Abstract
Due to the aging population, degenerative scoliosis is a growing clinical problem. It is associated with back pain and radicular symptoms. The pathogenesis of degenerative scoliosis lies in degenerative changes of the spinal structures, such as the intervertebral disc, the facet joints and the vertebrae itself. Possibly muscle weakness also plays a role. However, it is not clear what exactly causes the decompensation to occur and what determines the direction of the curve. It is known that in the normal spine a pre-existing rotation exists at the thoracic level, but not at the lumbar level. In this retrospective study we have investigated if a predominant curve pattern can be found in degenerative scoliosis and whether symptoms are predominantly present at one side relative to the curve direction. The lumbar curves of 88 patients with degenerative scoliosis were analyzed and symptoms were recorded. It was found that curve direction depended significantly on the apical level of the curve. The majority of curves with an apex above L2 were convex to the right, whereas curves with an apex below L2 were more frequently convex to the left. This would indicate that also in degenerative scoliosis the innate curvature and rotational pattern of the spine plays a role in the direction of the curve. Unilateral symptoms were not coupled to the curve direction. It is believed that the symptoms are related to local and more specific degenerative changes besides the scoliotic curve itself.
Keywords: Lumbar spine, Spinal decompensation, Degenerative scoliosis, Curve pattern, Right–left distribution
Introduction
Scoliosis, newly developed in a skeletally mature patient, is termed degenerative scoliosis or de novo scoliosis. Prevalence and incidence of degenerative scoliosis increase with age. An overall increase is seen due to the demographic shift towards an aging society [17, 33, 39]. Women are mostly affected [1, 31]. Generally it is assumed that degenerative scoliosis is caused by asymmetric disc degeneration and facet joint degeneration [1, 3, 4, 7, 27, 30, 36] and that the onset is marked by disc degeneration [17, 25]. This etiology sets degenerative scoliosis apart from other types of scoliosis, such as adolescent idiopathic scoliosis and scoliosis secondary to neuromuscular disease. In addition, degenerative scoliosis develops most frequently in the lumbar spine, where degenerative changes are most prevalent, whereas in neuromuscular and idiopathic scoliosis the major curve is usually in the thoracic or thoracolumbar spine.
Kouwenhoven et al. [20] showed that the normal spine has a specific pattern of vertebral rotation, with a predominant rotation to the right at the mid-thoracic level. Most prevalent curve types of adolescent idiopathic scoliosis were found to demonstrate comparable patterns [8, 11, 12, 14, 21, 23, 32, 35, 40], which correspond to the predominant rotational pattern in the normal spine [19]. This implies that once the spine starts to decompensate, due to a still unknown cause, it follows this already built-in rotational tendency [19]. In degenerative scoliosis factors causing asymmetrical degeneration and the eventual disruption of the spinal equilibrium are still unidentified. Whether a pre-existing rotational pattern also plays a role in degenerative scoliosis is unknown. An argument against this is that the normal lumbar spine does not show an obvious rotation deviating from the midline [20]. We hypothesize that spinal decompensation based on degenerative changes is purely coincidental and that left and right curves in lumbar degenerative scoliosis are equally distributed. However, if the distribution of curve direction is unequal, this may point to a pre-existing rotational tendency that has so far not been demonstrated.
Symptoms of degenerative scoliosis are most frequently progressive back pain, radiculopathy and neurogenic claudication [1, 3, 7, 9, 13, 15]. Aging progressively affects all structures of the spinal units, eventually leading to degenerative instabilities such as spondylolisthesis, spinal stenosis and scoliosis [2]. Considering these multiple degenerative pathologies, identifying the exact pain source is very difficult. Relationships between scoliotic pattern and patient symptoms are unclear, although speculations on such relationships are frequently made [1, 3, 7, 30, 42]. Some authors suggest that pain at the convexity is caused by muscle fatigue of the paraspinal muscles [3, 30, 42]. Other authors claim that pain at the convexity can also be caused by facet joints [7]. Pain at the concavity of the scoliotic curve is thought to be caused by destruction of facet joints [42] and degenerative changes in disc spaces [30]. Radicular pain at the concavity can arise from narrowed foramen [1, 3, 42] or ruptured discs causing radiculopathy [1, 3]. Dynamic overstretch of a nerve root could also cause radicular pain at the convex side of the scoliosis [1]. None of these speculations are evidence based.
