Abstract
 A primary Echinococcus granulosus infection of the spine involving the vertebrae T8 and T9 of a 6-year-old child was treated elsewhere by thoracotomy, partial corporectomy, multiple laminectomies and uninstrumented fusion. Owing to inappropriate stabilization, severe deformity developed secondary to these surgeries. X-rays, CT and MRI scans of the spine revealed a severe thoracic kyphoscoliosis of more than 100° (Fig. 1) and recurrence of Echinococcus granulosus infection. The intraspinal cyst formation was located between the stretched dural sac and the vertebral bodies of the kyphotic apex causing significant compression of the cord (Figs. 2, 3, 4). A progressive neurologic deficit was reported by the patient. At the time of referral, the patient was wheelchair bound and unable to walk by herself (Frankel Grade C). Standard antiinfectious therapy of Echinococcus granulosus requires a minimum treatment period of 3 months. This should be done before any surgical intervention because in case of a rupture of an active cyst, the delivered lipoprotein antigens of the parasite may cause a potentially lethal anaphylactic shock. Owing to the critical neurological status, we decided to perform surgery without full length preoperative antiinfectious therapy. Surgical treatment consisted in posterior vertebral column resection technique with an extensive bilateral costotransversectomy over three levels, re-decompression with cyst excision around the apex and multilevel corporectomy of the apex of the deformity. Stabilisation and correction of the spinal deformity were done by insertion of a vertebral body replacement cage anteriorly and posterior shortening by compression and by a multisegmental pedicle screw construct. After the surgery, antihelminthic therapy was continued. The patients neurological deficits resolved quickly: 4 weeks after surgery, the patient had Frankel Grade D and was ambulatory without any assistance. After an 18-month follow-up, the patient is free of recurrence of infection and free of neurologically deficits (Frankel E). This case demonstrates that inappropriate treatment—partial resection of the cyst, inappropriate anterior stabilization and posterior multilevel laminectomies without posterior stabilization—may lead to severe progressive kyphoscoliotic deformity and recurrence of infection, both leading to significant neurological injury presenting as a very difficult to treat pathology.
A primary Echinococcus granulosus infection of the spine involving the vertebrae T8 and T9 of a 6-year-old child was treated elsewhere by thoracotomy, partial corporectomy, multiple laminectomies and uninstrumented fusion. Owing to inappropriate stabilization, severe deformity developed secondary to these surgeries. X-rays, CT and MRI scans of the spine revealed a severe thoracic kyphoscoliosis of more than 100° (Fig. 1) and recurrence of Echinococcus granulosus infection. The intraspinal cyst formation was located between the stretched dural sac and the vertebral bodies of the kyphotic apex causing significant compression of the cord (Figs. 2, 3, 4). A progressive neurologic deficit was reported by the patient. At the time of referral, the patient was wheelchair bound and unable to walk by herself (Frankel Grade C). Standard antiinfectious therapy of Echinococcus granulosus requires a minimum treatment period of 3 months. This should be done before any surgical intervention because in case of a rupture of an active cyst, the delivered lipoprotein antigens of the parasite may cause a potentially lethal anaphylactic shock. Owing to the critical neurological status, we decided to perform surgery without full length preoperative antiinfectious therapy. Surgical treatment consisted in posterior vertebral column resection technique with an extensive bilateral costotransversectomy over three levels, re-decompression with cyst excision around the apex and multilevel corporectomy of the apex of the deformity. Stabilisation and correction of the spinal deformity were done by insertion of a vertebral body replacement cage anteriorly and posterior shortening by compression and by a multisegmental pedicle screw construct. After the surgery, antihelminthic therapy was continued. The patients neurological deficits resolved quickly: 4 weeks after surgery, the patient had Frankel Grade D and was ambulatory without any assistance. After an 18-month follow-up, the patient is free of recurrence of infection and free of neurologically deficits (Frankel E). This case demonstrates that inappropriate treatment—partial resection of the cyst, inappropriate anterior stabilization and posterior multilevel laminectomies without posterior stabilization—may lead to severe progressive kyphoscoliotic deformity and recurrence of infection, both leading to significant neurological injury presenting as a very difficult to treat pathology.
Keywords: Echinococcus granulosus, Infection, Thoracic spine, Kyphotic deformity, Neurologic deficit
Case presentation
A 6-year-old Bulgarian girl was admitted to our institution with a primary Echinococcus granulosus infection of the spine which was treated elsewhere with various interventions, including thoracotomy, partial corporectomy, multiple laminectomies and uninstrumented fusion. Recurrence of infection due to incomplete cyst resection and inappropriate surgical measures caused a progressive kyphoscoliotic deformity, spinal stenosis, cord compression and neurologic injury. At the presentation at our institution, she was wheelchair bound. The clinical investigation revealed gibbus in the middle thoracic spine with Cobb angle of 110°, cord compression at the apex and paraplegia with Frankel Grade C.
