Abstract
Previous functional imaging studies that compared activity patterns in older and younger adults during non-linguistic tasks found evidence for two phenomena: older participants usually show more pronounced task-related positive activity in the brain hemisphere that is not dominant for the task and less pronounced negative task-related activity in temporo-parietal and midline brain regions. The combined effects of these phenomena and the impact on word-retrieval, however, have not yet been assessed.
We used functional magnetic resonance imaging to explore task-related positive (active task > baseline) and negative activity (baseline > active task) during semantic and phonemic verbal fluency tasks. Increased right-frontal positive activity during the semantic task and reduced negative activity in the right hemisphere during both tasks was associated with reduced performance in older subjects. No substantial relationship between changes in positive and negative activity was observed in the older participants, pointing towards two partially independent but potentially co-occurring processes. Underlying causes of the observed functional network inefficiency during word-retrieval in older adults need to be determined in the future.
Keywords: functional magnetic resonance imaging, language production, aging, verbal fluency, default network
1. Introduction
Even though the aging brain suffers from a substantial loss of grey and white matter, overall, healthy aging is not associated with a dramatic decline of cognitive functions (Park and Reuter-Lorenz, 2009). With the increasing proportion of elderly persons worldwide, a growing interest in understanding the neural mechanisms allowing the aging brain to compensate for these structural losses has emerged. Modern imaging techniques like functional magnetic resonance imaging (fMRI) can help to unravel the mechanisms associated with healthy or pathological aging (for a review see Crosson et al., 2010). However, even though a large number of studies used functional imaging techniques to compare activity patterns associated with memory, attentional or perceptual processes in younger and older adults (Park and Reuter-Lorenz, 2009), very few studies so far have addressed language production mechanisms (Wierenga et al., 2008; Sörös et al., 2009; Meinzer et al., 2009; Shafto et al., 2010). This is of interest as word-retrieval deficits are frequently observed even in healthy older adults (Burke and Shafto, 2004) and constitute an early symptom of dementia (Henry, 2004).
With regard to non-linguistic functions, two phenomena have consistently been reported in functional imaging studies of cognitive aging when comparing groups of younger and older adults: First, across a wide range of cognitive or perceptual tasks, healthy older adults may show less focal task-related positive activity (i.e., when comparing a given active task with a baseline task; Cabeza, 2002; Davis et al., 2008). In addition, there is frequently more pronounced activity in the hemisphere that is not dominant for the task, mostly in the pre-frontal cortex. This has been described as the hemispheric asymmetry reduction in older adults (HAROLD; Cabeza, 2002). The functional significance of additional activity in older people has been intensely debated, but in the majority of studies, it has been concluded that additional activity may represent an adaptive process of functional network reorganization, potentially to compensate for age-related structural brain deterioration (for review see Park and Reuter-Lorenz, 2009).
Second, when comparing activity between a given active task and a baseline task, a set of brain regions in midline anterior and posterior regions and in lateral temporo-parietal cortices consistently show task-related negative activity (i.e., these areas are more active during rest than during the active task). These regions overlap with the so-called “default network of brain activity” that is active during resting state functional imaging studies (Gusnard et al., 2001; Raichle et al., 2001; Buckner et al., 2008). Rapid switching from the unconstrained default mode to a more constrained set of task-relevant areas may represent allocation of processing resources, as components of this network are suppressed during active tasks and activity in the two systems becomes strongly anticorrelated (e.g., Fox, 2009; see Buckner et al., 2008 for review). In young adults, areas that show negative task-induced activity are functionally interconnected and their activity is modulated by task demands (Persson et al., 2007; Park et al., 2010), such that more difficult tasks are associated with stronger negative activity. In healthy older adults, and more so in persons with Alzheimer s disease, reduced task-related negative activity, less interconnectivity, and less modulation by task demands have been observed (Grady et al., 2006; Persson et al., 2007; Andrews-Hanna et al., 2007; Park et al., 2010). Moreover, reduced negative activity has been linked to impaired behavioral performance (Persson et al., 2007; Damoiseaux, et al., 2008; Park et al., 2010).
Recently, a growing interest in changes of the neural substrates supporting language functions in aging has emerged. Because many core aspects of language are strongly lateralized to the left hemisphere, they represent an ideal model to assess the role of non-task dominant brain activity. Behavioral and functional imaging research has shown that language production and comprehension may be affected to different degrees by aging (for review see Wingfield and Grossman, 2006; Burke and Shafto, 2008). In particular, language production mechanisms are frequently impaired in old age, resulting in word-retrieval failures and reduced verbal fluency (Burke and Shafto, 2004; Meinzer et al., 2009; Shafto et al., 2010). Word-retrieval problems are also among the earliest signs of dementia (Henry et al., 2004).
With regard to the HAROLD phenomenon and language functions, a slightly different picture emerged compared to previous studies in other cognitive domains: Integration of right frontal areas into a functional network may help to sustain performance in older adults, even during strongly left lateralized syntactic tasks (e.g., Tyler et al., 2010; for review see Wingfield and Grossman, 2006). On the other hand, a recent study by Peelle et al. (2010) indicated that during difficult tasks, additional task positive activity in areas outside of the core network activated by younger adults might be inefficient and is associated with impaired performance and reduced functional network connectivity. In line with these findings, two recent studies on word-retrieval demonstrated that additional non-task dominant positive activity in older adults might be detrimental to word-retrieval. Wierenga et al. (2008) showed that right frontal activity in low performing adults during a naming task was negatively correlated with performance. In a second study, Meinzer et al. (2009) assessed semantic and phonemic verbal fluency using fMRI in a group of older German adults. Compared to a younger control group, increased right frontal activity was found only during the semantic task where the older group evidenced lower performance. In addition, increased right frontal activity was negatively correlated with performance in the older group.