The first objective of this study is to assess whether a dominant direction of compensation exists in degenerative scoliosis. Our second objective is to investigate whether asymmetric symptoms are related to the curve direction, i.e. whether pain and radicular symptoms in degenerative lumbar scoliosis are predominantly present at one side relative to the curve direction.
Materials and methods
In this retrospective study we filtered the outpatient records of our institution for patients who presented between 1996 and 2007 and were diagnosed with degenerative scoliosis. Patients aged 50 and over at the initial presentation of degenerative scoliosis and had a scoliosis of at least 10° in the coronal plain were included. Patients were excluded if they had a previous history of idiopathic scoliosis, neuromuscular scoliosis, or scoliosis secondary to an underlying pathology.
The patient records were reviewed in order to obtain patient data about age, gender, length, height, weight, symptoms, physical examination and history. The lumbar standing radiographs of the patients were reviewed by two independent observers. For each patient, curve direction, apical level, apical vertebra, Cobb angle in the coronal plane, and length of the curve were determined.
The apex of the curve was determined in the coronal plane and the apical level was determined. The apical vertebra was defined according to the Scoliosis Research Society (SRS) as the vertebra with the greatest distance from the midline with the most rotation [34]. According to the SRS classification curves with an apex at the 12th thoracic vertebral body or the first lumbar vertebral body are thoracolumbar curves. Curves with an apex between the first lumbar disc and the fourth lumbar vertebral body are lumbar curves [22]. The Cobb angle is the angle between the two most tilted vertebrae within a scoliotic curve [6]. The length of the curves was taken as the number of vertebrae between these two most tilted vertebrae.
Patient symptoms recorded were low back pain, pain in the buttocks, leg pain, hypo- or hyperesthesia and weakness affecting the legs.
Statistical analysis
All statistical analyses were performed with SPSS 15.0 for windows (SPSS, Inc, Chicago, IL). The binominal test was used to compare the observed frequencies of right and left curves to an equal distribution. The tests were performed for all curves combined and also separately per apical level.
In addition, it was tested whether curve direction depended on apex level using the Chi-square test.
Whether the observed frequencies of ipsilateral and contralateral pain and/or radicular symptoms relative to the scoliotic curve direction differs from an equal distribution was tested using a binominal test.
Results
Curve patterns
Eighty-eight patients met the inclusion criteria. The group consisted of 71 women and 17 men with a mean age of 70 (70.1 ± 10.3; range 50–93). Of these patients 50 were ultimately treated conservatively and 38 were treated surgically.
All patients had a single curve in the thoracolumbar range, except one patient who had a double curve. For this double curve, the curve with the largest Cobb angle was considered in the analyses. Of the total group, 39 curves (44%) were to the right and 49 (56%) were to the left (p = 0.337). Apical levels ranged from the 12th thoracic disc to the 4th lumbar vertebra (median apex, 2nd lumbar disc). The mean Cobb angle was 25° (25.4 ± 13.0; range 10°–83°). The median curve length was 4 vertebrae (range 2–6). Eighty-three patients had lumbar curves and five patients had thoracolumbar curves according to the SRS classification. Fifty-eight percent (49) of the lumbar curves were convex to the left (p = 0.156). Eighty-three percent (5) of the thoracolumbar curves were convex to the right (p = 0.375). The apical vertebrae of the curves varied from L1 to L4 (Table 1), with L3 the median apical vertebra.
Table 1.
