph of the sacrum taken. If the needle appeared to be in the epidural space the needle was aspirated to assess for blood, if no blood was drawn back 2 ml of Omnipaque (Iohexol 300 mg/ml, GE Healthcare) was injected to confirm correct placement. If the needle was correctly placed, as confirmed by the radio-contrast, the injection is classed as a “Hit” A “Miss” was recorded if the needle tip was not in the epidural space or if the radio-opaque dye did not flow freely or result in a characteristic epidurogram. The algorithm for the procedure is outlined in Fig. 1.
Fig. 1.
Algorithm for assessing the accuracy of caudal epidural needle placement
With the needle radiologically confirmed as being within the epidural space (and not within an epidural vein) a further 3 ml of Omnipaque was injected. The image intensifier is used to confirm cephalad flow of the dye to the level of the documented pathology. Only at this stage is the therapeutic injection of 80 mg of the steroid kenolog (Triamcinolone acetonide, Sigma Pharmaceuticals), 10 ml 0.25% Bupivocaine and 3 ml N saline (15 ml total) injected. A final radiograph of the level of the injected materials was taken and the level reached was recorded.
If the dye did not reach the documented level of pathology, the therapeutic injection of local anaesthetic and steroid was not given. In these circumstances, there is assumed to be a significant obstruction to the flow of the injected dye. The caudal route was therefore abandoned and an inter-laminar lumbar epidural with epidurogram was performed.
At the end of the procedure an adhesive dressing was applied and the patient was allowed home when the sedation had worn off and they were mobilizing safely.
All the procedures were carried out by two consultant spine surgeons, an associate specialist and three specialist registrars. All of the non-consultant grade surgeons had performed at least ten epidurals prior to the study and were deemed to be proficient in the technique by the supervising consultant. This conforms to the recommendations of The British Society for Rheumatology’s “Guidelines on Epidural Steroids for Spinal Pain” [2].
Basic patient demographics, the name and grade of surgeon performing the case, the underlying diagnosis with the level of pathology on MRI scans were recorded. A “Hit” or a “Miss” with the injection and the level the epidurogram reached following injection of the local anaesthetic and steroid was also documented.
Results
One hundred and forty-seven consecutive patients listed for caudal epidural were studied, of whom 5 did not attend for their injection. In two patients, we were unable to enter the sacral hiatus, which was assumed to be due to anatomical variation (calcified sacral hiatus or agenesis of the hiatus). These cases were converted to inter-laminar lumbar epidural injections. In three cases, there was insufficient data recorded and they have been excluded from the study. Information from the remaining 137 patients was analysed.
Of the 137 who remain, there were 71 females (52%) and 66 males (48%). The age range was 29–88 with a mean age of 56 years. 48 patients had their epidural for radicular leg pain (sciatica) with documented disc prolapses at L5/S1 (20 patients) L4/L5 (27 patients) and L2/L3 (1 patient). 76 patients had epidurals for stenotic symptoms, including nine cases of spondylolysthesis. Of these 7 had stenosis at L5/S1, 35 at L4/L5, 21 at L3/L4 and 4 patients had stenosis at L2/L3, the remaining 9 had multilevel spinal stenosis. Five had epidurals to alleviate leg pain in relation to failed back surgery and eight patients had multiple diagnoses that could account for their leg pain (i.e. spinal stenosis on MRI with concurrent osteoarthritis of hip or knee, vascular insufficiency, etc.) and received diagnostic epidurals to help delineate what proportion of their leg symptoms where attributable to their degenerate spine.
When assessed radiologically with radio-opaque contrast, 102 of the 137 had the needle correctly sited by the surgeon. In the other 35 patients, the needle was found to be outside the spinal canal either after initial fluoroscopic image or after injection of Omnipaque dye. The miss rate was 26% (95% confidence interval 18–33%).
Consultant surgeons performed 75 cases (56%); they had accurate blind placement of the needle in 57 of the 75 cases, giving them a miss rate of 24%. Middle grade surgeons performed the remaining 62 cases with accurate needle placement in 45, giving a miss rate of 27%. The difference in miss rate between consultants and non-consultant grades was not statistically different (chi-square p = 0.65).
Following the injection of contrast agent, it was noted that in two cases, the spinal needle was found to be within and epidural vein and gave an epidural venogram. This was noted despite negative aspirate to blood. The needles were repositioned within the caudal epidural space and further dye injected.
The epidurogram was used to assess the cephalad limit of the injected materials. In 94% of cases (129 of 137), the dye reached the documented level of spinal pathology. In the other 6% of cases (8 of 137), the epidurogram failed to reach the level of the pathology implying a blockage or restriction to flow. In these cases, the caudal route was abandoned and a standard lumbar inter-laminar epidural delivered to the level of pathology. This was also confirmed with epidurogram.
Discussion
Our study confirms that there can be miss rate of up to 26% in non-radiologically guided caudal epidurals. Our miss rate is similar to previous studies which demonstrate miss rates varying from 25 through to 36% in trained physicians performing this procedure regularly [3–7].