With regard to task-related negative activity and language functions, several studies reported differences during semantic tasks between young adults and older adults who are healthy (Persson et al., 2004; 2007) and adults with Alzheimer s disease (Lustig et al., 2003; 2004; McGeown et al., 2009). Moreover, Persson et al., (2007) reported better task performance in older participants who showed a pattern of task-related negative activity that was more similar to that of a younger control group. However, previous studies have not assessed the functional impact of altered negative activity during language production tasks. Furthermore, patterns of positive and negative task-induced activity in older adults have rarely been assessed in the same participants. The latter is of particular importance, as attenuation of negative activity may be associated with increased right-frontal activity in older participants (Persson et al., 2008) and changes in connectivity between “default network” regions and right pre-frontal areas have been observed (Grady et al., 2009).
Thus, in the present study we used a similar semantic and phonemic fluency design as in our previous study (Meinzer et al., 2009). Both verbal fluency tasks have previously been used to assess frontally mediated language processes and functional imaging studies in younger adults consistently reported strongly left lateralized activity in dorsolateral frontal cortices (Costafreda et al., 2006). Aims of the study were to (a) replicate our previous findings in an English speaking sample, (b) to assess task-induced positive and negative activity in the same younger and older participants, (c) to assess whether differences in task-related positive and negative activity in the older participants are related to performance (i.e., word-retrieval performance during the two fluency tasks) and, (d) to elucidate whether there is a relationship between changes in positive and negative activity in the older group.
2. Methods
The study was approved by the Institutional Review Board of the University of Florida and conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all participants prior to study inclusion.
2.1. Participants
Fourteen healthy older adults and fourteen younger adults were recruited from the University of Florida and Gainesville, Florida communities (older group: 69.2±5.8 years, range 61–80; younger group: 24.6±4.4, range 19–32). Groups were matched for gender (7 females and males in each group) and education (see Table 1). All participants were native English speakers and strongly right handed as determined by the Edinburgh Inventory (Oldfield, 1971).
Table 1.
Demographic and psychometric characteristics of the participants
| YOUNGER GROUP (N=14, 7 females) | OLDER GROUP (N=14, 7 females) | |
|---|---|---|
| Age (years) | 24.6 ± 4.4 | 69.2 ± 5.8 |
| Education (years) | 15.9 ± 2.1 | 16.4 ± 2.2 |
| MMSE (max. 30) | 29.1 ± 0.9 | 29.1 ± 0.9 |
| Neuropsychological testing | ||
| D-KEFS | ||
| semantic fluency (total animals/boys) | 44.5 ± 7.8 | 41.4 ± 6.7 |
| phonemic fluency (total F/A/S) | 48.0 ± 7.0 | 44.4 ± 11.2 |
| Ambigouos sentences (max. 39) | 36.4 ± 2.2 | 34.2 ± 7.1 |
| Pyramids and Palms (max. 52) | 50.5 ± 1.2 | 50.8 ± 1.0 |
| Boston Naming Test (max. 31) | 30.2 ± 1.6 | 30.4 ± 1.2 |
| Digit span | ||
| Forward (max. 16 points) | 11.9 ± 2.2 | 12.0 ± 1.7 |
| Backward (max. 14 points) | 9.6 ± 2.0 | 8.8 ± 2.2 |
| California Verbal Learning Test (max. 16) | ||
| correct recall (after learning trial 5) | 13.7 ± 0.9 | 11.5 ± 2.6 * |
| short delay free recall | 12.9 ± 3.1 | 10.0 ± 2.1 * |
| short delay cued recall | 13.4 ± 2.8 | 11.7 ± 2.6 |
| long delay free recall | 12.6 ± 3.5 | 10.9 ± 3.7 |
| long delay cued recall | 13.6 ± 2.7 | 11.4 ± 3.1 |
| long delay recognition hits | 15.4 ± 1.1 | 15.0 ± 0.9 |
Mean values of raw scores with standard deviations. Asterisks indicate significant differences between age groups at p< .05
None of the participants had previous or current neurological or psychiatric conditions, cardiovascular disease, uncontrolled hypertension or substance abuse as determined by a brief clinical interview and a standard health questionnaire. Additionally, all participants were screened with a brief cognitive test battery (Mini Mental State Exam, MMSE, Folstein et al., 1975) and with the Beck Depression Inventory (BDI, Beck et al., 1996). None of the participants reported subjective memory complaints in everyday life and no indicators of cognitive impairment were found during the cognitive screening (MMSE: all ≥ 27/30 points both old/young: 29.1±0.9). All subjects scored within the normal range on the BDI.
