Abstract
Epidemiological and clinical studies of people with low back pain (LBP) commonly measure the incidence of recovery. The pain numerical rating scale (NRS), scores from 0 to 10, and Roland Morris disability questionnaire (RMDQ), scores from 0 to 24, are two instruments often used to define recovery. On both scales higher scores indicate greater severity. There is no consensus, however, on the cutoff scores on these scales that classify people as having recovered. The aim of this study was to determine which cutoff scores most accurately classify those who had recovered from LBP. Subjects from four clinical studies were categorized as ‘recovered’ or ‘unrecovered’ according to their self-rating on a global perceived effect scale. Odd ratios were calculated for scores of 0, 1, 2, 3 and 4 on the NRS and RMDQ to predict perceived recovery. Scores of 0 on the NRS and ≤2 on the RMDQ most accurately identify patients who consider themselves completely recovered. The diagnostic odds ratio (OR) for predicting recovery was 43.9 for a score of 0 on the NRS and 17.6 for a score of ≤2 on the RMDQ. There was no apparent effect of LBP duration or length of follow-up period on the optimal cutoff score. OR for the NRS were generally higher than those for RMDQ. Cutoffs of 0 on the NRS and 2 on the RMDQ most accurately classify subjects as recovered from LBP. Subjects consider pain more than disability when determining their recovery status.
Keywords: Low back pain, Recovery, Pain numerical rating scale, Roland Morris disability questionnaire
Introduction
Studies on low back pain (LBP) commonly measure the incidence of recovery to make determinations of the effect of treatment [12, 16, 31, 33] or prognosis [11, 20, 25, 28]. However, recovery is a complex construct and there is currently no consensus on how it should be defined or measured. This makes interpretation and comparison of studies problematic.
Two measures that are frequently used and have been endorsed as measures of outcome for LBP studies [9] are the 11-point numerical rating scale for pain [7] (NRS) and the Roland Morris disability questionnaire [30] (RMDQ). These scales can be used to track the course of pain and disability. Importantly, NRS and RMDQ scores are also used to classify people as having recovered or not from an episode of back pain [2, 3, 8, 14–17].
If NRS or RMDQ are to be used to classify people as recovered or not, it is necessary for researchers to set a cutoff value, below which people are considered recovered and above which people are considered not recovered. Currently, this choice of cutoff is made arbitrarily. Thus, while some studies define recovery as having NRS scores of 0 [23], others define recovery as having NRS scores of ≤1 [18], and still others define recovery as having NRS scores of ≤2 [5]. The RMDQ score has also been used to define recovery, but similar inconsistencies apply. Cutoff scores used for the RMDQ have included ≤2 [2], ≤3 [23] and ≤4 [15]. At this point, there are no studies that detail the relationship of LBP patients’ perceptions of their own recovery with cutoff scores on these commonly used instruments and, hence, no data to guide selection of the most suitable cutoff.
Recovery may also be assessed more directly by asking patients whether they perceive they have recovered. The response options may be binary [19], or on a Likert scale [21], or a variant of the global perceived effect scale [24] (GPE). Studies that concurrently consider patient self-report measures of recovery and measure NRS or RMDQ, therefore, offer an opportunity to consider what score on the NRS or RMDQ best equates to patients’ perceptions of recovery. That is the approach we have used in the present study.
Patients with LBP are commonly categorized on the basis of how long they have experienced their symptoms. The European Guidelines for management of LBP [32] suggest classification as follows: acute (<6 weeks of symptom duration), subacute (6–12 weeks) and chronic (>12 weeks). It is not known if the duration of the episode of LBP influences patients’ ratings of recovery. Such information could provide guidance as to whether measurement of recovery should be adapted to suit different situations.
Recovery is a complex construct [1, 22, 27] and it is possible that construct-specific instruments such as the NRS and RMDQ may capture different aspects of recovery to direct reports of perceived recovery. Both construct-specific instruments and direct reports offer advantages and disadvantages. While the latter enables the individual to incorporate whatever constructs they consider important into their rating of recovery, such ratings are inherently idiosyncratic, which could make between-subject comparisons difficult. On the other hand, defining recovery in terms of pain and disability may limit individual relevance, but facilitate interpretation.