Diagnostic imaging section
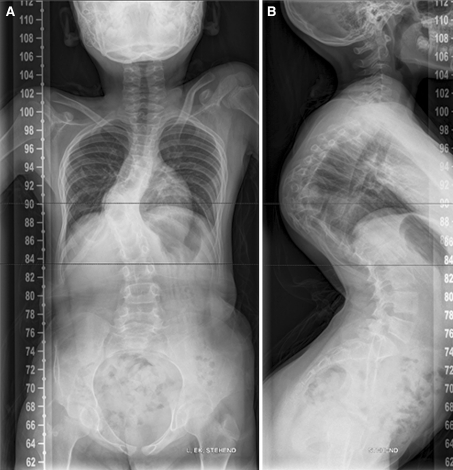
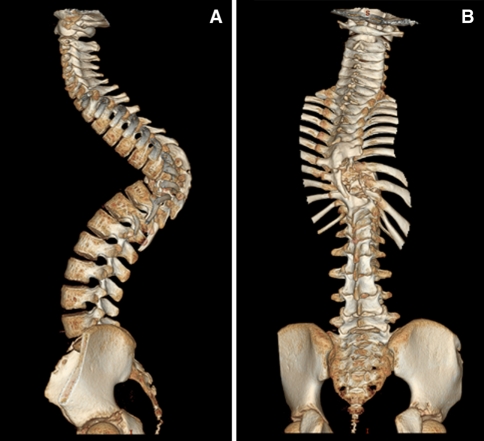
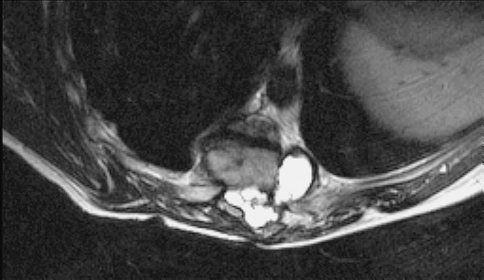
Anterior–posterior and lateral radiographs of the whole spine showed a severe thoracic kyphoscoliotic deformity with a kyphosis of 110° and a scoliosis of 35° with the apex located at T9 (Fig. 1). A computer tomographic (CT) reconstruction of the whole spine revealed that the apex of the deformity was located at the level of the previously partial corporectomy and multilevel laminectomies (Figs. 2, 3) Magnetic resonance imaging (MRI) confirmed a recurrence of the Echinoccocus cyst. The intraspinal cyst formation was located between the stretched dural sac and the vertebral bodies of the kyphotic apex causing significant compression of the cord (Figs. 4, 5).
Fig. 1.
X-rays of the patient showing a kyhoscoliotic deformity. a ap view, b lateral view
Fig. 2.
CT reconstruction of the whole spine showing the apex of the deformity is located in the area of the previous surgeries
Fig. 3.
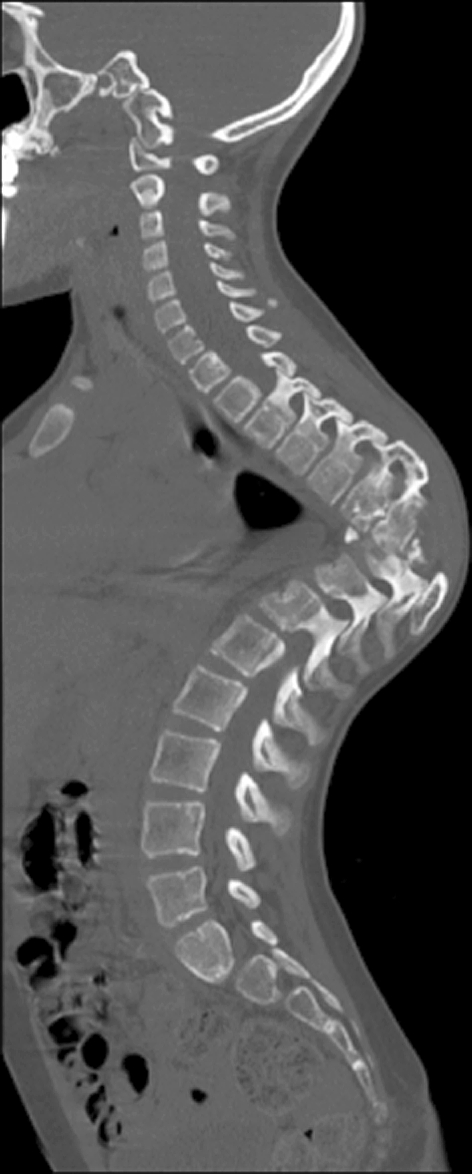
Sagittal CT-cut showing the bone bloc at the apex with a translation deformity
Fig. 4.