2.2. Neuropsychological Testing (see Table 1 for details of the results)
Additional neuropsychological testing was administered to all participants to ensure normal cognitive functions in old and young adults. The battery comprised the California Verbal Learning Test (CVLT-2, Delis et al., 2000) and the Digit Span subtest of the Wechsler Adult Intelligence Scale (WAIS-R; Wechsler, 1997) as objective tests of memory function. Additionally, executive functions of language and semantic processing (including confrontation naming) were assessed with the Boston Naming Test (BNT, Kaplan et al., 1983), the Delis – Kaplan Executive Functions System (D-KEFS Verbal Fluency Tests, Delis et al., 2004), the Test of Language Competence (TLC-E, Ambiguous Sentences subtest, Wiig and Secord, 1989), and the Pyramids and Palm Trees Test (Howard and Patterson, 1992). The tests were administered on a day prior to the MRI and the entire test battery took approximately one hour to administer.
Consistent with previous reports, the younger subjects performed better on two indices of the CVLT (correct recall after five trials, short delay free recall) and the verbal fluency tests (D-KEFS); however, the latter were not statistically significant. When considering age corrected norms, the older group performed within normal age ranges on all CVLT indices. No significant differences were found between the age groups on the Digit Span Test, which suggests a high level of functioning in our group of older adults. With respect to confrontation naming (BNT) and semantic processing (Ambiguous Sentences, Pyramids and Palm Trees) the groups performed equally well (see Table 1).
2.3. Experimental task and stimulus characteristics
Similar to our previous study (Meinzer et al., 2009), two overt verbal fluency tasks were implemented to be performed in the scanner (semantic and phonemic fluency). Participants were told that they would see either different categories or different initial letters at the center of a video screen. Their task was to generate different exemplars for each respective category (semantic fluency) or words beginning with a particular letter (phonemic fluency). Repetitions were counted as incorrect. A total of eight different categories and letters were used (categories: body parts, types of music, clothing, insects, colors, spices, beverages, criminal acts; letters: M, J, S, K, T, Q, P, K).
The fMRI task employed an externally paced paradigm and a temporal sparse-sampling technique (Hall et al., 1999). Here, the overt verbal response was assessed in the scanner during an off-phase of data acquisition and the hemodynamic response was acquired after a short time delay to avoid artifacts due to the articulation process. This procedure has been shown to be well suited to assess overt naming and verbal fluency in the scanner in several previous studies of healthy older adults and patients with acquired language disorders after stroke (Meinzer et al., 2006, 2007, 2008, 2009). Moreover, it has been shown that paced compared to unpaced paradigms elicit more robust activity in frontal brain areas contributing to word-retrieval and working memory (Basho et al., 2007).
Stimuli were presented visually by an fMRI compatible projector and a system of mirrors via the Eloquence Functional Imaging System (Invivo Corporation, Gainesville, Fl) using E-Prime Version 1 software (Psychology Software Tools, Inc., Pittsburgh, PA). Each category and letter was presented for three seconds during which the participants were required to respond overtly (i.e., out loud) with one exemplar of the given category or a word beginning with the given letter. Afterwards, the stimulus disappeared and was replaced by a black screen (2.53 seconds). The participants were instructed to say the word pass if they could not come up with a correct exemplar, to control for effects of motor cortex activity and hearing of their own voice. No overt responding was allowed after stimulus offset. A single whole-brain functional MR volume was acquired 0.27 seconds after stimulus offset (temporal sparse sampling). Verbal responses were transmitted from a microphone in the scanner to a speaker, and subsequently transcribed and analyzed.
Each condition was introduced on the visual display with a speech bubble (5.8 seconds). Then, the first trial for the presented condition was displayed (i.e. semantic or phonemic fluency, or the word rest ). During scanning, both experimental conditions (category and phonemic fluency) were presented in alternating blocks of 10 consecutive trials (i.e., the same category/letter was repeated 10 times within each block; total block length 63.8 seconds). Additionally, complex baseline blocks (five consecutive trials of saying the word rest aloud; block length 34.8 seconds) were interspersed between category and letter fluency blocks.
The eight categories and letters were presented during a single scanning session on the same day split into two runs, with a short break in between runs (four of the categories/letters were presented during each run). The same categories and initial letters were used for all participants. The order of presentation (semantic vs. phonemic fluency) was counterbalanced among participants and the order of appearance of categories and letters was randomized within runs. Prior to the first scan, a training session outside of the scanner was performed to familiarize the participants with the experimental design. A different set of categories and letters was used for this training session.
2.4. fMRI set-up and acquisition parameters
Scanning was conducted at the McKnight Brain Institute of the University of Florida using a 3-Tesla Philips Achieva MR-System. Stimulus presentation, participant response, and scanning took place while the participants were lying in a supine position on the scanner gantry. The participants head was stabilized with foam padding to minimize head movements.
For functional MRI scanning, a T2*-weighted Fast-Field Echo, Echo-Planar-Imaging (FFE-EPI) sequence utilizing a parallel scanning technique (SENSE) was used with the following parameters: TR=5.8 sec.; TA=2.53 sec.; TE=30 msec.; 38 transverse slices, interleaved acquisition, slice-thickness: 3 mm, no interslice gap; in-plane resolution: 3×3 mm; FOV: 240×240×114, acquisition matrix: 80×79. A total of 240 functional, whole brain volumes were acquired during the two sessions (80 for each of the fluency tasks, 80 baseline volumes). Additionally, a high resolution (1×1×1 mm) anatomical image was acquired to facilitate normalization of individual images and to ensure that participants did not have gross anatomical abnormalities.