This study involved a secondary analysis of data from four clinical studies of people with LBP. In each study, subjects reported NRS and RMDQ scores and directly reported whether they perceived they had recovered via a GPE scale. The primary aim was to determine the cutoffs for NRS and RMDQ that most accurately identified when LBP patients consider themselves recovered. A secondary aim was to assess whether the optimal cutoff varied according to the duration of LBP at entry into the study or to the length of follow-up period within the study.
Methods
Data sets
All subjects had non-specific LBP and were enrolled in one of four clinical studies. One was an observational study [4] in which patients were followed for 2 weeks while receiving a course of physiotherapy treatment. The other three studies were RCTs investigating the effects of McKenzie exercises [26], exercise and advice [29] or mobilization and stabilization exercises [10]. Symptom duration and follow-up points varied across the studies (Table 1).
Table 1.
Included studies
We grouped studies according to the duration of participants’ LBP [32] at entry into the study: acute (<6 weeks of symptom duration), subacute (6–12 weeks) and chronic (>12 weeks). Follow-up times were defined as the time from the entry into the study or randomization until follow-up and grouped as follows: short term (<12 weeks), intermediate term (12–26 weeks) and long term (>26 weeks).
Measures
All subjects completed (a) an 11-point NRS to score their pain, either averaged over the previous 24 h or over the previous week depending on the study, (b) the 24-point RMDQ to rate their disability, and (c) a GPE scale to rate recovery at each follow-up point. A full description of the measures is presented in “Appendix”. GPE was scored on an 11-point scale from completely recovered (+5), through unchanged (0) to vastly worse (−5). Scores were dichotomized such that subjects who reported that they were completely recovered (+5) were classified as recovered and all others as unrecovered. It is acknowledged that selection of a recovery cutoff score of completely recovered (+5) on the GPE may be considered an arbitrary choice on behalf of the authors, but there is at least face validity for the assumption that subjects selecting the option ‘completely recovered’ perceived themselves to be recovered.
Analyses
We examined the performance of cutoffs of ≤0, 1, 2 and 3 for NRS scores and ≤0, 1, 2, 3 and 4 for RMDQ scores.
Data from all studies at all follow-ups were pooled. Subjects were categorized according to whether their NRS and RMDQ scores were ≤ or > each candidate cutoff score. Diagnostic odds ratios (OR) were calculated for each candidate cutoff score. OR is the odds of being recovered for someone who scores on or below the cutoff divided by the odds of being recovered for someone who scores above the cutoff.
To test whether the optimal cutoff score varied with duration of LBP or length of follow-up, OR were calculated for each category of LBP (acute, subacute, chronic) and for each follow-up period (short, intermediate and long term).
Results
Optimal cutoff scores
Data from all studies at all follow-up points were pooled. In 1,818 assessments, 7.98% patients assigned a GPE score of completely recovered.
The cutoff scores that best discriminated people who felt they were, or were not recovered were an NRS score of 0 and an RMDQ score of 2 (Table 2). For the NRS, the OR for the optimal NRS cutoff was substantially higher than for the other candidates’ cutoffs. That is, a cutoff of 0 was clearly superior to other cutoff scores for the NRS. In contrast for RMDQ, the ORs were similar for all candidate cutoffs, indicating that no cutoff was clearly superior to others for the RMDQ. ORs for pain scores were substantially higher than those for RMDQ.
Table 2.
Odds ratios of complete recovery based on cutoff score
| Cutoff | OR | |
|---|---|---|
| Numerical rating scale | 0 | 43.9 |
| ≤1 | 32.8 | |
| ≤2 | 20.9 | |
| ≤3 | 20.1 | |
| Roland Morris disability questionnaire | 0 | 14.5 |
| ≤1 | 15.5 | |
| ≤2 | 17.6 | |
| ≤3 | 15.5 | |
| ≤4 | 15.2 |
Cutoff scores that best discriminate between recovered and unrecovered participants are indicated in bold
OR Odds ratio
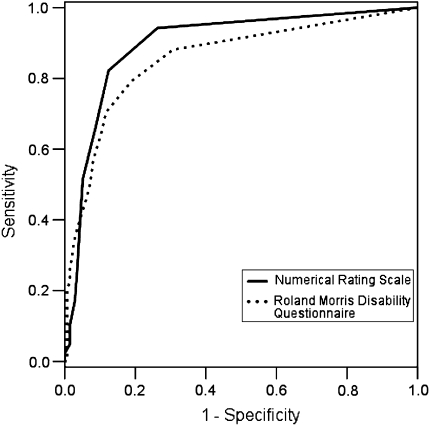
To compare the relative effectiveness of NRS and RMDQ in classifying recovery, we created ROC [6] curves (Fig. 1). Data were pooled from all assessments and areas under the curves [6] and (AUC) were 0.897 for the NRS and 0.856 for the RMDQ.