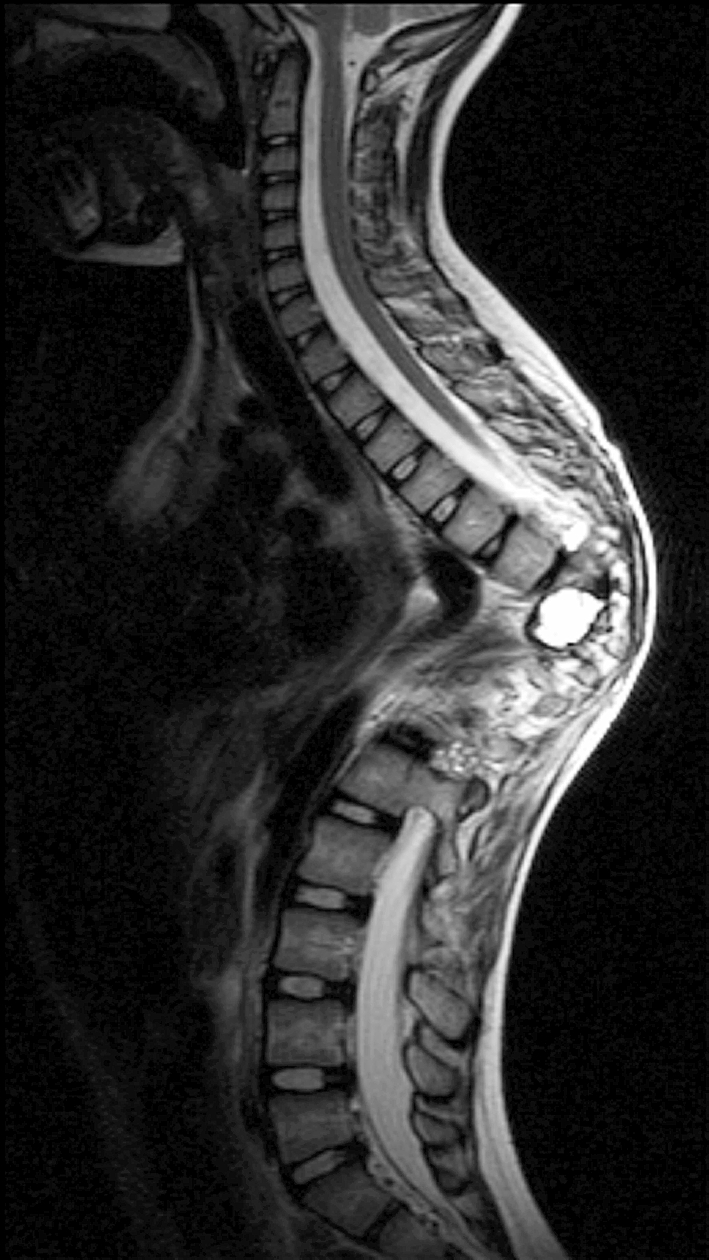
Sagittal T2-weighted MRI image showing the cystic formation at the apex
Fig. 5.
Axial T2-weighted MRI image showing the intraspinal cyst formation with spinal cord compression
Epidemiology, pathology
Hydatidosis caused by Echinococcus granulosus is an endemic parasitic disease in Mediterranean areas, such as North Africa, Spain, Greece, Turkey, Portugal, Middle East, Australia, New Zealand, South America, Baltic areas, the Philippines, Northern China and the Indian sub-continent [5, 9, 15]. In endemic areas, the incidence of Echinococcus granulosus infection in humans varies between 1 and 10 new cases per 100,000 inhabitants and year [19]. Its life cycle includes dogs as definitive and predominantly sheep as intermediate hosts. Therefore, it is known as the “sheep-dog-strain” [5, 8, 9, 15]. Humans are intermediate hosts. The infection is mainly transmitted directly by contact with the infected animal or indirectly by faecooral ingestion of the worm eggs [15, 16]. Once within human or any other intermediate host, the ingested eggs hatch in the duodenum to release oncospheres (true larvae) that burrow into the jejunal submucosa and enter veins or lymphatic vessels. They reach the liver, which acts as an effective filter for most of the larvae. However, if that barrier is overwhelmed, the larvae pass through the inferior vena cava into the right side of the heart and then to the lungs. If the worm is not lodged in liver or lungs, it may be trapped virtually anywhere in the body, such as peritoneum, spleen, kidney, heart, brain, spine, bony skeleton and muscles. Not every larva develops, over 90% are eliminated by the host reaction [4, 5, 19].
