Abstract
During anterior scoliosis instrumentation with a dual-rod system, the vertebrae are dissected anterolaterally. After surgery, some patients report a change in temperature perception and perspiration in the lower extremities. Sympathetic lesions might be an explanation for this. The aim of this clinical study was to investigate sympathetic function after anterior scoliosis instrumentation. A total of 24 female patients with idiopathic scoliosis (mean age at follow-up, 23.8 years) who had undergone anterior instrumentation on average 6.6 years earlier were included. Due to the suspected relevance of the sympathetic L2 ganglion, two groups were created: a T12 group, in which instrumentation down to T12 was carried out (n = 12), and an L3 group, in which instrumentation down to L3 was done (n = 12). Sympathetic function was assessed by measuring skin temperature at the back of the foot, a plantar ninhydrin sweat test and sympathetic skin responses (SSRs) following electrical stimulation. The side on which the surgical approach was carried out was compared with the contralateral, control side. Health-related quality of life was investigated using the Scoliosis Research Society SRS-22 patient questionnaire. In the T12 group, mean temperatures of 29.6°C on the side of the approach versus 29.5°C on the control side were measured (P > 0.05); in the L3 group, the mean temperatures were 33.2°C on the approach side versus 30.5°C on the control side (P = 0.001). A significant difference between the T12 group and the L3 group (P < 0.001) was observed on the approach side, but not on the control side (P = 0.15). The ninhydrin sweat test showed reduced perspiration in 11 of 12 patients in the L3 group on the approach side in comparison with the control side (P = 0.002). In the T12 group, no significant differences were noted between the left and right feet. SSRs differed significantly between the two groups (P = 0.005). They were detected in all nine analyzable patients in the T12 group on both sides. In the L3 group, they were found on the approach side only in 4 of 11 analyzable patients versus 11 patients on the control side. The results of the SRS-22 questionnaire did not show any significant differences between the two groups. In conclusion, anterior scoliosis instrumentation with a dual-rod system including vertebrae down to L3 regularly leads to lesions in the sympathetic trunk. These are detectable with an increase in temperature, reduced perspiration and reduced SSRs. The caudal level of instrumentation (T12 vs. L3) has an impact on the extent of impairment, supporting the suspected importance of the L2 ganglion. The clinical outcome does not seem to be significantly limited by sympathetic trunk lesions.
Keywords: Scoliosis, Anterior correction and instrumentation, Sympathetic chain, Complication
Introduction
Anterior correction and instrumentation of idiopathic scoliosis is a procedure originally developed by Dwyer et al. [13], which has been improved and has become established as a routine method during the last few years [16, 17, 26, 64]. The reported risks and complications of anterior instrumented correction include pseudarthrosis and rod breakages, minimal loss of correction and screw loosening in the cranial part of the instrumentation. However, incidences of these have been reduced considerably due to the development from a flexible single-rod system over rigid single-rod systems to dual-rod systems [16, 20, 26, 33, 53]. Another reported risk is injury to the aorta, due to its close vicinity to the screw tips [8, 24]. The corrective potential for the primary curve in the frontal plane of anterior idiopathic scoliosis instrumentation has been reported to range between 43 and 84% for single-rod instrumentations and between 43 and 71% for dual-rod instrumentations [53].
The neurological risk associated with anterior instrumented correction was earlier reported to be lower than the risk with posterior scoliosis surgery, but more recent morbidity and mortality data from the Scoliosis Research Society now no longer show this, with reported risks of approximately 0.5% for both techniques [11, 16]. Several studies have observed no neurological complications at all [33].
Approach-related side effects that have been reported include a more negative effect on pulmonary function in anterior transthoracic scoliosis surgery in comparison to posterior surgery; however, this pulmonary impairment in anterior thoracic scoliosis surgery improved significantly during the first 2 years of follow-up [16, 27, 28, 35]. Further approach-related side effects and complications include atelectasis, pleural effusion, pneumothorax and chylothorax [16, 26]. Sympathectomy has been described as a possible complication [16], but no systematic research is available on this topic nor does literature provide any clear incidence rates.
In the authors’ department, anterior scoliosis surgery has been an established procedure since the early 1990s. During the follow-up after surgery, some patients reported differences in temperature and perspiration between the lower extremities. As the vertebral bodies are dissected anterolaterally followed by instrumentation with plates and rods during the surgical approach for anterior correction and instrumentation in idiopathic scoliosis, lesions of the sympathetic chain and plexus that surround the spine caused during surgery might be a possible explanation for these findings.
A review of literature did not identify any systematic investigations of sympathetic lesions caused by anterior instrumentation for scoliosis. The present study was therefore conducted to investigate the sympathetic function in patients following anterior scoliosis surgery.
Materials and methods
Patients
A total of 24 female patients with idiopathic scoliosis (mean age at follow-up 23.8 ± 5.0 years, range 18.3–40.6 years), who had undergone anterior correction and instrumentation at a single institution 2.3–12.3 years previously (mean 6.6 ± 2.9 years) at a mean age of 17.2 ± 4.3 years (range 13.5–34.6 years), were included in this clinical investigation.
Due to the relevance of the L2 sympathetic ganglion (Fig. 1), two groups were created: a T12 group, with instrumentation down to T12 = thoracic scoliosis (n = 12; mean age at surgery 16.1 ± 2.3 years, range 13.5–19.8 years; mean follow-up 5.9 ± 3.1 years, range 2.3–12.3 years); and an L3 group, with instrumentation down to L3 = thoracolumbar scoliosis (n = 12; mean age at surgery 18.2 ± 5.6 years, range 13.6–34.6 years; mean follow-up 7.4 ± 2.6 years, range 3.4–11.2 years). Groups 1 and 2 did not differ significantly with regard to age at surgery or duration of follow-up (Mann–Whitney U test: P > 0.05). The raw data for the patients, including the major results, are listed in Table 1. In the T12 group, all patients had a right convex primary curve and consequently a right-sided double thoracotomy approach. In the L3 group, 11 patients had a left convex primary curve with a left-sided thoracolumbotomy approach; one patient (with instrumentation from T9 to L3) received a right-sided approach due to a right convex primary curve. Figure 2 presents an example of pre- and postoperative radiographs of a patient of group 1.
Fig. 1.
The anatomy of the lumbar sympathetic system. All sympathetic ganglia in the chain (yellow) below ganglion L2 receive their information from spinal cord levels L2 and above [6, 42, 45]. The L2 sympathetic ganglion bundles all sympathetic fibers of the lower extremities, pelvis, and pelvic organs. “L2”, spinal cord at level L2; IvF L2, intervertebral foramen at L2, with the L2 nerve root passing through it; SCGgl L2, L2 sympathetic chain ganglion; Postggl. fibers, postganglionic fibers (purple dotted line), running to the periphery with spinal nerves or running with and supplying blood vessels (BV). Blue dotted line: preganglionic fibers
Table 1.