Fig. 1.
ROC curves for numerical rating scale and Roland Morris disability questionnaire classifying recovery
Effect of LBP duration on cutoff
Separate analyses were conducted on subjects with acute LBP (476 assessments), subacute LBP (697 assessments) and chronic LBP (645 assessments). An NRS score of 0 had the highest OR for acute and chronic populations, while a score of 1 had the highest OR in subacute populations. RMDQ scores of 0, 1 and 2 had the highest ORs for subacute, acute and chronic populations, respectively (Table 3). There was no clear pattern of higher optimal cutoff scores with increasing LBP duration for either instrument. As with the data from the whole cohort, optimal cutoffs for NRS have much larger ORs than other candidates’ cutoffs, whereas ORs for all candidates are much more similar for RMDQ. Optimal pain cutoffs had higher absolute ORs for predicting recovery than optimal RMDQ cutoffs.
Table 3.
Odds ratios of complete recovery based on cutoff score and LBP duration
| Cutoff | Acute | Subacute | Chronic | |
|---|---|---|---|---|
| Numerical rating scale | 0 | 42.5 | 47.9 | 23.5 |
| ≤1 | 17.1 | 62.2 | 17.8 | |
| ≤2 | 12.8 | 31.8 | 11.8 | |
| ≤3 | 15.1 | 49.1 | 6.3 | |
| Roland Morris disability questionnaire | 0 | 21.0 | 11.4 | 7.2 |
| ≤1 | 23.8 | 10.5 | 11.0 | |
| ≤2 | 23.1 | 11.0 | 14.7 | |
| ≤3 | 16.4 | 11.2 | 11.2 | |
| ≤4 | 19.9 | 10.4 | 8.4 | |
| Percentage recovered (%) | 10.11 | 11.91 | 2.16 |
Cutoff scores that best discriminate between recovered and unrecovered participants are indicated in bold
OR Odds ratio
Effect of length of follow-up on cutoff
Candidate cutoffs were tested separately for all subjects at short-term follow-up (929 assessments), intermediate-term follow-up (447 assessments) and long-term follow-up (442 assessments). NRS scores of 0 or 1 and RMDQ scores of 0 and 4 had the highest ORs. There was no clear pattern of higher optimal cutoff scores with increasing length of follow-up for either instrument (Table 4). As with the data from the whole cohort, optimal cutoffs for NRS have much larger ORs than other candidates, whereas ORs for all candidates are much more similar for RMDQ. Optimal pain cutoffs had higher absolute ORs for predicting recovery than optimal RMDQ cutoffs.
Table 4.
Odds ratios of complete recovery based on cutoff score and follow-up period
| Cutoff | Short term | Intermediate term | Long term | |
|---|---|---|---|---|
| Numerical rating scale | 0 | 46.2 | 25.9 | 73.7 |
| ≤1 | 25.3 | 27.5 | 86.5 | |
| ≤2 | 18.5 | 16.8 | 35.5 | |
| ≤3 | 17.2 | 14.7 | 42.6 | |
| Roland Morris disability questionnaire | 0 | 18.0 | 13.6 | 13.0 |
| ≤1 | 14.6 | 11.7 | 28.0 | |
| ≤2 | 18.7 | 9.6 | 35.1 | |
| ≤3 | 18.5 | 7.3 | 26.7 | |
| ≤4 | 19.2 | 5.7 | 44.3 | |
| Percentage recovered (%) | 8.18 | 6.70 | 8.82 |
Cutoff scores that best discriminate between recovered and unrecovered participants are indicated in bold
OR Odds ratio
Discussion
This study provides the first data-based recommendations for cutoffs to define recovery from LBP using NRS and RMDQ scores. Patients who consider themselves completely recovered are most accurately classified by a score of 0 on a pain NRS or a score of 2 or less on the 24-point RMDQ. These cutoffs provide a high level of discrimination between people who consider themselves completely recovered and those who do not. While we do not suggest that pain or disability measures in isolation capture the full complexity of recovery, the AUCs from the ROC curves provide some assurance that NRS and RMDQ are useful in defining recovered subjects.