After infection of the oncospheres of Echinococcus granulosus to first clinical symptoms, incubation periods that vary from months to years can be observed. Many cases remain asymptomatic for years or permanently. Clinical symptoms have a high variation due to slow expansionary growth of the worm hydatides [19].
In 60% hydatid cysts affect the liver, in 30% the lung and in 5% the peritoneum. Spinal hydatid cysts account for 1% of all cases of hydatid disease [18]. An osseous location is seen 0.5–2% and approximately half of those are located vertebrally [20].
The thoracic spine is the most common location of spinal manifestations (52%), followed by the lumbar spine (37%), the sacral and the cervical spine (5.5% each). One case of thoracic involvement extending from the seventh rib has been reported [6, 15, 18]. The disease usually spreads to the spine by direct extension from pulmonary, abdominal, or pelvic manifestations and affects predominantly the dorsal region of the spine [5, 6, 8, 9, 15–19]. Inside the vertebrae, the parasites spread along the intratrabecular space and medullary canal causing bony destruction [9]. A growth rate of approximately 7 mm per month has been reported [1, 18]. Intervertebral discs are usually preserved, because the disease tends to spread under the periosteum and ligaments [2]. The growing cysts lead to local bone destruction, deformity and affect adjacent bones. Three mechanisms for the intraosseous invasion have been described [15] (Table 1).
Table 1.
Potential mechanism for intraosseous invasion of a hydadid disease
| Mechanism | Pathophysiology | Affected structures | Effect |
|---|---|---|---|
| Mechanical process | Expansion, compression, dislocation, repression | Surrounding tissues | Atrophy of the bone, atrophy of the vascular nerve tissues, rarefying osteitis |
| Ischaemic process | Obstruction, compression | Nutrient vessels | Bony necrosis, formation of sequestra |
| Cellular process | Osteoclast proliferation | Compressed bone tissue | Destruction |
Intraspinal echinococcosis appears only sporadically worldwide, whereas extradurally located cysts have been reported more frequently than intradurally ones [10, 11]. If spinal hydatidosis causes clinical symptoms, they are initially non-specific and patients usually present with disease-associated complications. The most common symptoms and signs in vertebral involvement are paraparesis (62%), increasing back or radicular pain (55%), sensory loss of disturbance (36%), sphincter disturbance (30%) and paraplegia (26%) [6, 9, 15]. The time period between the onset of the symptoms and medical consultation varies between 6 and 15 months [12, 15].
Diagnosis, differential diagnosis
Radiologically, the spinal manifestations can be classified into five different types according to Braithwaite and Lees [4, 18, 19]. The primary cyst can be located intramedullary, intradural-extramedullary, extradural, hydatid cyst of the vertebrae, and paravertebral lesions extending to spinal structures.
The radiographic characteristics of spinal hydatid disease are similar to primary and secondary tumours, Pott disease, and pyogenic infection of the spine [20]. An MRI is the best imaging tool for diagnosing spinal hydatid disease, as it images the relation to anatomic structures such as the spinal cord and its extension into the surrounding soft tissue [20]. Characteristic MRI findings of hydatid cysts are two dome-shaped ends, no debris in the lumen, and a “sausage-like” appearance [7]. CT scans may be more convenient and more appropriate to show osteolytic lesion and bony deformities associated with the disease.
Any kind of fine-needle aspiration should be avoided to reduce the risk of anaphylaxis or spreading of the disease [9]. Generally, routine laboratory tests do not show specific values. Eosinophilia is mostly limited (<15%) or absent. An intradermal Casoni test result is positive in 95%, but there are up to 40% false-positive results. Indirect hemagglutination test and enzyme-linked immunosorbent assay are established methods for the detection of anti-Echinococcus antibodies (immunoglobulin G). In spinal involvement, the serological sensitivity of antigens is 25%, whereas it is 80–100% in liver involvement [13]. Characteristic histological findings of Echinococcus granulosus are a multilayered cyst wall containing hooklet-bearing scolices. The external wall is usually thicker, laminated, may calcify and consists of layers of chitin. The innermost germinal layer produces hydatid fluid and may include numerous embryonal scolices termed “hydatid sand” [18].