Patients’ raw data
| Patient number | Lenke curve type | Cobb main curve (degree) | Instrumented vertebrae | Sex | Age | Side of approach | SRS-22 | Temperature (°C) | SSR | Ninhydrin test (color reaction) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop. | Follow-up | At surgery (years) | At follow-up (years) | Total score | Side of approach | Control side | Side of approach | Control side | Side of approach | Control side | |||||
| T12 group | |||||||||||||||
| 1 | 1A− | 55 | 18 | T6–T12 | F | 19.8 | 27.2 | Right | 4.14 | 27.2 | 28.2 | n.a. | n.a. | 1 | 3 |
| 2 | 1BN | 73 | 38 | T6–T12 | F | 18.1 | 20.3 | Right | 4.09 | 32.6 | 31.4 | n.a. | n.a. | 2 | 1 |
| 3 | 1AN | 70 | 26 | T6–T12 | F | 16.3 | 20.4 | Right | 4.36 | 28.7 | 31.3 | n.a. | n.a. | 3 | 1 |
| 4 | 1B− | 53 | 20 | T6–T12 | F | 16.7 | 19.1 | Right | 4.55 | 28.3 | 31.3 | Present | Present | 0 | 1 |
| 5 | 1B+ | 57 | 35 | T6–T12 | F | 14.0 | 19.2 | Right | 4.68 | 29.0 | 28.0 | Present | Present | 1 | 2 |
| 6 | 1AN | 65 | 26 | T6–T12 | F | 13.5 | 23.0 | Right | 3.95 | 29.7 | 29.6 | Present | Present | 3 | 3 |
| 7 | 1CN | 55 | 22 | T6–T12 | F | 17.8 | 21.3 | Right | 4.27 | 29.1 | 28.1 | Present | Present | 1 | 2 |
| 8 | 1BN | 50 | 23 | T7–T12 | F | 19.7 | 25.0 | Right | 4.05 | 31.4 | 29.1 | Present | Present | 1 | 1 |
| 9 | 1CN | 68 | 27 | T5–T12 | F | 13.9 | 26.1 | Right | 4.36 | 30.0 | 30.3 | Present | Present | 1 | 2 |
| 10 | 1CN | 68 | 27 | T5–T12 | F | 14.4 | 23.7 | Right | 3.00 | 32.3 | 31.3 | Present | Present | 2 | 1 |
| 11 | 1CN | 60 | 23 | T6–T12 | F | 15.3 | 19.8 | Right | 4.23 | 26.4 | 26.0 | Present | Present | 3 | 3 |
| 12 | 1CN | 55 | 26 | T6–T12 | F | 13.7 | 18.3 | Right | 2.23 | 30.8 | 29.6 | Present | Present | 1 | 3 |
| Average | 61 | 26 | 16.1 | 21.9 | 3.99 | 29.6 | 29.5 | 1.6 | 1.9 | ||||||
| L3 group | |||||||||||||||
| 13 | 5CN | 50 | 33 | L1–L3 | F | 14.7 | 22.2 | Left | 3.77 | 33.3 | 30.8 | Absent | Present | 0 | 1 |
| 14 | 6C+ | 70 | 35 | T12–L3 | F | 16.2 | 22.9 | Left | 4.00 | 31.8 | 32.2 | n.a. | n.a. | 1 | 3 |
| 15 | 5CN | 45 | 17 | T11–L3 | F | 17.2 | 28.1 | Left | 4.55 | 33.5 | 28.8 | Absent | Present | 1 | 3 |
| 16 | 5CN | 40 | 4 | T11–L3 | F | 13.6 | 24.2 | Left | 3.14 | 30.5 | 28.9 | Absent | Present | 0 | 1 |
| 17 | 5CN | 50 | 6 | T11–L3 | F | 18.5 | 23.2 | Left | 4.09 | 33.3 | 29.7 | Present | Present | 0 | 1 |
| 18 | 5CN | 52 | 17 | T11–L3 | F | 17.7 | 25.1 | Left | 4.05 | 31.3 | 28.8 | Absent | Present | 2 | 2 |
| 19 | 5CN | 55 | 15 | T10–L3 | F | 16.9 | 20.3 | Left | 4.60 | 33.3 | 31.8 | Present | Present | 1 | 2 |
| 20 | 6CN | 49 | 10 | T11–L3 | F | 16.0 | 24.8 | Left | 3.91 | 34.7 | 30.3 | Present | Present | 0 | 3 |
| 21 | 5CN | 50 | 14 | T11–L3 | F | 15.8 | 23.6 | Left | 3.95 | 35.2 | 30.9 | Absent | Present | 0 | 2 |
| 22 | 5CTL | 78 | 48 | T11–L3 | F | 34.6 | 40.6 | Left | 3.45 | 34.9 | 31.5 | Absent | Present | 0 | 1 |
| 23 | 5CN | 65 | 25 | T10–L3 | F | 15.4 | 19.6 | Left | 4.14 | 33.8 | 30.2 | Absent | Present | 1 | 2 |
| 24 | 6C+ | 70 | 36 | T9–L3 | F | 22.4 | 33.5 | Right | 3.95 | 33.2 | 32.0 | Present | Present | 1 | 2 |
| Average | 56 | 22 | 18.2 | 25.7 | 3.97 | 33.2 | 30.5 | 0.6 | 1.9 | ||||||
| Overall average | 59 | 24 | 17.2 | 23.8 | 3.98 | 31.4 | 30.0 | 1.1 | 1.9 | ||||||
n.a. not available
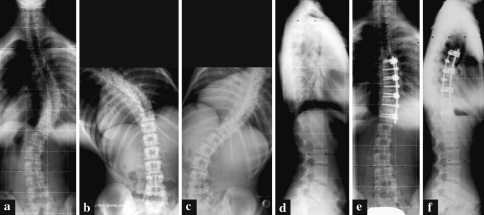
Fig. 2.