We expected that subjects with longer LBP duration would consider themselves recovered despite higher levels of pain and disability (that is, we expected they would have higher cutoff scores) but this was not the case. There was no clear evidence that the optimal cutoff score was higher with increasing LBP duration or with increasing length of follow-up. While the optimal cutoff for RMDQ was higher (≤4) for long-term follow-up than for the complete data set, it was also higher (4) for the short-term follow-up, and lower (0) for the intermediate term. This apparently random finding may reflect statistical imprecision associated with the low number of recovered subjects.
Odds ratios for optimal NRS cutoffs were always higher than those for the RMDQ. We did not use interferential statistics to investigate the significance of these differences, because observations were clustered by study and there was more than one observation on some subjects and so we could not assume independence of observations. To some extent, this difference may be due to the different number of response options for the 2 scales, i.e., 11 in the case of the NRS and 25 for the RMDQ. Nonetheless, it appears that NRS pain scores align better with patients’ perceptions of recovery than do RMDQ scores. This contention is supported by the AUCs for the ROC curves, which were higher for the NRS than the RMDQ.
Various statistics can be used to quantify test performance. These include accuracy, sensitivity and specificity, positive and negative predictive values, likelihood ratios and Youden’s index. Accuracy, the percentage of observations that are correctly classified, was not used because high levels of accuracy can be achieved by chance alone when the prevalence of recovered subjects is high or, as in our study, low. We used the OR to quantify performance because, unlike sensitivity and specificity or likelihood ratios, OR provide a single summary statistic. An alternative single summary statistic is Youden’s index (sensitivity plus specificity minus 1); the disadvantage of Youden’s index in this instance is that the statistic is difficult to interpret [13]. The OR has been recommended on the grounds of its interpretability [13], but we know of no compelling reason to prefer Youden’s or ORs for the purposes of our study. To investigate the sensitivity of our findings in the choice of summary statistic, we also estimated optimal cutoffs using Youden’s index (data not reported). These analyses yielded similar, but not identical, findings. Optimal cutoffs as determined by Youden’s index were 1 for both NRS and RMDQ.
The difference in ORs for the candidate cutoffs for the RMDQ was quite small. That is, there is relatively little difference in accuracy of the optimal cutoff and the scores adjacent to it. Given that this is the case, it seems unreasonable to make concrete recommendations about the choice of cutoff scores for this measure. Instead, researchers should consider, for each particular study question, the consequences of choosing a higher or lower cutoff on the RMDQ. This choice would involve a trade-off between sensitivity and specificity.
By using the OR as a measure of test performance, we implicitly assumed that sensitivity and specificity were equally important. Selecting a higher cutoff will improve the sensitivity (that is, a greater proportion of subjects who consider themselves recovered will be correctly classified). However, use of a higher cutoff will also result in a larger proportion of unrecovered subjects being incorrectly classified as recovered. The opposite applies if a lower cutoff is selected.
In summary, there are several implications that arise for future studies. LBP researchers who decide to categorize their subjects as recovered or unrecovered on the basis of NRS or RMDQ score should use cutoffs of 0 and ≤2, respectively. If a choice is to be made between the two scales, it is likely that NRS-based categorization will align better with patients’ perceptions of their recovery. These findings do not appear to be sensitive to the duration of LBP symptoms.
There are some limitations that should be considered when determining the utility of these findings. First, the data were collected from patients with LBP; it is unclear whether the optimal cutoffs are transferable to patients with other conditions. Second, there was some inconsistency in the optimal cutoffs for different duration of LBP and follow-up periods; this may be due to the fact that relatively small numbers of subjects were completely recovered at certain follow-up points. It is acknowledged that the complete recovery rates in the source studies (10% or less) are lower than that expected, possibly due to the stringent criterion used for recovery. We have no reason to believe that the samples in these studies are substantially different from those typically enrolled in the primary care studies of LBP. Consequently, we are confident regarding the generalization of these findings. Lastly, the data structure precluded formal statistical inference and calculation of 95% confidence intervals around the OR estimates.