Rationale for treatment and evidence-based literature
Successful treatment of the spinal hydatidosis is challenging because of its invasiveness and its potentially severe complications. Surgical removal is the first and most effective option [1]. The total eradication of the cyst without rupture should be the primary goal of the surgery [2]. Radical excision of cysts in the spine is challenging because of the absence of distinct anatomic planes, an extensive bone invasion and the closeness of neural structures. Like in tumour surgery, complete excision of the cysts without rupture should be performed to minimise spreading of pathogenic elements probably causing recurrence [18]. If a cyst ruptures, multiple additional cysts and a serious anaphylactic reaction induced by spillage of cystic fluid may occur. In the case of intraoperative cyst rupture, the surgical area should be irrigated with hypertonic saline. However, this seems relatively ineffective, as it is known, that if any cyst ruptures into the intradural space, reoccurrence is unavoidable [4]. Intraoperative cyst ruptures with the risk of anaphylaxis are reported in approximately 45% [5, 15, 18]. There is no specific surgical technique to avoid this complication. Most cysts rupture during laminectomy, especially if they are located extradurally [1].
Various surgical interventions have been performed to treat hydatid disease of the spine, including anterior, posterior and combined procedures. In neurological impairment anterior or posterior decompression procedures are required. Based on the medical records submitted from Bulgaria, where the patient was operated several times including two thoracotomies with partial corporectomies at Th8 and Th9 without anterior or posterior stabilization and multiple posterior decompression procedures because of neurologic impairment. Obviously, these interventions were insufficient to control infection and due to the lack of instrumentation an instable situation caused a rapidly progressing kyphotic deformity. The bony apex was continuously causing anterior pressure on the cord causing neurological deterioration with episodes of paraplegia. Anterior compression was falsely treated with posterior laminectomies further destabilizing the spine and accelerating the kyphotic deformation. Spinal column deformity often occurs in children after multilevel laminectomies for spinal tumours and its incidence in these patients has been reported to occur in 24–100% [14]. According to the literature, several cases of partial hydatid cyst resection, inappropriate anterior stabilization and posterior multilevel laminectomies without posterior stabilization have led to progressive deformities of the thoracic spine. No cases of reoperations due to spinal deformity were found when cyst resection and posterior laminectomy were combined with anterior or posterior stabilization [6, 8, 9, 11, 13, 16, 18].
Appropriate surgical treatment would include complete cyst resection without violation of the wall, appropriate decompression of the cord, correction of the deformity and appropriate anterior and posterior instrumented stabilization techniques. Regarding the approach a posterior intervention was performed because all pathologies which should be addressed were located posteriorly. The advantage of the posterior approach in the current case is that all important steps of the procedure, including decompression of the cord, resection of the intraspinaI cysts, vertebral column resection and deformity correction can be done by one approach under direct visualisation of the cord. In a kyphotic deformity the posterior approach with costotransversectomy gives a perfect view of the operative field especially for the resection of the posterior wall of the bony apex. An anterior approach via thoracotomy would provide a better vessel control in cases of vascular injury however it is inappropriate in terms of apical resection and cord control.
Standard antiinfectious therapy of Echinococcus granulosus with albendazole (10 mg/kg bodyweight/day) requires a minimum treatment period of 3 months. This should be done before any surgical intervention already to prevent the patient from a potentially lethal anaphylactic shock in case of a rupture of an active cyst, where the lipoprotein antigens of the parasite are delivered [8, 10, 11, 14, 20]. Albendazole blocks glucose uptake and depletes the glycogen stores of the parasite. Besides, a temporary minor elevation of the liver enzymes no long-term side effects of albendazole has been reported. Several studies recommended mean course duration of 3–4 months [1].
However, in cases of neurologic dysfunction immediate surgery is indicated to prevent permanent damage. In the current case, the patient was paraplegic and based on anamnestic evaluation the motoric dysfunction was deteriorating in the past 3 weeks. In these cases, the increased risk of anaphylactic reactions caused by insufficient preoperative antiinfectious treatment must be weighted against the risk of neurologic deterioration. In the present case, the authors decided to perform immediate surgery followed by antiinfectiouus therapy to provide chances for neurologic recovery.
In general, spinal Echinococcus cysts are characterised by high recurrence rate of 50–100% [9, 11, 15, 18, 19].
Some extradural cases have been treated with CT-guided needle aspiration and irrigation using hypertonic saline eliminate to collapse the spinal cysts and to minimise spinal cord compression. The so-called “PAIR” approach (puncture, aspiration of the cyst, injection of hypertonic saline and re-aspiration) is a widely used non-surgical technique for the treatment of hepatic hydatid cysts. Ultrasonography guided PAIR technique has been also used for the treatment for paraspinal manifestation [3]. Potential complications of this technique are anaphylaxis and the risk of iatrogenic spreading.