2.5. Functional MRI data analysis
Pre-processing of functional MRI data was performed using Statistical Parametric Mapping (SPM5, Wellcome Department of Imaging Neuroscience, London, UK). Pre-processing of data included correction for slice-time differences and spatial alignment to the first volume in the image series to adjust for head movements. Functional volumes were normalized to standard MNI space using SPM5 default parameters (i.e., affine and non-linear warping, trilinear interpolation) and smoothed with a Gaussian Kernel of 6×6×6 mm full-width-at-half-maximum. To generate individual statistical maps, data were modeled using a finite impulse response function (FIR, 1st order; Gaab et al., 2007). The design matrix for the statistical analysis comprised the three covariates-of-interest (semantic and phonemic fluency trials, baseline trials) as well as covariates-of-no-interest (movement parameters obtained during realignment) to improve overall model fit to the empirical data and to reduce residual error variance. Regressors were entered in a session specific manner. The effects of the conditions were determined in a single statistical model at the first level to account for session specific effects (e.g., different noise levels). Before estimating the modeled regressors, a high-pass filter with a cut-off period of 128 seconds was applied to the data. After estimation of the overall model for each participant, planned contrasts-of-interest were calculated. These included separate comparisons of category and phonemic fluency runs with the baseline condition (i.e., positive task-related activity: semantic fluency > baseline, phonemic fluency > baseline) and the inverse contrasts (i.e., negative task-related activity: baseline > semantic fluency, baseline > phonemic fluency). All responses were included. For the group analysis, a random effect model was calculated that included the above mentioned contrasts. Four different analyses were performed:
2.5.1. Within-group comparison positive and negative activity task-related activity
To assess patterns of positive and negative task-related activity, we compared the two experimental conditions with the baseline separately for each age group (positive activity). To assess task negative activity, the inverse contrast was calculated (baseline > experimental tasks). To ensure a meaningful comparison between task-related positive and negative activity, and between age groups, the highest corrected threshold was chosen for where there was at least one significant cluster found for each of the comparisons and age groups (voxel level p< .005, false discovery rate corrected, FDR; Genovese et al., 2002). Only clusters that were significant at a corrected cluster threshold of p< .05 are reported.
2.5.2. Between-group comparisons of task-related positive and negative activity
To assess the impact of age on positive and negative activity we directly contrasted the activity obtained from analysis (2.5.1.) (i.e., tasks > baseline; baseline > tasks) between the two groups. As reviewed in the introduction, previous studies have reported differences between older and younger subjects with regard to task-related positive and negative activity. Thus, it is conceivable that some areas that show positive/negative activity in one group could show the opposite trend in the other group, which may bias the comparison. Therefore, each comparison (2-sample t-tests) was inclusively masked by the respective pattern of positive/negative activity of each age group (e.g., the comparison of positive activity old > young was inclusively masked by the contrast activity > baseline in the old group to ensure that potential areas were indeed active in the old group). Group differences are reported at a FDR-corrected voxel threshold of p< .05 and a corrected cluster threshold of p< .05.
2.5.3. Correlations between performance and task-related positive and negative activity in differentially active areas in the older group
Significantly greater positive activity was only found in the older group, while significantly greater negative activity was only found in the younger group (please see results). To explore the functional relevance of these differences for the older group we performed a region-of-interest (ROI) analysis using these clusters of significant difference in activity. As in our previous study (Meinzer et al., 2009) we correlated average normalized (z-transformed) beta activity in the differentially active clusters (entire clusters) with the individual performance as obtained in the scanner for the two verbal fluency tasks. Specifically, these analyses allow us to assess how performance in the older group is affected by (a) increased positive activity in areas not active in the younger group and (b) reduced negative activity of the older participants within the normal pattern of negative activity in the younger group.
2.5.4. Correlations between right frontal positive activity and negative activity
The final analysis aimed to explore whether increased activity in right frontal areas in the older group is coupled with the loss of negative activity. To this end, we correlated the individual subjects degree of negative activity from the differentially active clusters that were correlated with behavior for each fluency task (see Table 2) with the degree of positive activity in the two right hemisphere clusters active in the fluency-baseline contrast for each respective condition (i.e., semantic/phonemic fluency > baseline). [Please note: Activity from the fluency-baseline comparison was chosen as no differences in right frontal areas were found between the two groups during the phonemic task].
Table 2.