The distribution of curve direction for each apical vertebra
| Apical vertebra of curve | n | Number of curves convex to the right | Number of curves convex to the left | p (binominal test) |
|---|---|---|---|---|
| L1 | 6 | 5 (83%) | 1 (17%) | 0.219 |
| L2 | 35 | 20 (57%) | 15 (43%) | 0.500 |
| L3 | 39 | 12 (31%) | 27 (69%) | 0.024* |
| L4 | 8 | 2 (25%) | 6 (75%) | 0.289 |
The p level indicates whether the distribution deviates significantly from an equal left–right distribution
*Statistically significant (p < 0.05)
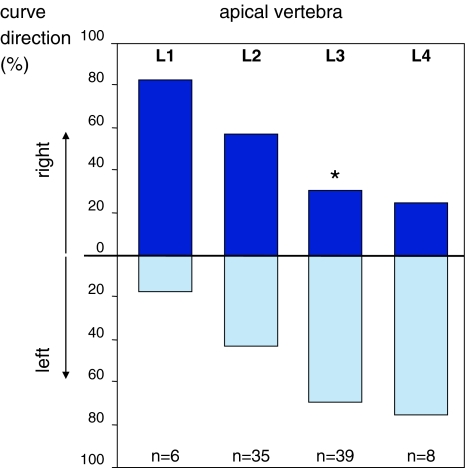
Left and right distributions were also tested per apical vertebra (Table 1). For apical level L1 an inclination for convexity to the right was observed. Eighty percent (4) of patients with the apex of the curve at L1 had a convexity to the right (p = 0.219). In 35 patients L2 was the apical vertebra. In this group no tendency to left or right was observed. A small majority, 57% (20) of curves were convex to the right (p = 0.500). Curves with an apex at L3 (n = 39), however, showed a significant inclination to the left. In this group 69% (27) had a curve convexity to the left (p = 0.024). Also most (75%, n = 6) of the curves with the apex at L4 were convex to the left (p = 0.289). The curve direction depended significantly on the apical level (p = 0.011) and on the level of the apical vertebra (p = 0.017) (Fig. 1).
Fig. 1.
Distribution of left and right convex curves per apical vertebra. The direction of the curve depends significantly on the apical vertebra (Chi-square, p = 0.017). When tested separately per level, the left–right distribution for curves with an apex at L3 differed significantly from a random distribution (binominal, p = 0.024)
Symptoms
All patients had low back pain. Only four patients (5%) had unilateral back pain. This was not related to the curve direction, since two patients had pain at the same, and two at the contralateral side of curve convexity. Eighty-two percent (72) of all patients had radicular leg pain. Of these patients 57% (41) had unilateral leg pain, the others had pain in both legs. The side at which unilateral radicular leg pain occurred was unrelated to the curve direction; 21 patients (51%) had symptoms ipsilateral to the curve convexity and 20 (49%) patients contralateral to the scoliotic curve (p = 1.000). Pain in the buttocks was reported by one patient. Seventy-two patients had symptoms of the lower extremities of which 22 had weakness, 1 hyperesthesia, 6 hypoesthesia and 11 showed neurological symptoms during physical examination.
Discussion
In this retrospective radiographic study we have assessed the direction of curve decompensation in a group of 88 patients with degenerative lumbar scoliosis. It was found that curve direction depended significantly on the apical level of the curve. Interestingly, the majority of curves with an apex above L2 were convex to the right, whereas curves with an apex below L2 were more frequently convex to the left. The SRS classification does not differentiate in this respect, since the transition from left to right-sided curves falls within the class of lumbar curves. Hence, when looking at all lumbar curves combined, no predominant curve direction is found.
Vertebral rotation and lateral deviation of the spine are coupled phenomena, with the rotation of vertebral bodies directed into the convexity of the curve [41]. It was previously demonstrated that in the normal non-scoliotic spine a predominant left-sided rotation exists in the high thoracic vertebrae, whereas the mid- and lower thoracic vertebrae are predominantly rotated to the right [20]. This prevalent rotational pattern in the normal spine corresponds with the predominance of right-sided thoracic and thoracolumbar curves in idiopathic and neuromuscular scoliosis [19, 38]. The lumbar vertebrae of the normal spine do not show a predominant rotation. However, in idiopathic and neuromuscular scoliosis often a left-sided compensatory curve is seen at the lumbar level [19, 38]. Although in lumbar degenerative scoliosis, the scoliotic curve is essentially at the lumbar level, it apparently does show the same predominant direction as the compensatory lumbar curve in idiopathic or neuromuscular scoliosis. The strong relationship between apical level and curve direction does indicate that also in degenerative scoliosis the innate curvature of the spine plays a role in the direction of the curve. In this study we have analyzed curve directions in the coronal plane, although scoliosis is essentially a complex 3D deformation [26]. Nevertheless, a good impression can be obtained from 2D images because lateral deviation is coupled to rotational deviation [20, 38].