A 16.7-year-old female patient (patient no. 4) with a Lenke-type 1B- curve. a Preoperative frontal projection. b Preoperative left bending. c Preoperative right bending. d Preoperative lateral projection. e Postoperative frontal projection following anterior double-rod instrumentation T6–T12. f Postoperative lateral projection
Technique of anterior instrumentation
The patients in this study underwent anterior double-rod Halm–Zielke instrumentation (HZI) for the thoracolumbar region and Halm–Liljenqvist instrumentation (HL) for the thoracic region (Depuy Spine, Kirkel, Germany), a modification of Zielke’s ventral derotation spondylodesis (VDS). The surgical technique has been presented by our research group in this journal previously [17, 26]. Following a typical lateral thoracotomy or thoracolumbotomy, including incision of the pleura and ligation of the segmental vessels, the lateral aspects of the vertebrae requiring instrumentation were dissected (Fig. 3a) and the discs were removed via a lateral approach. Caudally to the diaphragm, the psoas muscle was detached from the lateral vertebrae from the anterior to the posterior side with a Cobb rasp. Lid plates were fixed to the lateral aspect of the vertebral bodies with a sunk screw and a top-loading screw (Fig. 3b). The top-loading screws were placed posteriorly and the sunk screws anteriorly. For screw insertion, the holes were prepared with an awl and explored with a probe. The top-loading screws were interconnected with a 4-mm flexible rod and, after partial correction, a solid fluted rod with a diameter of 6 mm was pre-bent and inserted and deformity correction was completed. The parietal pleura could be completely closed with a running suture.
Fig. 3.
a The lateral appearance of the thoracolumbar spine following dissection before instrumentation. The black arrows indicate the sympathetic nerves. b The lateral aspect of the thoracolumbar spine of the same patient after resection of the discs and instrumentation of the vertebrae with lid plates in the course of anterior double-rod instrumentation
Measurement of sympathetic function
All patients were systematically investigated with regard to their sympathetic function in collaboration with the neurological department. This included: (1) measurement of skin temperature at the back of the foot, (2) a plantar ninhydrin sweat test, and (3) assessment of sympathetic skin responses (SSRs) following electrical stimulation. The side of the surgical approach was compared with the contralateral side in each patient as an intra-individual control. To minimize the risk of bias, a random choice was made using QuickCalcs Online Randomization Software (GraphPad Software, Inc., 2002–2005) on whether to begin the investigation on the left or right side. In addition, the results for the T12 group were compared with those of the L3 group.
Measuring skin temperature is a common method of monitoring lumbar sympathetic blocks [14, 29, 60]. In this study, cutaneous temperature at the back of the foot was measured in centigrade using the Braun ThermoScan® Pro 4000 infrared contact thermometer (Welch Allyn, Inc., Skaneateles Falls, New York, USA). Temperature was measured three times on each foot, and mean values were calculated.
The Moberg ninhydrin sweat test [30] is easy to perform and provides striking results that allow qualitative evaluation of perspiration disturbances [15, 49] (Fig. 4). Unilateral anhidrosis was described with this test following therapeutic lumbar sympathectomy as early as 1966 [48]. The clean soles of both feet pressed against paper (white quality copy paper) left a print that could be dyed with a 1% ninhydrin solution (combination of 0.5 mg ninhydrin per analysis, 50 mL acetone SeccoSolv® dried, 1 Pasteur pipette of acetic acid 100% anhydrous per analysis; all chemicals provided by Merck KGaA, Darmstadt, Germany). The paper was dried and warmed for 5 min at about 60°C in an incubator [51]. The amino acids in the sweat react on contact with ninhydrin to form a bluish-purple colorant. The results were evaluated separately by two different independent examiners who were blinded to the side of approach in each patient; they compared the intensity of the chemical color reaction on the two sides. Four qualitative categories of perspiration were separately applied to the right and left foot: 0 = no perspiration, 1 = minor, 2 = moderate and 3 = major perspiration. In each patient, the surgical approach side was directly compared with the control side, leading to a pair of categories (e.g., right foot 0, left foot 3, in a case of no perspiration on the right vs. major perspiration on the left).
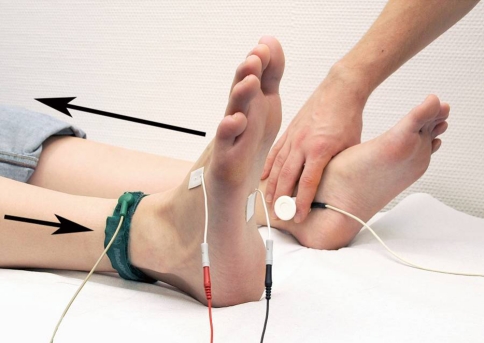
The SSR was first described by Tarchanoff in 1890 [55] and can be recorded to monitor and assess the effect of lumbar sympathetic blocks [50]. Measurement of SSRs after an electrical stimulus, as the most common stimulation modality [59], was carried out using a standardized electromyography device (Medtronic Keypoint® Portable, Software Version V5.06, Medtronic, Inc., Minneapolis, Minnesota, USA). Following the method described by Claus and Schondorf, Ag/AgCl surface electrodes were positioned at the sole of the foot (active electrode) and at the back of the foot (indifferent electrode) on the extremity being studied [10]. Electrical stimulus was applied above the contralateral tibial nerve (Fig. 5). The duration of stimulation was 0.2 s at 30 mA. Registration parameters were: duration of measurement 5–10 s, lower frequency limit 0.1–1.0 Hz, upper limit 100–2000 Hz, amplification 0.05–3.0 mV/division [10]. After SSRs had been measured six times for each limb, it was evaluated categorically whether an SSR was present or not. The reproducibility of the response is relatively poor with regard to response amplitude and latency [19, 59]. Following recommendations by several research groups [52, 54], this study therefore intentionally focused on the qualitative presence or absence of the response, rather than on amplitude or latency.
Fig. 4.
An example of a ninhydrin test in a patient in the L3 group (instrumentation down to L3), showing reduced perspiration on the left side (the approach side)
Fig. 5.
Surface electrodes were positioned on the sole (active electrode) and on the back of the foot (indifferent electrode) on the extremity being studied (i.e., the right side in this example) [10]. The electrical stimulus was applied above the contralateral tibial nerve. The black arrows indicate the direction of the electrical pathways
The validated German version of the Scoliosis Research Society SRS-22 questionnaire was used to assess health-related quality of life for the patients included in the study [36]. The SRS-22 consists of five domains, with the numbers of questions in each domain as follows: “function/activity” (5), “pain” (5), “self-image/appearance” (5), “mental health” (5) and “satisfaction with management” (2) [1]. For each question, five possible answers are available, rated one to five, with five representing a normal healthy status. The overall total from all domains for each patient is calculated as the “total score”.