Procedure imaging section
Procedure (surgery, intervention)
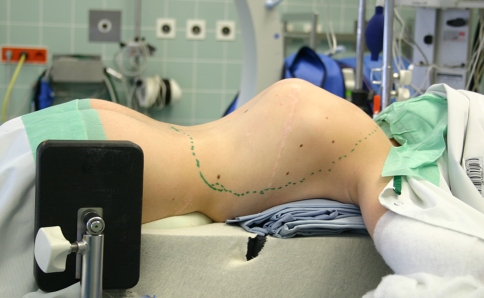
Under general anaesthesia and spinal cord monitoring, the patient was placed in a prone position (Fig. 6). A posterior midline exposure was carried out from T3 to L4. Pedicle screws were inserted bilaterally in the upper and lower thoracic spine (Fig. 7). A vertebral column resection technique was done by a posterior approach to directly control the course of the cord and to localise and resect the intraspinal cyst formations. A re-decompression with a right side laminectomy of the remaining laminas from T7 to T11 was performed the expose the cord. All hydatid cysts located posterolaterally causing local compression were excised. A three level bilateral costotransversectomy was performed to expose the apex.
Fig. 6.
Preoperative image showing the kyphotic deformity (gibbus)
Fig. 7.
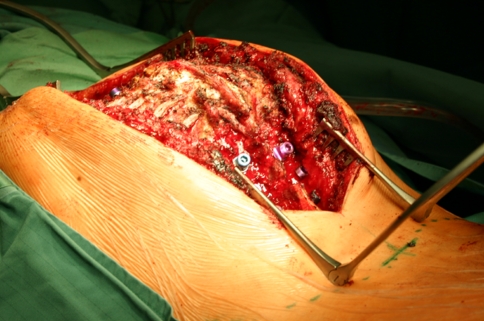
Intraoperative image showing the kyphotic apex (gibbus) of the iatrogenic deformity. Pedicle screws have been placed and the ribs were exposed to perform the costotransversectomy
The corporectomy in the apex of the deformity was carried out from posterolateral by resection of the pedicles and resection of the former bony fusion mass using a high-speed burr. A unilateral rod was placed temporarily to stabilize the spine during vertebral column resection procedure. The lung and the big vessels were protected by Harmon retractors. After bilateral resection of the lateral and anterior parts of the fused bone bloc in the apex of the deformity the posterior wall was carefully removed. This is the most critical step of the procedure because the cord was stretched over the apex of the deformity. In addition, severe compression was caused by cysts located between the posterior wall of the apex and the cord. First, the posterior wall was thinned out from anterior with the high-speed burr. A dissector was carefully inserted between the cord and the posterior wall. With anteriorly directed pressure, the bony wall was broken away from the cord. This procedure was done under spinal cord monitoring showing normal signals during the whole procedure. After the VCR manoeuvre, the spine was completely mobile and ready for correction (Fig. 8). A distractible vertebral body replacement device (Synex II, Synthes, Swiss, Oberdorf) was placed anteriorly into the corporectomy defect to serve as a hypomochlion (Fig. 9). The temporary rod was carefully removed and two new rods with a physiological kyphotic curvature were inserted. The deformity was reduced slowly under continuous spinal cord monitoring from 110° to 45° by a cantilever technique. Autologous bone graft from the previously resected ribs and homologous bone graft were placed lateral to the vertebral body replacement device and posterolaterally to achieve anterior and posterior bone fusion. Suction drains were inserted and the wound was closed (Fig. 10).
Fig. 8.
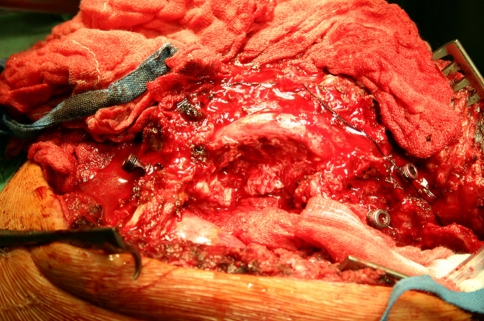
Intraoperative image after left side costotransversectomy. The lung and the big vessels are retracted, the cord has been exposed and is stretched over the apex of the deformity. Anteriorly, the apex has already been removed with a high-speed burr
Fig. 9.