In idiopathic scoliosis, it is assumed that biomechanical factors play a role in the development and progression of the curvature. It is thought that a spine with scoliosis experiences greater loading on the concave side and that this asymmetrical loading causes asymmetrical growth and progression of deformity [37]. Similar processes may play a role in degenerative scoliosis, only in this case, the greater loads at the concave side may induce degenerative changes which could result in a further progression of the scoliosis. These degenerative changes, however, can be diverse, ranging from degenerative changes in the intervertebral discs to spondylolysis or spondylolisthesis, rotatory dislocations and destruction of facet joints, depending on the “weakest link” [28]. It is known that disc degeneration temporarily induces segmental instability [16, 24], making the spinal construct more vulnerable to forces that can increase a slight pre-existing rotatory pattern, such as dorsally directed shear loads (DDSL’s) [5, 18]. The fact that degenerative scoliosis occurs most often in the lumbar spine, where most vertebrae are subject to these DDSL’s supports this assumption.
In our patient population, we found that all patients presented with back pain and most patients experienced radicular leg pain. No relationship existed between curve direction and the direction of one-sided symptoms. Several possible mechanisms have been put forward to explain the causes of radicular pain in degenerative scoliosis. Radicular pain may result from impingement of the nerve root due to decreased foraminal width or overstretching of the neural elements. The intervertebral foraminal width changes during spinal motion [10, 16]. It decreases during extension, ipsilateral lateral side bending, and ipsilateral axial rotation and increases during flexion, contralateral lateral side bending and contralateral axial rotation. Based on this, in scoliosis a decreased foraminal width can be expected at the concavity of the curve. Although this was corroborated in an MRI study of Ploumis et al. [29], they found that the foraminal width at the concave side was still within the normal range. They concluded that ligamentum flavum hypertrophy, posterior disc bulging, and bony overgrowth are more likely to contribute to stenosis irrespective of scoliosis. This would also explain why in our study radicular leg pain is not related to the direction of the curve. Hence, for a better understanding of the symptoms related to degenerative scoliosis the underlying local deformations should be studied in more detail.
Conclusion
One of the potential effects of severe degeneration is scoliosis. In 88 patients with degenerative lumbar scoliosis we found a significant relationship between curve direction and the apex of the curve. Curves with an apex above L2 were mostly convex to the right, whereas curves with an apex below L2 were more frequently convex to the left. This indicates that in degenerative scoliosis the innate curvature or rotatory pattern of the spine is an important factor for the direction of spinal decompensation. Although the normal spine does not show a pre-existing rotation at the lumbar level, the asymmetry of the whole spine is presumably directive for the decompensatory changes. The primary symptoms were back pain and radicular leg pain. The side of unilateral symptoms is unrelated to the curve direction. This supports the concept that symptoms are the result of specific degenerative changes irrespective of the scoliotic curve.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Aebi M. The adult scoliosis. Eur Spine J. 2005;14(10):925–948. doi: 10.1007/s00586-005-1053-9. [DOI] [PubMed] [Google Scholar]
- 2.Benoist M. Natural history of the aging spine. Eur Spine J. 2003;12(Suppl 2):S86–S89. doi: 10.1007/s00586-003-0593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birknes JK, White AP, Albert TJ, Shaffrey CI, Harrop JS. Adult degenerative scoliosis: a review. Neurosurgery. 2008;63(3 Suppl):94–103. doi: 10.1227/01.NEU.0000325485.49323.B2. [DOI] [PubMed] [Google Scholar]