Statistical analysis was performed using SPSS 15.0 (SPSS Inc., Chicago, Illinois, USA). Before statistical testing, the normal distribution of each continuous variable was analyzed on an exploratory basis (using the Kolmogorov–Smirnov test). In addition to descriptive analysis, the following tests were applied: Mann–Whitney U test, Wilcoxon test, chi-square test, marginal homogeneity test and Pearson correlation coefficients. Differences were considered significant at P < 0.05.
Results
Temperature measurements at the back of the patients’ feet showed a mean temperature on the surgical approach side of 29.6 ± 1.9°C in the T12 group and 33.2 ± 1.4°C in the L3 group. On the contralateral control side, the temperature averaged 29.5 ± 1.7°C in the T12 group and 30.5 ± 1.3°C in the L3 group. A significant difference in skin temperature on the approach side was found between the T12 group and the L3 group (Mann–Whitney U test: P < 0.001), but there was no difference on the control side (Mann–Whitney U test, P = 0.147). In the L3 group, a significant increase in temperature was found on the approach side in comparison with the control side (Wilcoxon test, P = 0.001). This was not seen in the T12 group (Wilcoxon test, P = 0.531).
The two examiners’ evaluations were 100% concordant for the ninhydrin sweat tests for all the patients included in the study. The results of the ninhydrin test are listed in Table 2. In the L3 group, 11 of the 12 patients showed a decrease in perspiration on the surgery side in comparison with the control side; one patient had equal perspiration. In the L3 group, statistical analysis showed that perspiration differed significantly on the approach side in comparison with the control side (marginal homogeneity test, P = 0.002). In the T12 group, only 6 patients had less perspiration on the approach side in comparison with the control side, and no significant differences between the two sides were detected (marginal homogeneity test, P = 0.346).
Table 2.
Results of the ninhydrin test

|
It should be noted that in the T12 group, patients are distributed on both sides of the gray diagonal (equal perspiration), whereas in the L3 group, all but one of the patients are located to the left of the gray diagonal, implying a deterioration on the surgical approach side
SSR testing was performed in 9 patients in the T12 group and 11 in the L3 group. In the T12 group, an SSR was found in all 9 patients on the approach side as well as on the control side. In the L3 group, it was found in only 4 out of 11 patients (36.4%) on the approach side, but in all patients on the control side (Fig. 6). Statistical analysis showed a significant difference between the two groups with regard to detectable SSRs on the approach side (chi-square test, P = 0.005).
Fig. 6.
Example from a patient in the L3 group. Top: the surgical approach side, showing no sympathetic skin response (SSR). Bottom: the contralateral control side, showing a clear SSR
The results of the SRS-22 questionnaire are presented in Table 3. Statistical analysis (Mann–Whitney U test) did not identify any significant differences between the two groups either in relation to each domain or the total score.
Table 3.
Results of the SRS-22 questionnaire
| T12 group | L3 group | P | |
|---|---|---|---|
| Function/activity | 4.08 ± 0.73 | 3.98 ± 0.43 | 0.641 |
| Pain | 4.03 ± 0.80 | 4.25 ± 0.70 | 0.485 |
| Self-image/appearance | 3.82 ± 0.78 | 3.63 ± 0.61 | 0.187 |
| Mental health | 3.93 ± 0.78 | 3.92 ± 0.62 | 0.560 |
| Satisfaction with management | 4.25 ± 0.97 | 4.12 ± 0.58 | 0.422 |
| Total score | 3.99 ± 0.70 | 3.97 ± 0.40 | 0.224 |
The sum of points in each domain and also in the total score was divided by the number of questions answered
Seven patients in the L3 group (58.3%) had a subjective feeling that the leg on the approach side was relatively warmer than that on the control side. This observation was not made by any of the patients in the T12 group, showing a significant difference between the two groups (chi-square test, P = 0.005). Patients in the L3 group who had a subjective feeling that the leg on the approach side was relatively warmer than that on the control side had an objective increase in the temperature of the foot on the approach side, at 33.7°C versus 30.7°C on the control side, while patients who did not report a subjective temperature difference also had an objective increase in the temperature of the foot on the approach side, at 32.6°C versus 30.3°C on the control side. However, these differences between the two subgroups of the L3 group were not statistically significant (Mann–Whitney U test).
The duration of follow-up and age at surgery did not correlate significantly with temperature measurements on the approach side or control side, or with temperature differences between the two sides. The Mann–Whitney U test did not show any significant differences between patients with and without reduced perspiration on the approach side with regard to the duration of follow-up and age at surgery. No significant differences with regard to the duration of follow-up or age at surgery were detected between patients with or without detectable SSRs on the approach side in the L3 group.
None of the patients reported any pain corresponding to postsympathectomy neuralgia.
Discussion
The surgical approach in anterior instrumentation for scoliosis using the technique described may result in lesions of the sympathetic nerves surrounding the vertebrae. Sympathetic lesions differ depending on the level of instrumentation (T12 group, down to T12 vs. L3 group, down to L3). There was a significant increase in the skin temperature of the foot on the approach side in the L3 group. This is the result of vasodilation due to sympathicolysis and unopposed vasodilation by parasympathetic fibers [45]. On the basis of the available literature, it can be accepted that temperature differences of more than one standard deviation from the mean temperature of homologous control regions, i.e., between two opposing backs of the foot, can be regarded as pathologic [58]. In addition, reduced perspiration in the feet and a reduced SSR on the approach side in comparison with the control side were found in the L3 group. The health-related quality of life measured with the SRS-22 did not differ significantly between the two groups, indicating that lesions of the sympathetic system in these patients do not appear to restrict the general subjective health-related quality of life. This is an important clinical result that should be emphasized. The SRS-22 results in this study showed good correspondence with the available literature on scoliosis patients [9, 12, 36].
The significance of the L2 ganglion
In the sympathetic nervous system of the lower extremities, the preganglionic myelinated neurons originate from the lateral horn of the lower thoracic and upper lumbar spinal cord, which is located in the lower thoracic spine. After a short course through the spinal nerve, they travel through the white rami communicantes to the sympathetic trunk. There, they continue caudally in the interganglionic branches and are switched to postganglionic neurons at different levels of the sympathetic trunk ganglia (Fig. 1) [45]. The number and location of ganglia in the lumbar sympathetic chain may vary [32, 43]. However, the most constant and the largest of the lumbar ganglia is described as being associated with the second lumbar vertebra [56]. The postganglionic neurons travel through the gray unmyelinated rami communicantes to the spinal nerves, which guide them to the periphery [42, 45]. The gray rami are found at all levels of the sympathetic chain, while the white rami are found only on the thoracic and upper two lumbar levels [45]. All sympathetic ganglia in the chain below the L2 ganglion receive their information from spinal cord levels L2 and above [6, 42].