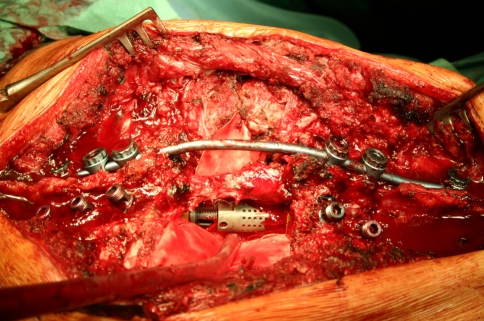
Intraoperative image showing a vertebral body replacement device placed into the anterior defect to serve as a hypomochlion. The posterior wall has been removed and the cord is completely decompressed
Fig. 10.
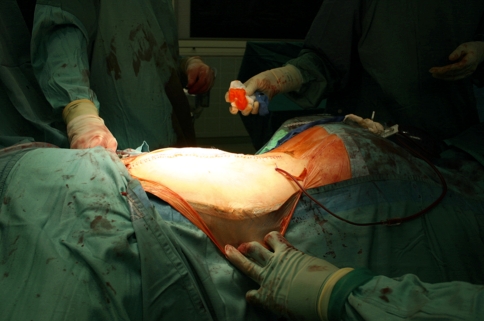
Postoperative image showing the patient’s back after the correction
Outcome and follow up
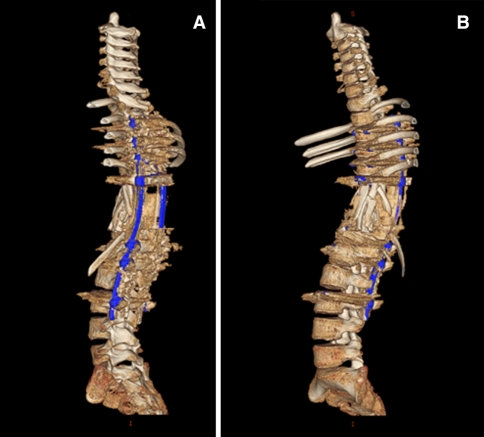
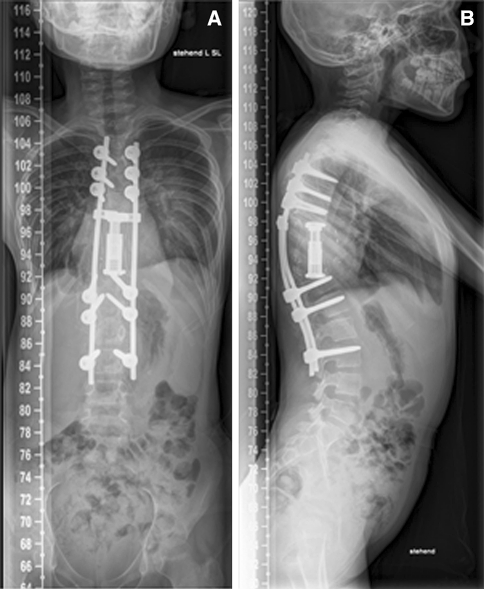
The neurological deficits resolved quickly; 2 weeks after surgery, the patient had deficits according to the Frankel Grade D and could walk without any assistance. Postoperative X-rays revealed a proper correction of the deformity. A postoperative CT and MRI scan were performed to verify the location of implants. Antihelminthic therapy was continued for 1 year postoperatively and a brace was recommended for 6 months. The patient was discharged 4 weeks after the surgery. Follow-up investigations were performed at 6 months and 18 months including MRI and CT scans. The radiological control examination showed no loss of correction (Figs. 11, 12). At the last flow-up, the patient was free of recurrence of infection and neurologically intact (Frankel E) and was able to return to her normal life, continued to go to school and perform her normal sport activities without any restrictions.
Fig. 11.
Postoperative 3D CT reconstruction of the whole spine at 18 months follow-up showing lateral views of the corrected spine
Fig. 12.