Statistical comparison of positive and negative task-related activity between younger and older participants (FDR-corrected p< .05); Note: no significant differences in positive activity were found for the phonemic fluency task even at p< .005 uncorrected
| POSITIVE ACTIVITY (OLD > YOUNG) – semantic fluency | |||||||
|---|---|---|---|---|---|---|---|
| Anatomical structure | Hemi | BA | k | Z | x | y | z |
| Postcentral gyrus | R | 3 | 80 | 5.1 | 27 | −38 | 46 |
| Precuneus | 7 | 3.5 | 18 | −47 | 44 | ||
| Superior parietal lobule | 7 | 3.3 | 33 | −47 | 47 | ||
| Medial frontal gyrus | R | 6 | 140 | 4.4 | 15 | 6 | 55 |
| Middle frontal gyrus | 6 | 4.3 | 24 | −7 | 45 | ||
| Middle frontal gyrus | R | 11 | 53 | 4.3 | 39 | 40 | −15 |
| Inferior frontal gyrus | 4.0 | 45 | 32 | 4 | |||
| Medial frontal gyrus | R | 9 | 142 | 4.2 | 27 | 36 | 20 |
| Middle frontal gyrus | 9/8 | 4.1 | 30 | 17 | 49 | ||
| Posterior cingulate gyrus | R | 30 | 119 | 3.9 | 12 | −63 | 14 |
|
NEGATIVE ACTIVITY (YOUNG > OLD) – semantic fluency | |||||||
| Postcentral gyrus | R | 3/2 | 72 | 5.1 | 27 | −38 | 46 |
| Inferior parietal lobule | 40 | 4.0 | 39 | −38 | 57 | ||
| Middle temporal gyrus | R | 39 | 136 | 4.5 | 39 | −75 | 15 |
| Precuneus | 7 | 3.5 | 24 | −65 | 34 | ||
| Parahippocampal gyrus | R | 36 | 95 | 4.2 | 21 | −41 | −11 |
| Lingual gyrus | 19 | 3.8 | 18 | −59 | −5 | ||
| Precuneus | R | 7/31 | 87 | 3.9 | 15 | −44 | 44 |
| Cingulate gyrus | 31 | 3.6 | 18 | −33 | 34 | ||
|
NEGATIVE ACTIVITY (YOUNG > OLD) – phonemic fluency | |||||||
| Posterior cingulate gyrus | R | 31 | 345 | 4.5 | 15 | −36 | 35 |
| Precuneus | L | 31 | 4.0 | −15 | −48 | 30 | |
| Superior temporal gyrus | R | 13 | 262 | 4.4 | 45 | −48 | 22 |
| Middle temporal gyrus | 39 | 4.0 | 39 | −66 | 23 | ||
| Medial frontal gyrus | L | 30 | 100 | 4.1 | −3 | −9 | 56 |
| Cingulate gyrus | 24 | 4.1 | −6 | −4 | 44 | ||
| Posterior cingulate gyrus | R | 30 | 62 | 4.1 | 6 | −55 | 6 |
| 29 | 3.5 | 9 | −46 | 5 | |||
| Middle temporal gyrus | L | 39 | 144 | 4.1 | −33 | −69 | 23 |
| Superior temporal gyrus | 39 | 3.9 | −45 | −51 | 25 | ||
| Postcentral gyrus | R | 2/1 | 75 | 3.9 | 50 | −24 | 45 |
| Supramarginal gyrus | 40 | 3.8 | 59 | −39 | 32 | ||
R = right, L = left; Hemi = hemisphere, BA = Brodman area; k = cluster extent (voxels); Z = Z-value; x/y/z = coordinates of peak voxels in significant clusters in Talairach space; bold = peak voxel in significant cluster; smoothness 9.6 × 9.5 × 10 mm, resel count positive activity: 1392.2 resels, resel = 33.71, negative activity: 1394.0 resels, resel = 33.67
Anatomic localization of significant voxels within clusters was conducted using the Talairach Daemon software (Lancaster et al., 2000) with the nearest grey matter option enabled. For presentation of the results, the data are superimposed on a standard brain template (Montreal Brain).
3. Results
3.1. Intrascanner performance during the verbal fluency tasks
Both groups produced significantly more exemplars during the semantic fluency task than during the phonemic fluency task (young/old: F(1,26)=32.9/49.2, p< .001). Consistent with the results of our previous study (Meinzer et al., 2009) the younger subjects produced significantly more correct category exemplars during the semantic fluency task than the older subjects (young: 73.9±2.5, old 71.56±1.5; F(1,26)=9.1, p= .006). Even though the younger group on average produced more exemplars during the phonemic task, there was no statistically significant difference between the groups (young: 66.6±4.7, old: 64.3±3.6; F(1,26)=0.9, p= .34).
3.2. fMRI results
3.2.1. Within group analysis of task-related positive (Figure 1 and Supplementary Table 1) and negative activity (Figure 2 and Supplementary Table 2)
Figure 1.
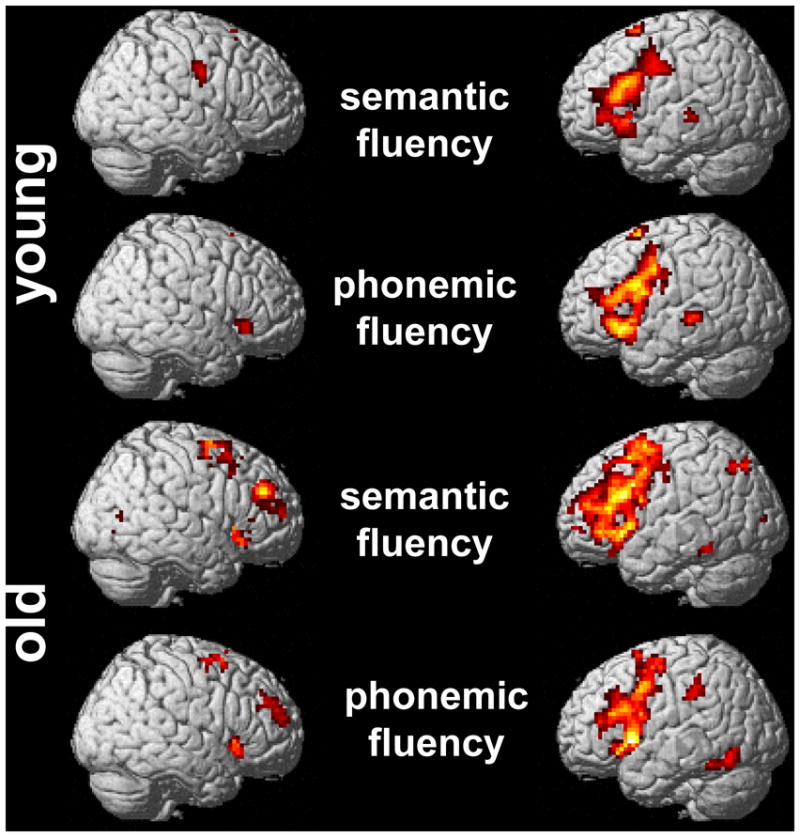
Patterns of task-related positive activity in older and younger participants during the semantic and phonemic fluency tasks (p< .005 FDR-corrected)
Figure 2.