Conflict of interest statement
The authors have no conflict of interest to declare.
Appendix
Global perceived effect scale
Compared to when this episode first started, how would you describe your back these days?![]()
Pain numerical rating scale
I would like you to rate your pain on a scale from 0 to 10 where 0 is no pain and 10 is the worst pain possible. Please give a number to describe your pain at the present time.![]()
Roland Morris disability questionnaire
When your back hurts, you may find it difficult to do some of the things you normally do.
This list contains some sentences that people have used to describe themselves when they have back pain. When you read them, you may find that some stand out because they describe you today. As you read the list, think of yourself today. When you read a sentence that describes you today, fill the box to the left of the sentence. If the sentence does not describe you, then leave the box blank and go onto the next one. Remember, only mark the sentence if you are sure that it describes you today.
References
- 1.Beaton DE, Tarasuk V, Katz JN, Wright JG, Bombadier C. Are you better? A qualitative study of the meaning of recovery. Arthritis Care Res. 2001;45:270–279. doi: 10.1002/1529-0131(200106)45:3<270::AID-ART260>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Burton AK, McClune TD, Clarke RD, Main CJ. Long-term follow-up of patients with low back pain attending for manipulative care: outcomes and predictors. Man Ther. 2004;9:30–35. doi: 10.1016/S1356-689X(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 3.Collins DL, Evans JM, Grundy RH. The efficiency of multiple impulse therapy for musculoskeletal complaints. J Manip Physiol Ther. 2006;29:162.e1–162.e9. doi: 10.1016/j.jmpt.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Costa LOP, Maher CG, Latimer J, Ferreira PH, Ferreira ML, Pozzi GC, Freitas LMA. Clinimetric testing of three self-report outcome measures for low back pain patients in Brazil. Which one is the best? Spine. 2008;33:2459–2463. doi: 10.1097/BRS.0b013e3181849dbe. [DOI] [PubMed] [Google Scholar]
- 5.Coste J, Lefranc G, Guillemin F, Pouchot J. Prognosis and quality of life in patients with acute low back pain: insights from a comprehensive inception cohort study. Arthritis Rheum. 2004;51:168–176. doi: 10.1002/art.20235. [DOI] [PubMed] [Google Scholar]
- 6.Deyo R, Centor R. Assessing responsiveness of functional scales to clinical change: an analogy to diagnostic test performance. J Chronic Dis. 1986;39:897–906. doi: 10.1016/0021-9681(86)90038-X. [DOI] [PubMed] [Google Scholar]
- 7.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Andersen JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–381. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn KM, Jordan K, Croft PR. Characterizing the course of low back pain: a latent class analysis. Am J Epidemiol. 2006;163:754–761. doi: 10.1093/aje/kwj100. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad A, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tolett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira ML, Ferreira PH, Latimer J, Herbert RD, Hodges PW, Jennings MD, Maher CG, Refshauge KM. Comparison of general exercise, motor control exercise and spinal manipulative therapy for chronic low back pain: a randomized trial. Pain. 2007;131:31–37. doi: 10.1016/j.pain.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Gheldof ELM, Vinck J, Vlaeyen JWS, Hidding A, Crombez G. Development of and recovery from short- and long-term low back pain in occupational settings: a prospective cohort study. Eur J Pain. 2007;11:841–854. doi: 10.1016/j.ejpain.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Giles LGF, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490–1502. doi: 10.1097/00007632-200307150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PMM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/S0895-4356(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 14.Grotle M, Brox JI, Vøllestad NK. Concurrent comparison of responsiveness in pain and functional status measurements used for patients with low back pain. Spine. 2005;29:E492–E501. doi: 10.1097/01.brs.0000143664.02702.0b. [DOI] [PubMed] [Google Scholar]