- 4.Bradford DS, Tay BK, Hu SS. Adult scoliosis: surgical indications, operative management, complications, and outcomes. Spine. 1999;24(24):2617–2629. doi: 10.1097/00007632-199912150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Castelein RM, van Dieen JH, Smit TH. The role of dorsal shear forces in the pathogenesis of adolescent idiopathic scoliosis—a hypothesis. Med Hypotheses. 2005;65(3):501–508. doi: 10.1016/j.mehy.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Cobb JR. Outline for the study of scoliosis. Instructional course lectures. Ann Arbor, MI: The American Academy of Orthopaedic Surgeons; 1948. pp. 261–275. [Google Scholar]
- 7.Davies A, Saifuddin A. Imaging of painful scoliosis. Skeletal Radiol. 2008;38(3):207–223. doi: 10.1007/s00256-008-0517-5. [DOI] [PubMed] [Google Scholar]
- 8.Dickson RA, Lawton JO, Archer IA, Butt WP. The pathogenesis of idiopathic scoliosis. Biplanar spinal asymmetry. J Bone Jt Surg Br. 1984;66(1):8–15. doi: 10.1302/0301-620X.66B1.6693483. [DOI] [PubMed] [Google Scholar]
- 9.Epstein JA, Epstein BS, Jones MD. Symptomatic lumbar scoliosis with degenerative changes in the elderly. Spine. 1979;4(6):542–547. doi: 10.1097/00007632-197911000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara A, An HS, Lim TH, Haughton VM. Morphologic changes in the lumbar intervertebral foramen due to flexion-extension, lateral bending, and axial rotation: an in vitro anatomic and biomechanical study. Spine. 2001;26(8):876–882. doi: 10.1097/00007632-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg C, Dowling FE. Handedness and scoliosis convexity: a reappraisal. Spine. 1990;15(2):61–64. doi: 10.1097/00007632-199002000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg CJ, Moore DP, Fogarty EE, Dowling FE. Left thoracic curve patterns and their association with disease. Spine. 1999;24(12):1228–1233. doi: 10.1097/00007632-199906150-00010. [DOI] [PubMed] [Google Scholar]
- 13.Grubb SA, Lipscomb HJ. Diagnostic findings in painful adult scoliosis. Spine. 1992;17(5):518–527. doi: 10.1097/00007632-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, Chau WW, Chan YL, Cheng JC. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. Results of disproportionate endochondral–membranous bone growth. J Bone Jt Surg Br. 2003;85(7):1026–1031. doi: 10.1302/0301-620X.85B7.14046. [DOI] [PubMed] [Google Scholar]
- 15.Gupta MC. Degenerative scoliosis. Options for surgical management. Orthop Clin North Am. 2003;34(2):269–279. doi: 10.1016/S0030-5898(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 16.Kirkaldy-Willis WH, Farfan HF (1982) Instability of the lumbar spine. Clin Orthop Relat Res(165):110–123 [PubMed]
- 17.Kobayashi T, Atsuta Y, Takemitsu M, Matsuno T, Takeda N. A prospective study of de novo scoliosis in a community based cohort. Spine. 2006;31(2):178–182. doi: 10.1097/01.brs.0000194777.87055.1b. [DOI] [PubMed] [Google Scholar]
- 18.Kouwenhoven JW, Castelein RM. The pathogenesis of adolescent idiopathic scoliosis: review of the literature. Spine. 2008;33(26):2898–2908. doi: 10.1097/BRS.0b013e3181891751. [DOI] [PubMed] [Google Scholar]
- 19.Kouwenhoven JW, Van Ommeren PM, Pruijs HE, Castelein RM. Spinal decompensation in neuromuscular disease. Spine. 2006;31(7):E188–E191. doi: 10.1097/01.brs.0000208131.42824.c3. [DOI] [PubMed] [Google Scholar]
- 20.Kouwenhoven JW, Vincken KL, Bartels LW, Castelein RM. Analysis of preexistent vertebral rotation in the normal spine. Spine. 2006;31(13):1467–1472. doi: 10.1097/01.brs.0000219938.14686.b3. [DOI] [PubMed] [Google Scholar]