If the L2 ganglion or its link to the spinal cord is definitively damaged, as is the case in the L3 group, there is no further sympathetic outflow from the spinal cord to the lumbar sympathetic ganglia supplying the limb on the side concerned. This leads to impairment of sympathetic function, as shown in the present results. If the L2 ganglion and its link to the spinal cord are untouched, as is the case in the T12 group, the lumbar sympathetic ganglia still receive information via ganglia L1 and L2, and sympathetic function on the side concerned is preserved. It can be assumed that surgery rarely leads to a complete 100% destruction of a sympathetic ganglion and its links. In patients comparable to the L3 group, this leads to a situation ranging between the two extremes mentioned above with a partial damage of the sympathetic structures. The different and complex sympathetic innervation of pelvic organs that is mainly performed via fibers accompanying blood vessels instead of fibers in peripheral nerves is not addressed in the present study.
The role of surgical technique
Could surgical dissection of the lateral vertebral bodies be modified to reduce injuries to the sympathetic trunk? Particularly when the psoas muscle is pushed off the lateral vertebrae from anterior to posterior, the direct nervous connection between the spinal cord and sympathetic ganglia, i.e., the white rami communicantes, may be disrupted, as they are situated in the very close vicinity underneath the muscle tissue. In some cases, nervous fibers may be spared, but the size of these sympathetic fibers varies and they may be very small. In addition, a certain amount of dissection is inevitable to allow placement of the lid plates. However, soft tissues should not be dissected in multiple small portions resulting in massive destructions and should rather be removed en bloc preserving nervous interconnections within the soft tissues. In addition, blunt dissection should be favored. Uncritical extensive coagulation and cautery of the soft tissues covering the lateral vertebrae should be avoided. One surgical option for minimizing damage to the sympathetic trunk is to push back the soft tissue attaching the vertebral body, including the sympathetic trunk, subperiosteally from anterior to posterior and thus free the bone to place the implant.
Recovery of sympathetic function
According to literature, postoperative sympathetic chain lesions leading to temperature variations, absence of SSRs and a reduction in perspiration tend to recover over time in some patients; however, there are hardly any reliable data on the time needed for recovery, which varies between 3 weeks and 1 year [3, 22, 34, 39, 41, 61]. Among 25 patients with postoperative sympathetic dysfunction after anterior lumbosacral surgery in a series including 412 patients, most of the affected patients experienced resolution of symptoms within 6 months [22]. The present study includes patients with different follow-up periods. It can be assumed that the extent and number of sympathetic chain lesions immediately after surgery are greater than they were at the time of investigation. This study did not identify a significant impact of the length of the follow-up period on the outcome parameters. However, due to the small number of patients and variations in the follow-up, this result needs to be interpreted with caution and prospective investigations in comparable patients to study the restriction of sympathetic function over time will be needed to assess patients’ ability to recover.
As the literature shows [63], there are no treatment options for a sympathetic injury following surgery.
Experience with sympathectomy in hyperhidrosis and arterial occlusive disease
Sympathectomy has been described as a common form of treatment in patients with hyperhidrosis [2, 5, 18, 34, 42] and advanced arterial occlusive disease [25, 61], even with complex regional pain syndrome (CRPS) [29]. The effects in these studies are similar to those observed in the scoliosis patients described here. Lumbar sympathectomy (ablation of the L2–4 ganglia and their connections) has been described in the treatment of plantar hyperhidrosis [31]. Rieger and Pedevilla performed a bilateral lumbar resection of the sympathetic trunk between the L2–3 and L4–5 levels in 16 patients with plantar hyperhidrosis and observed persistent anhydrosis after a mean follow-up of 17 weeks [42]. As the right and left sides were treated on separate days, this group was able to identify an average temperature increase of 2.8°C in the skin on the feet on the treated side.
Postsympathectomy neuralgia is a known phenomenon, described in literature as having a frequency of up to 50% [7, 40, 42]. However, this was not reported by the patients in the present study.
Weyland et al. [61] investigated 34 patients with arterial occlusive disease who underwent chemical lumbar sympathectomy at vertebrae L2 and L3, and noted an increase of skin temperature of 1.12°C in comparison with the untreated side 21 days after sympathectomy.
The role of sympathetic lesions in complications of anterior spine surgery
Complication rates in anterior spine surgery vary considerably. Morbidity and mortality data from the Scoliosis Research Society found an overall complication rate of 5.2% for anterior instrumentation and fusion of adolescent idiopathic scoliosis including an incidence of 0.26% for neurologic complications [11]. On the other hand, in a review, Ikard [21] described exposure-related overall complication rates of 10–50% and neurologic complication rates of 1–25% in thoracic and lumbar anterior spine surgery. Neurologic complications of lumbar anterior spine exposure include spinal cord ischemia, injury to the hypogastric sympathetic plexus leading to retrograde ejaculation, impotence and even neurogenic priapism, lumbosacral plexus injury and sympathetic dysfunction including a warm lower extremity [21, 22, 39, 41, 45–47].
The reported incidence of sympathetic dysfunctions following anterior lumbar/lumbosacral surgery varies in literature. In a series of 471 anterior lumbar interbody fusion (ALIF) patients, Sasso et al. [47] only described a single case of retrograde ejaculation, but no cases of additional sympathetic dysfunction. Two larger series found rates of 6 and 10% for temperature variation (with the ipsilateral side being warmer due to a lack of sympathetic vasoconstriction), dysesthesias, discoloration and swelling of the lower extremity [22, 39]. In a review including 182 patients, Regan et al. [41] reported an 8.8% incidence of patients with “warm leg”.
Most reports on complications resulting from anterior surgery in the thoracic and lumbar spine have evaluated patients in whom degenerative diseases, rather than idiopathic scoliosis, led to surgery [22, 39].
As only women were included in the present study, the problem of ejaculatory dysfunction did not arise. However, special care should be taken in male patients undergoing anterior scoliosis instrumentation. Lumbar sympathectomy is reported to lead to temporary or permanent ejaculatory dysfunction (retrograde ejaculation) in 0–54% of cases, as sperm emission is controlled via sympathetic fibers originating from the lower thoracic and upper lumbar spinal cord [4, 22, 38, 41, 42, 44, 46, 47, 57, 62].