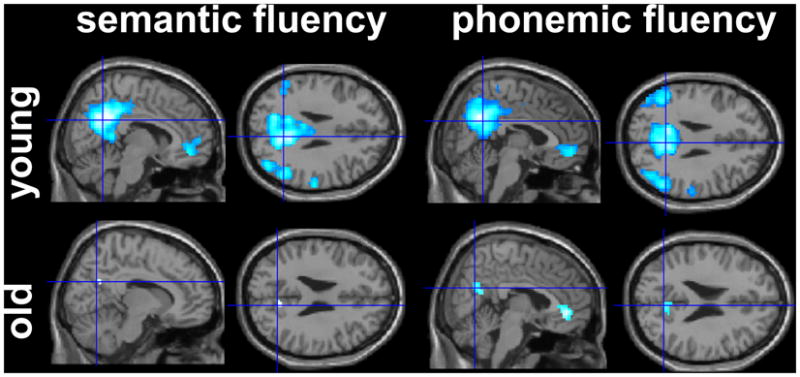
Patterns of task-related negative activity in older and younger participants during the semantic and phonemic fluency tasks (p< .005 FDR-corrected)
In line with our previous study, for both tasks the strongest activity clusters in the young group were found bilaterally in the medial superior frontal area (BA 6) and the anterior rostral cingulate zone (BA 32). Lateral frontal activity for both tasks was strongly left lateralized and comprised several areas in the inferior and middle frontal gyri (see Figure 1 and Supplementary Table 1 for details of peak activity patterns). Right hemisphere activity in the younger group during the semantic fluency task was limited to the precentral gyrus, the lingual and parahippocampal gyri, and the caudate nucleus. Only during the phonemic fluency task, an additional cluster in the right inferior frontal gyrus (BA 47) was found in the younger group.
Compared to the younger group, the older participants demonstrated a very similar pattern of activity during both fluency tasks, except for larger clusters in the bilateral medial frontal and lateral frontal lobes. Some additional areas that were not active or even showed negative activity in the younger group were found to be positively active in the older group (left superior and inferior parietal cortex, posterior cingulate gyrus). The older group showed activity in a similar area in the right inferior frontal gyrus (BA 47) as the young group during the phonemic task. In contrast, additional clusters in the right middle frontal gyrus (BA 46, semantic fluency) and superior and middle frontal gyri (BA 10, phonemic fluency) were only found in the older group.
Consistent with the literature on task-related negative activity during language and other cognitive tasks (Buckner et al., 2008) the younger participants showed extensive bilateral negative activity during both fluency tasks in anterior and posterior midline areas with peak activity in the medial frontal cortex and the precuneus. Additional clusters during the semantic task were found bilaterally in temporal and parietal areas (superior and middle temporal, supramarginal and angular gyri), the right parahippocampal gyrus and the right putamen. During the phonemic fluency task a very similar pattern emerged with peak activity in the superior and middle temporal gyri and the left and right parahippocampal gyrus.
Compared to the younger group, the older group had fewer clusters of negative activity and the extent of negative activity was greatly reduced. Task-related negative activity was limited to the two midline areas that also showed negative activity in the younger group and was centered in the posterior cingulate gyrus (semantic and phonemic task) and the pre-genual medial frontal cortex (phonemic fluency only).
3.2.2. Between group comparison of task-related positive and negative activity (see Table 2)
In line with our own previous study (Meinzer et al., 2009), group differences in task-related positive activity during the whole brain analysis were found only during the semantic fluency task. Moreover, more pronounced activity in the group comparison was only found in the old group and located exclusively in the right hemisphere. Three of these clusters were located in the right frontal cortex (medial, middle and inferior frontal areas). Two additional clusters were found in the right posterior parietal lobe and the posterior cingulate gyrus. It is significant to note that some of these areas overlapped with clusters showing negative activity in the younger group. However, the difference between old and young participants cannot solely be explained by more pronounced negative activity in the younger group, because we inclusively masked the comparison with the positive activity pattern of the older group during the semantic task.
For both fluency tasks the group comparison revealed more pronounced negative activity only in the younger group. For the semantic task, four clusters in the right hemisphere showed more pronounced negative activity in the younger group with peak negative activity located in the postcentral/inferior parietal cortex, the middle temporal gyrus, the parahippocampal/lingual gyrus and the precuneus (see Table 2 for details). Significantly stronger negative activity was substantiated in the younger group during the phonemic fluency task and located bilaterally in the posterior midline area (cingulate gyrus/precuneus), the left medial frontal and middle temporal gyrus and three additional right hemisphere clusters (posterior superior/middle temporal gyrus, posterior cingulate gyrus and the postcentral/supramarginal gyrus).