- 15.Grotle M, Brox JI, Glomsrød B, Lønnc JH, Vøllestad NK. Prognostic factors in first-time care seekers due to acute low back pain. Eur J Pain. 2007;11:290–298. doi: 10.1016/j.ejpain.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Hancock MJ, Maher CG, Latimer J, McLachlan AJ, Cooper CW, Day RO, Spindler MF, McAuley JH. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomised controlled trial. Lancet. 2007;370:1638–1643. doi: 10.1016/S0140-6736(07)61686-9. [DOI] [PubMed] [Google Scholar]
- 17.Hancock MJ, Maher CG, Latimer J, Herbert RD, McAuley JH. Independent evaluation of a clinical prediction rule for spinal manipulative therapy: a randomised controlled trial. Eur Spine J. 2008;17:936–943. doi: 10.1007/s00586-008-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancock MJ, Maher CG, Latimer J, Herbert RD, McAuley JH. Can rate of recovery be predicted in patients with acute low back pain? Development of a clinical prediction rule. Eur J Pain. 2009;13:51–55. doi: 10.1016/j.ejpain.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Heneweer H, Aufdemkampe G, Tulder MW, Kiers H, Stappaerts KH, Vanhees L. Psychosocial variables in patients with (sub)acute low back pain: an inception cohort in primary care physical therapy in the Netherlands. Spine. 2007;32:586–592. doi: 10.1097/01.brs.0000256447.72623.56. [DOI] [PubMed] [Google Scholar]
- 20.Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, York J, Das A, McAuley JH. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. Br Med J. 2008;337:154–157. doi: 10.1136/bmj.a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heymans MW, Vet HCW, Bongers PM, Knol DL, Koes BW, Mechelen W. The effectiveness of high-intensity versus low-intensity back schools in an occupational setting: a pragmatic randomized controlled trial. Spine. 2006;31:1075–1082. doi: 10.1097/01.brs.0000216443.46783.4d. [DOI] [PubMed] [Google Scholar]
- 22.Hush JM, Refshauge KM, Sullivan G, Souza L, Maher CG, McAuley JH. Recovery: what does this mean to patients with low back pain? Arthritis Rheum. 2009;61(1):124–131. doi: 10.1002/art.24162. [DOI] [PubMed] [Google Scholar]
- 23.Jensen TS, Albert HB, Sorensen JS, Manniche C, Leboeuf-Yde C. Magnetic resonance imaging findings as predictors of clinical outcome in patients with sciatica receiving active conservative treatment. J Manip Physiol Ther. 2007;30:98–108. doi: 10.1016/j.jmpt.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17:163–170. doi: 10.1179/jmt.2009.17.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linton SJ, Boersma K. Early identification of patients at risk of developing a persistent back problem: the predictive validity of the Orebro musculoskeletal pain questionnaire. Clin J Pain. 2003;19:80–86. doi: 10.1097/00002508-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Machado LAC, Maher CG, Herbert RD, Clare H, McAuley JH (2010) The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med 8(10). [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 27.Norman GR. Hi! How are you? Response shift, implicit theories and differing epistemologies. Qual Life Res. 2003;12:239–249. doi: 10.1023/A:1023211129926. [DOI] [PubMed] [Google Scholar]
- 28.Oleske DM, Neelakantan J, Andersson GB, Hinrichs BG, Lavender SA, Morrissey MJ, Zold-Kilbourn P, Taylor E. Factors affecting recovery from work-related, low back disorders in autoworkers. Arch Phys Med Rehabil. 2004;85:1362–1364. doi: 10.1016/j.apmr.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Pengel LHM, Refshauge KM, Maher CG, Nicholas MK, Herbert RD, McNair P. Physiotherapist-directed exercise, advice, or both for subacute low back pain: a randomized trial. Ann Intern Med. 2007;146:787–796. doi: 10.7326/0003-4819-146-11-200706050-00007. [DOI] [PubMed] [Google Scholar]
- 30.Roland M, Morris RA. A study of the natural history of low-back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Steenstra I, Anema J, Bongers P, Vet HC, Knol D, Mechelen W. The effectiveness of graded activity for low back pain in occupational healthcare. Occup Environ Med. 2006;63:718–725. doi: 10.1136/oem.2005.021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tulder MW, Becker A, Bekkering T, Breen A, del Real MTG, Hutchison A, Koes BW, Laerum E, Malmivaara A. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15:S169–S191. doi: 10.1007/s00586-006-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vroomen P, Krom MC, Wilmink J, Kester A, Knottnerus J. Lack of effectiveness of bed rest for sciatica. New Engl J Med. 1999;340:418–423. doi: 10.1056/NEJM199902113400602. [DOI] [PubMed] [Google Scholar]