Postoperative X-rays at 18 months follow-up. a ap view, b lateral view
References
- 1.BM Altinörs N, Caner HH, Erdogan B. Central nervous system hydatidosis in Turkey: a cooperative study and literature survey analysis of 458 cases. J Neurosurg. 2000;93:1–8. doi: 10.3171/jns.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 2.Bhojraj SY, Shetty NR. Primary hydatid disease of the spine: an unusual cause of progressive paraplegia: case report and review of the literature. J Neurosurg. 1999;91:216–218. doi: 10.3171/spi.1999.91.2.0216. [DOI] [PubMed] [Google Scholar]
- 3.Bilgic S, Kose O, Sehirlioglu A, Safaz I, Ozkan H. Primary paraspinal hydatid cyst treated with puncture, aspiration, injection and re-aspiration (PAIR) technique: a case report. Eur Spine J. 2008;18:165–167. doi: 10.1007/s00586-008-0737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braithwaite PA, Lees RF. Vertebral hydatid disease: radiological assessment. Radiology. 1981;140:763–766. doi: 10.1148/radiology.140.3.7280247. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Vicuna R, Carvajal I, Ortiz-Garcia A, Lopez-Robledillo JC, Laffon A, Sabando P. Primary solitary Echinococcosis in cervical spine. Postsurgical successful outcome after long-term albendazole treatment. Spine. 2000;25:520–523. doi: 10.1097/00007632-200002150-00021. [DOI] [PubMed] [Google Scholar]
- 6.Govender TS, Aslam M, Parbhoo A, Corr P (2000) Hydatid disease of the spine. A long-term followup after surgical treatment. Clin Orthop Relat Res 143–147 [PubMed]
- 7.Güneçs M, Akdemir H, Tuğcu B, Günaldi O, Gümüçs E, Akpinar A. Multiple intradural spinal hydatid disease: a case report and review of literature. Spine. 2009;20:346–350. doi: 10.1097/BRS.0b013e3181a01b0f. [DOI] [PubMed] [Google Scholar]
- 8.Hamdan TA, Al-Kaisy MA. Dumbbell hydatid cyst of the spine: case report and review of the literature. Spine. 2000;25:1296–1299. doi: 10.1097/00007632-200005150-00018. [DOI] [PubMed] [Google Scholar]
- 9.Herrera A, Martinez AA, Rodriguez J. Spinal hydatidosis. Spine. 2005;30:2439–2444. doi: 10.1097/01.brs.0000184688.68552.90. [DOI] [PubMed] [Google Scholar]
- 10.Islekel S, Zileli M, Ersahin Y. Intradural spinal hydatid cysts. Eur Spine J. 1998;7:162–164. doi: 10.1007/s005860050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalkan E, Cengiz SL, Cicek O, Erdi F, Baysefer A. Primary spinal intradural extramedullary hydatid cyst in a child. J Spinal Cord Med. 2007;30:297–300. [PMC free article] [PubMed] [Google Scholar]
- 12.Khazim R, Fares Y, Heras-Palou C, Ruiz Barnes P. Posterior decompression of spinal hydatidosis: long term results: Fundacion Jimenez Diaz, Madrid, Spain. Clin Neurol Neurosurg. 2003;105:209–214. doi: 10.1016/S0303-8467(03)00013-1. [DOI] [PubMed] [Google Scholar]
- 13.Kotil K, Tatar T, Bilge T. Spinal hydatidosis accompanied by a secondary infection: case report. J Neurosurgery Spine. 2007;6:585–590. doi: 10.3171/spi.2007.6.6.13. [DOI] [PubMed] [Google Scholar]
- 14.Papagelopoulos PJ, Peterson HA, Ebersold MJ, Emmanuel PR, Choudhury SN, Quast LM. Spinal column deformity and instability after lumbar or thoracolumbar laminectomy for intraspinal tumors in children and young adults. Spine. 1997;22:442–451. doi: 10.1097/00007632-199702150-00019. [DOI] [PubMed] [Google Scholar]
- 15.Papanikolaou A. Osseous hydatid disease. Trans R Soc Trop Med Hyg. 2008;102:233–238. doi: 10.1016/j.trstmh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Prabhakar MM, Acharya AJ, Modi DR, Jadav B. Spinal hydatid disease: a case series. J Spinal Cord Med. 2005;28:426–431. doi: 10.1080/10790268.2005.11753843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sapkas GS, Machinis TG, Chloros GD, Fountas KN, Themistocleous GS, Vrettakos G. Spinal hydatid disease, a rare but existent pathological entity: case report and review of the literature. South Med J. 2006;99:178–183. doi: 10.1097/01.smj.0000199747.81684.54. [DOI] [PubMed] [Google Scholar]
- 18.Schnepper GD, Johnson WD. Recurrent spinal hydatidosis in North America. Case report and review of the literature. Neurosurg Focus. 2004;17:1–6. doi: 10.3171/foc.2004.17.6.8. [DOI] [PubMed] [Google Scholar]
- 19.Spies C, Weisskopf M, Ohnsorge JA. Intraspinal echinococcosis within the lumbar spine of an 18-year-old male patient. Z Orthop Unfall. 2008;146:463–467. doi: 10.1055/s-2008-1038543. [DOI] [PubMed] [Google Scholar]
- 20.Tuzun M, Hekimoğlu B. CT findings in skeletal cystic echinococcosis. Acta Radiol. 2002;43:533–538. doi: 10.1034/j.1600-0455.2002.430515.x. [DOI] [PubMed] [Google Scholar]