3.2.3. Correlations between performance and task-related positive and negative activity in differentially active areas in the older group
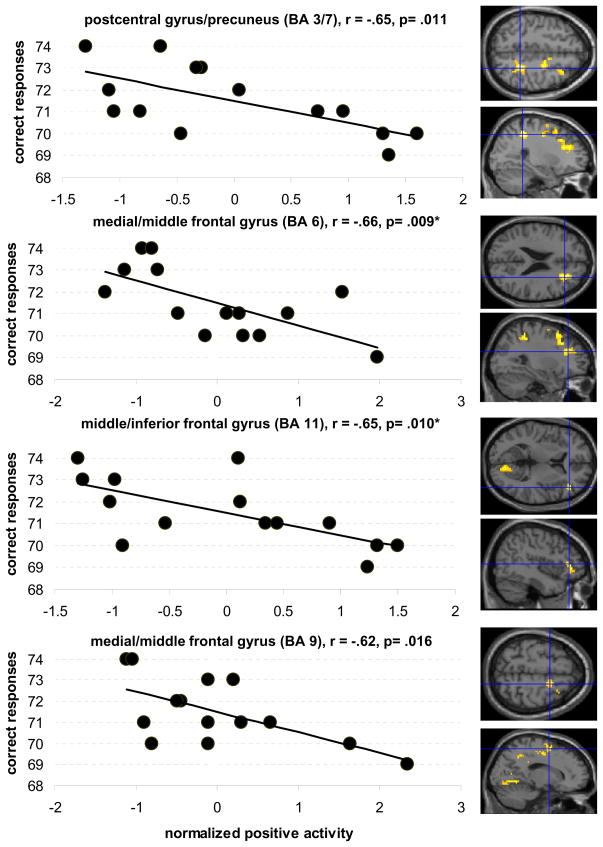
To assess the functional significance of more pronounced right hemisphere activity we correlated activity in the differentially active areas (Table 2) with performance of the older group. For the semantic fluency task there were strong negative correlations between activity in four of the five differentially active clusters (postcentral gyrus/precuneus: r= −.65, p= .011; medial/middle frontal gyrus: r= −.66, p= .009; middle/inferior frontal gyrus: r= −.65, p= .010; medial frontal gyrus: r= −.62, p= .016; see Figure 3). That is, older participants with greater right hemisphere activity produced fewer category exemplars. The cluster in the posterior cingulate gyrus was not correlated with performance (r= −.05, p= .85).
Figure 3.
shows the correlations between intrascanner performance during the semantic task (max. 80 correct responses) and the degree of positive activity in areas more activated by older participants (crosshairs on axial and sagittal images illustrate the location of the respective clusters). All clusters are located in the right hemisphere. Uncorrected threshold p= .05, * refers to correlations that survived after correcting for multiple comparisons (p= .01)
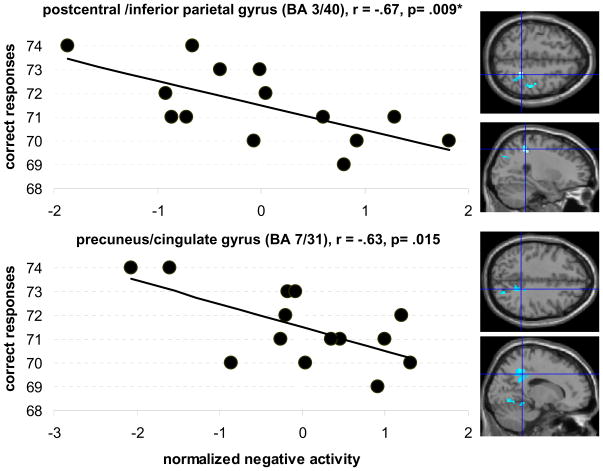
For both fluency tasks the younger participants exhibited more negative activity than the older group. Thus, we correlated activity in these differentially active areas (Table 2) with performance in the older group to assess whether more pronounced negative activity in these areas in the older group is related to performance. Stated differently, we were interested in discovering whether a pattern that more closely resembles that of the younger participants is beneficial to performance. Indeed, more pronounced negative activity in two of the four clusters predicted better performance in the older participants during the semantic task (right postcentral/inferior parietal gyrus: r= −.67, p= .009; right precuneus/cingulate gyrus: r= −.63, p= .015; see Figure 4). The degree of negative activity in the remaining clusters was not correlated with performance (right parahippocal/lingual gyrus r= −.27, p= .33; right middle temporal gyrus: r= −.50, p= .064; please note that the marginally significant correlation for the middle temporal cluster was driven by an outlier; removing this data point reduced the correlation to r= −.27, p= .36).
Figure 4.