- 21.Lenke LG, Betz RR, Clements D, et al. Curve prevalence of a new classification of operative adolescent idiopathic scoliosis: does classification correlate with treatment? Spine. 2002;27(6):604–611. doi: 10.1097/00007632-200203150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Lowe T, Berven SH, Schwab FJ, Bridwell KH. The SRS classification for adult spinal deformity: building on the King/Moe and Lenke classification systems. Spine. 2006;31(19 Suppl):S119–S125. doi: 10.1097/01.brs.0000232709.48446.be. [DOI] [PubMed] [Google Scholar]
- 23.Millner PA, Dickson RA. Idiopathic scoliosis: biomechanics and biology. Eur Spine J. 1996;5(6):362–373. doi: 10.1007/BF00301963. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki M, Hong SW, Yoon SH, et al. Kinematic analysis of the relationship between the grade of disc degeneration and motion unit of the cervical spine. Spine. 2008;33(2):187–193. doi: 10.1097/BRS.0b013e3181604501. [DOI] [PubMed] [Google Scholar]
- 25.Murata Y, Takahashi K, Hanaoka E, et al. Changes in scoliotic curvature and lordotic angle during the early phase of degenerative lumbar scoliosis. Spine. 2002;27(20):2268–2273. doi: 10.1097/00007632-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 26.Negrini S, Negrini A, Atanasio S, Santambrogio GC. Three-dimensional easy morphological (3-DEMO) classification of scoliosis, part I. Scoliosis. 2006;1:20. doi: 10.1186/1748-7161-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oskouian RJ Jr, Shaffrey CI (2006) Degenerative lumbar scoliosis. Neurosurg Clin N Am 17(3):299–315, vii [DOI] [PubMed]
- 28.Piat C, Laredo JD, Tassin JL. Degenerative vertebral dislocation. Ann Radiol (Paris) 1995;38(4):214–220. [PubMed] [Google Scholar]
- 29.Ploumis A, Transfeldt EE, Gilbert TJ, Jr, et al. Degenerative lumbar scoliosis: radiographic correlation of lateral rotatory olisthesis with neural canal dimensions. Spine. 2006;31(20):2353–2358. doi: 10.1097/01.brs.0000240206.00747.cb. [DOI] [PubMed] [Google Scholar]
- 30.Ploumis A, Transfledt EE, Denis F. Degenerative lumbar scoliosis associated with spinal stenosis. Spine J. 2007;7(4):428–436. doi: 10.1016/j.spinee.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Pritchett JW, Bortel DT. Degenerative symptomatic lumbar scoliosis. Spine. 1993;18(6):700–703. doi: 10.1097/00007632-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Sahlstrand T. An analysis of lateral predominance in adolescent idiopathic scoliosis with special reference to convexity of the curve. Spine. 1980;5(6):512–518. doi: 10.1097/00007632-198011000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine. 2005;30(9):1082–1085. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 34.Scoliosis Research Society. Glossary. Available at http://www.srs.org/patients/glossary.php. Accessed 2009
- 35.Sevastik JA, Aaro S, Normelli H (1984) Scoliosis. Experimental and clinical studies. Clin Orthop Relat Res(191):27–34 [PubMed]
- 36.Smith JS, Shaffrey CI, Kuntz C, Mummaneni PV. Classification systems for adolescent and adult scoliosis. Neurosurgery. 2008;63(3 Suppl):16–24. doi: 10.1227/01.NEU.0000320447.61835.EA. [DOI] [PubMed] [Google Scholar]
- 37.Stokes IA. Analysis and simulation of progressive adolescent scoliosis by biomechanical growth modulation. Eur Spine J. 2007;16(10):1621–1628. doi: 10.1007/s00586-007-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stokes IA, Sangole AP, Aubin CE. Classification of scoliosis deformity three-dimensional spinal shape by cluster analysis. Spine. 2009;34(6):584–590. doi: 10.1097/BRS.0b013e318190b914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderpool DW, James JI, Wynne-Davies R. Scoliosis in the elderly. J Bone Jt Surg Am. 1969;51(3):446–455. [PubMed] [Google Scholar]
- 40.Weinstein SL. Natural history. Spine. 1999;24(24):2592–2600. doi: 10.1097/00007632-199912150-00006. [DOI] [PubMed] [Google Scholar]
- 41.White AA III, Panjabi MM (1979) Practical biomechanics of scoliosis. Clinical biomechanics of the spine. Lippencott, Philadelphia, PA, pp 91–114
- 42.Winter RB, Lonstein JE, Denis F. Pain patterns in adult scoliosis. Orthop Clin North Am. 1988;19(2):339–345. [PubMed] [Google Scholar]