Limitations of the study
Limitations of the present study include the relatively small number of patients. In addition, the study setting included patients with a variety of follow-up periods. Further prospective investigations with larger populations and multiple follow-up measurements to study the course of impairment, including the tendency to recover over time, are desirable. Another important issue to be studied in the future is the long-term effect of sympathetic dysfunction in relation to trophic sympathetic restrictions. On the basis of an awareness that sympathectomy impairs wound healing and causes a negative balance of bone metabolism [23, 37], it will be of major interest whether scoliosis patients following anterior instrumentation down to L3 will develop impairments of wound and bone healing and also of bony integration of implants in the affected lower extremities.
However, despite certain limitations, this investigation presents clear results that are of clinical importance. Patients should therefore be informed about possible effects on the sympathetic nerve system prior to anterior scoliosis surgery.
Conclusions
Anterior instrumentation of scoliosis including vertebrae down to L3 regularly led to lesions of the sympathetic trunk in this investigation.
These sympathetic lesions lead to a significant increase in temperature, reduction of perspiration and reduced SSRs in the lower extremity on the approach side.
The caudal level of anterior instrumentation (T12 vs. L3) has an impact on the extent of impairment of sympathetic function, supporting the suspected importance of the L2 ganglion.
Patients should be informed about these regular complications prior to surgery.
Clinical outcome in terms of the SRS-22 is not significantly restricted by sympathetic trunk lesions.
Prospective investigations should be carried out to further study sympathetic lesions in detail in relation to surgical techniques for minimizing lesions, to follow potential neurological recovery tendencies during follow-up and to investigate potential long-term trophic restrictions.
Footnotes
The authors Martin Marziniak and Viola Bullmann contributed equally to the study.
References
- 1.Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society-22 patient questionnaire for idiopathic scoliosis. Spine (Phila Pa 1976) 2003;28(1):63–69. doi: 10.1097/00007632-200301010-00015. [DOI] [PubMed] [Google Scholar]
- 2.Asik ZS, Orbey BC, Asik I. Sympathetic radiofrequency neurolysis for unilateral lumbar hyperhidrosis: a case report. Agri. 2008;20(3):37–39. [PubMed] [Google Scholar]
- 3.Beisse R, Muckley T, Schmidt MH, Hauschild M, Buhren V. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2(2):128–136. doi: 10.3171/spi.2005.2.2.0128. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi C, Ballard JL, Abou-Zamzam AM, Teruya TH, Abu-Assal ML. Anterior retroperitoneal lumbosacral spine exposure: operative technique and results. Ann Vasc Surg. 2003;17(2):137–142. doi: 10.1007/s10016-001-0396-x. [DOI] [PubMed] [Google Scholar]
- 5.Bonde P, Nwaejike N, Fullerton C, Allen J, McGuigan J. An objective assessment of the sudomotor response after thoracoscopic sympathectomy. J Thorac Cardiovasc Surg. 2008;135(3):635–641. doi: 10.1016/j.jtcvs.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Braus H, Elze C (1960) Periphere Leitungsbahnen II, Centrales Nervensystem, Sinnesorgane. In: Braus H, Elze C (eds) Anatomie des Menschen, 2nd edn., vol 3. Springer-Verlag, Berlin, pp 350–363
- 7.Buche M, Randour P, Mayne A, Joucken K, Schoevaerdts JC. Neuralgia following lumbar sympathectomy. Ann Vasc Surg. 1988;2(3):279–281. doi: 10.1016/S0890-5096(07)60015-6. [DOI] [PubMed] [Google Scholar]
- 8.Bullmann V, Fallenberg EM, Meier N, Fischbach R, Schulte TL, Heindel WL, Liljenqvist UR. Anterior dual rod instrumentation in idiopathic thoracic scoliosis: a computed tomography analysis of screw placement relative to the aorta and the spinal canal. Spine. 2005;30(18):2078–2083. doi: 10.1097/01.brs.0000179083.84421.64. [DOI] [PubMed] [Google Scholar]
- 9.Bunge EM, Juttmann RE, de Kleuver M, van Biezen FC, de Koning HJ. Health-related quality of life in patients with adolescent idiopathic scoliosis after treatment: short-term effects after brace or surgical treatment. Eur Spine J. 2007;16(1):83–89. doi: 10.1007/s00586-006-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claus D, Schondorf R. Sympathetic skin response. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:277–282. [PubMed] [Google Scholar]
- 11.Coe JD, Arlet V, Donaldson W, Berven S, Hanson DS, Mudiyam R, Perra JH, Shaffrey CI. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2006;31(3):345–349. doi: 10.1097/01.brs.0000197188.76369.13. [DOI] [PubMed] [Google Scholar]
- 12.Deviren V, Patel VV, Metz LN, Berven SH, Hu SH, Bradford DS. Anterior arthrodesis with instrumentation for thoracolumbar scoliosis: comparison of efficacy in adults and adolescents. Spine (Phila Pa 1976) 2008;33(11):1219–1223. doi: 10.1097/BRS.0b013e318170fce0. [DOI] [PubMed] [Google Scholar]
- 13.Dwyer AF, Newton NC, Sherwood AA. An anterior approach to scoliosis. A preliminary report. Clin Orthop Relat Res. 1969;62:192–202. doi: 10.1097/00003086-196901000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Frank SM, El-Rahmany HK, Tran KM, Vu B, Raja SN. Comparison of lower extremity cutaneous temperature changes in patients receiving lumbar sympathetic ganglion blocks versus epidural anesthesia. J Clin Anesth. 2000;12(7):525–530. doi: 10.1016/S0952-8180(00)00207-5. [DOI] [PubMed] [Google Scholar]
- 15.Gurtler B. Ninhydrin test and sensory nerve action potentials in the clarification of peripheral nerve lesions. Comparative studies. Schweiz Arch Neurol Neurochir Psychiatr. 1967;100(2):399–410. [PubMed] [Google Scholar]