We found no significant difference in the miss rates between sub-populations of our cohort, in particular no difference was found with regard to patient sex, age, underlying diagnosis or body mass index. Patient body habitus has been shown to have an effect on the miss rate of caudal epidurals because of the difficulty in identifying the anatomical landmarks. Price et al. [3] felt that radiological guidance was required to confirm needle position during caudal epidural injections whatever the weight of the patient. We echo this sentiment even though we did not demonstrate a clear deleterious effect of obesity in our study.
There was no significant difference between the Hit/Miss rates of middle-grade surgeons and the consultant surgeons in our study. Renfrew et al. [7] demonstrated a clear learning curve in accuracy of needle placement in caudal epidurals. His study demonstrated significant increases in accuracy of needle placement as experience increases. Those who had performed less than 10 procedures had a miss rate 52.3%, those who had performed between 10 and 50 procedures had a miss rate of 46.6% and staff physicians had a miss rate of 38.3%. The findings from our study validate the recommendations of the The British Society for Rheumatology Guidelines on Epidural Steroids for Spinal Pain which states that trainees should observe 10 caudal injections and then perform 10 with supervision before embarking on unsupervised injections [2]. Our middle-grade surgeons demonstrated that the procedure could be performed safely after adequate training and a minimum of 10 supervised cases if radiological guidance is used to confirm needle placement.
The use of an epidurogram to confirm the level the dye/therapeutic agents reach is also important. In 6% of our cases the radio-opaque dye did not flow freely to the level of documented pathology. We therefore converted these cases to inter-laminar epidural injections with an epidurogram.
Our study also reminds us to be aware of anatomical variations of the sacral hiatus; we had two patients in whom we were unable to enter the sacral hiatus. In Senoglu’s study [8] of 96 dry sacral bones eight had abnormalities of the sacral hiatus (2 with posterior closure defect and six with agensis of the sacral hiatus).
Sekiguchi and colleagues [9] studied 92 sacra and found closed hiatus in 3% and absent hiatus in 4%. It could therefore be expected that between 5 and 10% of patients may have abnormality of the sacral hiatus making cannulation with a spinal needle difficult or impossible.
The physician should also be vigilant for inadvertent intra-vascular administration of the injected materials and be alert to the potential hazards and risks of systemic administration of local anaesthetic and steroids. The possibility of intravenous injection and its potential side effects should be covered in the consenting process [10].
We must also be aware that negative aspiration for blood does not guarantee that the needle is not within an epidural vein, which may “collapse” on aspiration. When we identified that we were in a vein with blood drawn back on aspiration the needle was repositioned. Despite this we still had a 1.5% intravascular injection rate. Intravascular injection of local anaesthetic agents can cause a variety of central nervous system and cardiovascular symptoms ranging from peri-oral tingling, drowsiness and facial flushing through hypotension to bradycardias and convulsions, which may result in cardio-respiratory arrest. Management of systemic toxicity should include early awareness of the complication, immediate cessation of the injection and appropriate management of the airway. Resuscitation equipment must be available [11]. The literature suggests rates of intravascular injection could be between 9 and 14% in non-radiologically guided caudal epidural injections [6, 7, 12].
We appreciate that the costs of caudal epidurals are increased when performed within a hospital setting with anaesthetic support and radiological guidance. However, we feel that we should strive to give our patients the best chance of adequate delivery of the therapeutic agents and we feel that radiological guidance and confirmatory epidurogram are important tools to achieve this goal. Other methods of identifying the sacral hiatus and confirming the needle is within the epidural space have been well documented. The use of ultrasound assisted guidance been shown to be accurate in identifying that sacral hiatus and can also be used to demonstrate flow of fluid within the spinal canal in children [13]. The use of ultrasound can be particularly beneficial where localised swelling makes palpation of the anatomical landmarks difficult [14]. The “Whoosh” test consists of injecting 2–3 ml of air into the epidural space with simultaneously auscultation proximally over the thoracic spine for the characteristic “whoosh” of the injected air. While this test is highly specific there is a risk of venous air embolism if the injection is inadvertently given into an epidural vein [15–17]. Both ultrasound guidance and the “Whoosh” test offer the advantage that there is no exposure to ionising radiation for either the patient or the surgeon. However, we feel that both have the disadvantage that there is no way of confirming effective delivery of the therapeutic agents to the level of pathology. This can only be done with an epidurogram and in our series 6% of cases had to be converted as the contrast dye did not reach the level of pathology.
It is also noted that fluoroscopic confirmation of needle position is a pre-requisite for any scientific study into epidural injections. A systematic review article recently excluded a study from its analysis as the investigators of the excluded paper had not used fluoroscopy to confirm the accuracy of the injection [1]. Even with the “gold standard” of radiological confirmation and epidurogram there could be some potential for error (i.e. Intra-dural injection) in delivery of the therapeutic agent.
Conclusion
Caudal epidural injections are used by a large number of physicians in the treatment of radicular leg pain and spinal stenosis. The vast majority of procedures are performed in an outpatient setting without the use of radiological guidance.
Our study confirms that even appropriately trained surgeons who perform these injections on a regular basis can miss the epidural space in 26% of cases when placing the spinal needle without radiological guidance. This misplacement error is well recognised and several ways of ensuring the needle is in the epidural space have been proposed, including ultrasound and injection of air and saline as a test.
We noted that in 6% of cases, even when the needle was optimally placed in the spinal canal, there was a failure of the therapeutic agents to reach the documented level of pathology.
Thus, if the surgical miss rate and the failure of flow of the injected materials are taken together it can be seen that up to a third of patients undergoing a non-radiologically guided caudal epidural injection may have no benefit from it. This is a particular concern as the further management of the patients spinal pathology can be significantly influenced by their response both the local anaesthetic and steroid components of the injection.
We therefore recommend radiological guidance and the routine use of a radio-contrast epidurogram as the gold standard for insuring adequate delivery of the therapeutic agents in caudal epidural injections.
References
- 1.Abdi S, Datta S, Trescot A, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007;10:185–212. [PubMed] [Google Scholar]
- 2.The British Society for Rheumatology. Guidelines on Epidural Steroids in Spinal Pain. http://www.rheumatology.org.uk/includes/documents/cm_docs/2009/e/epidural_steroids_for_spinal_pain.pdf
- 3.Price C, Rogers P, Prosser A, Arden N. Comparison of the caudal and lumbar approaches to the epidural space. Ann Rheum Dis. 2000;59:879–882. doi: 10.1136/ard.59.11.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White AH, Derby R, Wynne G. Epidural injections for the diagnosis and treatment of low back pain. Spine. 1980;5:78–86. doi: 10.1097/00007632-198001000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Stitz M, Sommer H. Accuracy of blind versus fluoroscopically guided epidural injection. Spine. 1999;24(13):1371–1376. doi: 10.1097/00007632-199907010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Manchikanti L, Cash K, Pampati V, McManus C, Damron K. Evaluation of fluoroscopically guided caudal epidural injections. Pain Physician. 2004;7(2):81–92. [PubMed] [Google Scholar]
- 7.Renfrew D, Moore T, Kathol M, el-Khoury G, Lemke J, Walker C. Correct placement of epidural steroid injections: fluoroscopic guidance and contrast administration. Am J Neuroradiol. 1991;12:1003–1007. [PMC free article] [PubMed] [Google Scholar]
- 8.Senoglu N, Senoglu M, Oksuz H, et al. Landmarks of the sacral hiatus for caudal epidural block: an anatomical study. Br J Anaesth. 2005;95(5):692–695. doi: 10.1093/bja/aei236. [DOI] [PubMed] [Google Scholar]
- 9.Sekiguchi M, Yabuki S, Satoh K, Kikuchi S. An anatomic study of the sacral hiatus: a basis for successful caudal epidural block. Clin J Pain. 2004;20(1):51–54. doi: 10.1097/00002508-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 10.General Medical Council Guidance on Consent. Patients and doctors making decisions together Part 2: making decisions about investigations and treatment. http://www.gmc-uk.org/guidance/ethical_guidance/consent_guidance/discussing_side_effects_complications_and_other_risks.asp
- 11.Royal College of Anaesthetists and The Pain Society. Recommendations on the use of epidural injections of back pain and leg pain of spin al origin. Royal College of Anaesthetists July 2002 Bulletin 14. http://www.britishpainsociety.org/epi_inj.pdf
- 12.Manchikanti L, Bakhit C, Pampati V. The role of epidurography in caudal neuroplasty. Pain Digest. 1998;8:277–281. [Google Scholar]
- 13.Chen C, Tang S, Hsu T, et al. Ultrasound guidance in epidural needle placement. Anaesthesiology. 2004;101:181–184. doi: 10.1097/00000542-200407000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz D, Raghunathan K, Dunn S, Conelly NR. Ultrasonography and paediatric caudals. Anesth Analg. 2008;106:97–99. doi: 10.1213/01.ane.0000287681.41646.b8. [DOI] [PubMed] [Google Scholar]
- 15.Lewis M, Thomas P, Wilson L, Mulholland R. The “Whoosh” test. A clinical test to confirm correct needle placement in caudal epidural injections. Anaesthesia. 1992;47:57–58. doi: 10.1111/j.1365-2044.1992.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 16.Eastwood D, Williams C, Buchan I. Caudal epidurals: the whoosh test. Anaesthesia. 1998;53:305–307. doi: 10.1046/j.1365-2044.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- 17.Saberski L, Kondamuri S, Osinubi O. Identification of the epidural space: is loss of resistance to air a safe technique? A review of the complications related to the use of air. Reg Anaesth. 1997;22(1):3–15. doi: 10.1016/S1098-7339(06)80050-7. [DOI] [PubMed] [Google Scholar]