- 16.Halm H, Richter A, Thomsen B, Koszegvary M, Ahrens M, Quante M. Anterior scoliosis surgery: state of the art and a comparison with posterior techniques. Orthopade. 2009;38(2):131–145. doi: 10.1007/s00132-008-1365-7. [DOI] [PubMed] [Google Scholar]
- 17.Halm HF, Liljenqvist U, Niemeyer T, Chan DP, Zielke K, Winkelmann W. Halm-Zielke instrumentation for primary stable anterior scoliosis surgery: operative technique and 2-year results in ten consecutive adolescent idiopathic scoliosis patients within a prospective clinical trial. Eur Spine J. 1998;7(5):429–434. doi: 10.1007/s005860050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han PP, Kenny K, Dickman CA. Thoracoscopic approaches to the thoracic spine: experience with 241 surgical procedures. Neurosurgery. 2002;51(5 Suppl):S88–S95. [PubMed] [Google Scholar]
- 19.Hoeldtke RD, Davis KM, Hshieh PB, Gaspar SR, Dworkin GE. Autonomic surface potential analysis: assessment of reproducibility and sensitivity. Muscle Nerve. 1992;15(8):926–931. doi: 10.1002/mus.880150810. [DOI] [PubMed] [Google Scholar]
- 20.Hurford RK, Jr, Lenke LG, Lee SS, Cheng I, Sides B, Bridwell KH. Prospective radiographic and clinical outcomes of dual-rod instrumented anterior spinal fusion in adolescent idiopathic scoliosis: comparison with single-rod constructs. Spine. 2006;31(20):2322–2328. doi: 10.1097/01.brs.0000238966.75175.2b. [DOI] [PubMed] [Google Scholar]
- 21.Ikard RW. Methods and complications of anterior exposure of the thoracic and lumbar spine. Arch Surg. 2006;141(10):1025–1034. doi: 10.1001/archsurg.141.10.1025. [DOI] [PubMed] [Google Scholar]
- 22.Kang BU, Choi WC, Lee SH, Jeon SH, Park JD, Maeng DH, Choi YG. An analysis of general surgery-related complications in a series of 412 minilaparotomic anterior lumbosacral procedures. J Neurosurg Spine. 2009;10(1):60–65. doi: 10.3171/2008.10.SPI08215. [DOI] [PubMed] [Google Scholar]
- 23.Kim LR, Whelpdale K, Zurowski M, Pomeranz B. Sympathetic denervation impairs epidermal healing in cutaneous wounds. Wound Repair Regen. 1998;6(3):194–201. doi: 10.1046/j.1524-475X.1998.60305.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuklo TR, Lehman RA, Jr, Lenke LG. Structures at risk following anterior instrumented spinal fusion for thoracic adolescent idiopathic scoliosis. J Spinal Disord Tech. 2005;18(Suppl):S58–S64. doi: 10.1097/01.bsd.0000123424.12852.75. [DOI] [PubMed] [Google Scholar]
- 25.Lee BY, Da Silva MC, Aquino-Chu G, Herz BL. Surgery of the sympathetic nervous system. J Spinal Cord Med. 1996;19(1):20–26. doi: 10.1080/10790268.1996.11719414. [DOI] [PubMed] [Google Scholar]
- 26.Liljenqvist UR, Bullmann V, Schulte TL, Hackenberg L, Halm HF. Anterior dual rod instrumentation in idiopathic thoracic scoliosis. Eur Spine J. 2006;15(7):1118–1127. doi: 10.1007/s00586-005-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonner BS, Auerbach JD, Estreicher M, Milby AH, Kean KE. Video-assisted thoracoscopic spinal fusion compared with posterior spinal fusion with thoracic pedicle screws for thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2009;91(2):398–408. doi: 10.2106/JBJS.G.01044. [DOI] [PubMed] [Google Scholar]
- 28.Lonner BS, Auerbach JD, Estreicher MB, Betz RR, Crawford AH, Lenke LG, Newton PO. Pulmonary function changes after various anterior approaches in the treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2009;22(8):551–558. doi: 10.1097/BSD.0b013e318192d8ad. [DOI] [PubMed] [Google Scholar]
- 29.Manjunath PS, Jayalakshmi TS, Dureja GP, Prevost AT. Management of lower limb complex regional pain syndrome type 1: an evaluation of percutaneous radiofrequency thermal lumbar sympathectomy versus phenol lumbar sympathetic neurolysis—a pilot study. Anesth Analg. 2008;106(2):647–649. doi: 10.1213/01.ane.0000298285.39480.28. [DOI] [PubMed] [Google Scholar]
- 30.Moberg E. Objective methods for determining the functional value of sensibility in the hand. J Bone Joint Surg Br. 1958;40-B(3):454–476. doi: 10.1302/0301-620X.40B3.454. [DOI] [PubMed] [Google Scholar]
- 31.Moran KT, Brady MP. Surgical management of primary hyperhidrosis. Br J Surg. 1991;78(3):279–283. doi: 10.1002/bjs.1800780306. [DOI] [PubMed] [Google Scholar]
- 32.Murata Y, Takahashi K, Yamagata M, Takahashi Y, Shimada Y, Moriya H. Variations in the number and position of human lumbar sympathetic ganglia and rami communicantes. Clin Anat. 2003;16(2):108–113. doi: 10.1002/ca.10069. [DOI] [PubMed] [Google Scholar]
- 33.Muschik MT, Kimmich H, Demmel T. Comparison of anterior and posterior double-rod instrumentation for thoracic idiopathic scoliosis: results of 141 patients. Eur Spine J. 2006;15(7):1128–1138. doi: 10.1007/s00586-005-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumayer C, Panhofer P, Zacherl J, Bischof G. Effect of endoscopic thoracic sympathetic block on plantar hyperhidrosis. Arch Surg. 2005;140(7):676–680. doi: 10.1001/archsurg.140.7.676. [DOI] [PubMed] [Google Scholar]
- 35.Newton PO, Upasani VV, Lhamby J, Ugrinow VL, Pawelek JB, Bastrom TP. Surgical treatment of main thoracic scoliosis with thoracoscopic anterior instrumentation: a five-year follow-up study. J Bone Joint Surg Am. 2008;90(10):2077–2089. doi: 10.2106/JBJS.G.01315. [DOI] [PubMed] [Google Scholar]
- 36.Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M. Validity and reliability of an adapted german version of Scoliosis Research Society-22 questionnaire. Spine. 2009;34(8):818–821. doi: 10.1097/BRS.0b013e31819b33be. [DOI] [PubMed] [Google Scholar]
- 37.Pagani F, Sibilia V, Cavani F, Ferretti M, Bertoni L, Palumbo C, Lattuada N, De Luca E, Rubinacci A, Guidobono F. Sympathectomy alters bone architecture in adult growing rats. J Cell Biochem. 2008;104(6):2155–2164. doi: 10.1002/jcb.21775. [DOI] [PubMed] [Google Scholar]
- 38.Quayle JB. Sexual function after bilateral lumbar sympathectomy and aorto-iliac by-pass surgery. J Cardiovasc Surg (Torino) 1980;21(2):215–218. [PubMed] [Google Scholar]
- 39.Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91(1 Suppl):60–64. doi: 10.3171/spi.1999.91.1.0060. [DOI] [PubMed] [Google Scholar]
- 40.Raskin NH, Levinson S, Hoffman PM, Pickett JB, 3rd, Fields HL. Postsympathectomy neuralgia. Amelioration with diphenylhydantoin and carbamazepine. Am J Surg. 1974;128(1):75–78. doi: 10.1016/0002-9610(74)90238-4. [DOI] [PubMed] [Google Scholar]
- 41.Regan JJ, Yuan H, McAfee PC. Laparoscopic fusion of the lumbar spine: minimally invasive spine surgery. A prospective multicenter study evaluating open and laparoscopic lumbar fusion. Spine. 1999;24(4):402–411. doi: 10.1097/00007632-199902150-00023. [DOI] [PubMed] [Google Scholar]
- 42.Rieger R, Pedevilla S. Retroperitoneoscopic lumbar sympathectomy for the treatment of plantar hyperhidrosis: technique and preliminary findings. Surg Endosc. 2007;21(1):129–135. doi: 10.1007/s00464-005-0690-8. [DOI] [PubMed] [Google Scholar]
- 43.Rocco AG, Palombi D, Raeke D. Anatomy of the lumbar sympathetic chain. Reg Anesth. 1995;20(1):13–19. [PubMed] [Google Scholar]
- 44.Rose SS. Lumbar sympathectomy. Acta Chir Belg. 1977;76(1):123–126. [PubMed] [Google Scholar]
- 45.Samudrala S, Khoo LT, Rhim SC, Fessler RG. Complications during anterior surgery of the lumbar spine: an anatomically based study and review. Neurosurg Focus. 1999;7(6):e9. doi: 10.3171/foc.1999.7.6.12. [DOI] [PubMed] [Google Scholar]
- 46.Saraph V, Lerch C, Walochnik N, Bach CM, Krismer M, Wimmer C. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J. 2004;13(5):425–431. doi: 10.1007/s00586-004-0722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasso RC, Best NM, Mummaneni PV, Reilly TM, Hussain SM. Analysis of operative complications in a series of 471 anterior lumbar interbody fusion procedures. Spine. 2005;30(6):670–674. doi: 10.1097/01.brs.0000155423.18218.75. [DOI] [PubMed] [Google Scholar]
- 48.Schiffter R, Schliack H. Erfahrungen mit dem Ninhydrin-Schweißtest nach Moberg in der Diagnostik peripherer Nervenläsionen. Fortschr Neurol Psychiat. 1966;34:331–346. [Google Scholar]
- 49.Schliack H, Schiffter R. Differential diagnostic possibilities in peripheral neurology with the aid of the ninhydrin test. Acta Neuroveg (Wien) 1967;30(1):512–521. doi: 10.1007/BF01239930. [DOI] [PubMed] [Google Scholar]
- 50.Schmid MR, Kissling RO, Curt A, Jaschko G, Hodler J. Sympathetic skin response: monitoring of CT-guided lumbar sympathetic blocks. Radiology. 2006;241(2):595–602. doi: 10.1148/radiol.2412051229. [DOI] [PubMed] [Google Scholar]
- 51.Schnider P, Moraru E, Kittler H, Binder M, Kranz G, Voller B, Auff E. Treatment of focal hyperhidrosis with botulinum toxin type A: long-term follow-up in 61 patients. Br J Dermatol. 2001;145(2):289–293. doi: 10.1046/j.1365-2133.2001.04349.x. [DOI] [PubMed] [Google Scholar]
- 52.Schondorf R. Skin potentials: normal and abnormal. In: Low PA, editor. Clinical autonomic disorders. Philadelphia: Lippincott Raven; 1997. pp. 221–231. [Google Scholar]
- 53.Shamji MF, Isaacs RE. Anterior-only approaches to scoliosis. Neurosurgery. 2008;63(3 Suppl):139–148. doi: 10.1227/01.NEU.0000325486.92090.DA. [DOI] [PubMed] [Google Scholar]
- 54.Spitzer A, Lang E, Birklein F, Claus D, Neundorfer B. Cardiac autonomic involvement and peripheral nerve function in patients with diabetic neuropathy. Funct Neurol. 1997;12(3–4):115–122. [PubMed] [Google Scholar]
- 55.Tarchanoff J. Über die galvanischen Erscheinungen in der Haut des Menschen bei Reizungen der Sinnesorgane und bei verschiedenen Formen der psychischen Thätigkeit. Pflügers Archiv. 1890;46:46–55. doi: 10.1007/BF01789520. [DOI] [Google Scholar]
- 56.Thorek P. Section 5: pelvis. In: Thorek P, editor. Anatomy in surgery. New York: Springer-Verlag; 1985. p. 630. [Google Scholar]
- 57.Tiusanen H, Seitsalo S, Osterman K, Soini J. Retrograde ejaculation after anterior interbody lumbar fusion. Eur Spine J. 1995;4(6):339–342. doi: 10.1007/BF00300293. [DOI] [PubMed] [Google Scholar]
- 58.Uematsu S, Jankel WR, Edwin DH, Kim W, Kozikowski J, Rosenbaum A, Long DM. Quantification of thermal asymmetry. Part 2: application in low-back pain and sciatica. J Neurosurg. 1988;69(4):556–561. doi: 10.3171/jns.1988.69.4.0556. [DOI] [PubMed] [Google Scholar]
- 59.Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13(4):256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- 60.Wetzel FT, LaRocca SH, Adinolfi M. The treatment of chronic extremity pain in failed lumbar surgery. The role of lumbar sympathectomy. Spine. 1992;17(12):1462–1468. doi: 10.1097/00007632-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Weyland A, Weyland W, Lamersdorf A, Ensink FB, Hildebrandt J, Kettler D. Neurolytic block of the lumbar sympathetic trunk in advanced stages of peripheral arterial occlusive disease. Anasthesiol Intensivmed Notfallmed Schmerzther. 1993;28(7):420–426. doi: 10.1055/s-2007-998956. [DOI] [PubMed] [Google Scholar]
- 62.Whitelaw GP, Smithwick RH. Some secondary effects of sympathectomy; with particular reference to disturbance of sexual function. N Engl J Med. 1951;245(4):121–130. doi: 10.1056/NEJM195107262450401. [DOI] [PubMed] [Google Scholar]
- 63.Zelle B, Zeichen J, Pape HC, Weissenborn K, Krettek C. Upper sympathetic trunk lesion after video-assisted fracture stabilization of the thoracic spine: a case report. J Spinal Disord Tech. 2002;15(6):502–506. doi: 10.1097/00024720-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Zielke K. Ventral derotation spondylodesis. Results of treatment of cases of idiopathic lumbar scoliosis (author’s transl) Z Orthop Ihre Grenzgeb. 1982;120(3):320–329. doi: 10.1055/s-2008-1051620. [DOI] [PubMed] [Google Scholar]