Correlations between intrascanner performance during the semantic task and the degree of negative activity in the older participants in areas that were differentially more deactivated in the younger group. The figure shows that older participants with more pronounced negative activity in these clusters performed better during the task (crosshairs on axial and sagittal images illustrate the location of the respective clusters). All clusters are located in the right hemisphere. Uncorrected threshold p= .05, * refers to correlations that survived after correcting for multiple comparisons (p= .0125)
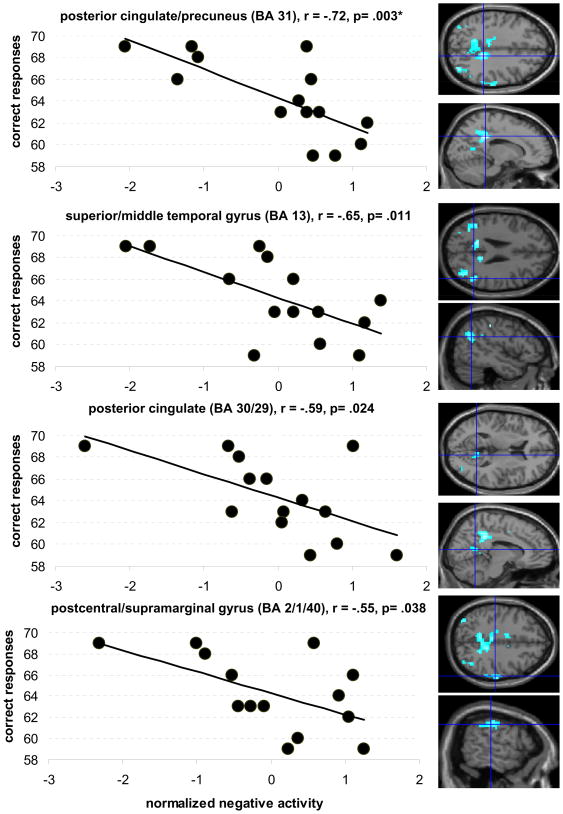
The degree of negative activity in the two differentially active left hemisphere clusters during the phonemic task was not correlated with performance (left medial frontal gyrus: r= −.25, p= .37; left middle/superior temporal gyrus: r= −.43, p= .119). On the other hand, strong positive correlations between the four remaining right hemisphere clusters (Table 2) and performance were present (left/right cingulate gyrus/precuneus: r= −.72, p= .003; right superior temporal: r= −.65, p= .011; right posterior cingulate: r= −.59, p= .024; right postcentral/supramarginal: r= −.55, p= .038; see Figure 5). Thus, more pronounced negative activity in the right hemisphere predicted better performance in our older participants.
Figure 5.
Correlations between intrascanner performance (max. 80 correct responses) during the phonemic task and the degree of negative activity in the older participants in areas that were differentially more deactivated in the younger group. The figure shows that older participants with more pronounced negative activity in these clusters performed better during the task (crosshairs on axial and sagittal images illustrate the location of the respective clusters). All clusters are located in the right hemisphere. Uncorrected threshold p= .05, * refers to correlations that survived after correcting for multiple comparisons (p= .008)
3.2.4. Correlations between right frontal positive activity and negative activity
For the semantic task we found a weak positive correlation between activity in the right middle frontal gyrus ROI (BA 46, k=46, peak 42/42/16) and the two clusters in the postcentral/inferior parietal gyrus (r= .54, p= .043) and the precunues/cingulate gyrus (r= .54, p= .043). Thus, reduced negative activity in these two areas predicted the degree of positive activity in the right middle frontal gyrus (MFG) ROI. Positive activity in the right inferior frontal gyrus (IFG, BA 47, k=207, peak voxel coordinates: 33/26/-4) was not correlated with the degree of negative activity in these two regions (r=.38/26, p= .18/.27). The same correlation analyses were performed for the differentially active negative clusters during the phonemic task and right frontal positive activity during this task in the older group (superior/middle frontal gyrus and inferior frontal gyrus, see Supplementary Table 1). No significant correlations were found.
4. Discussion
In the present study we used fMRI to assess task-related positive and negative activity in healthy younger and older participants during two verbal fluency tasks. The main findings can be summarized as follows: First, we replicated the main results of our previous study (Meinzer et al., 2009) that found more pronounced right frontal activity during the semantic task where performance was more affected by aging in a different sample of older adults who spoke a different language. Second, as in our previous study, more pronounced right frontal activity during the semantic task was negatively correlated with performance. Third, we extended our analyses to explore the relationships between task-induced negative activity and performance in the same participants. Here, we support the findings of previous studies that found reduced negative activity in the older group and its significant relationship with performance. Compared to previous studies that analyzed activity in pre-defined ROIs (e.g., Persson et al., 2008), we analyzed our data by assessing correlations within differentially activated areas between younger and older adults to elucidate the impact of these age related changes on performance. This approach is completely data driven and does not require any a priori assumption. Fourth, we assessed whether there is a relationship between changes in task positive and negative activity in older participants. The fact that we only found weak correlations for the semantic fluency task, and no correlations during the phonemic task, argues for two partially independent but potentially co-occurring processes in older adults. We will discuss each of these findings in more detail below:
With regard to positive activity, we found a strikingly similar pattern in younger and older native English speakers compared to our previous study with native German speakers (Meinzer et al., 2009). We also supported the previous finding of more pronounced task-related positive activity in the older group, mainly in right frontal brain areas during the semantic fluency task, where the older participants produced significantly fewer words than the younger group. Also consistent with our previous study, this relatively increased frontal activity was correlated with reduced performance. The replication of our previous findings using the same paradigm but across different languages strengthens the validity of our findings (Lieberman and Cunningham, 2009).
Task performance was worse during the phonemic compared to the semantic task in both groups. Even though younger adults produced more correct exemplars for the phonemic task, this difference was not significant. Relatively equivalent performance on this task in the older group was mirrored by a very similar pattern of task-related positive activity compared to the younger participants. Again, this is consistent with our previous study (Meinzer et al., 2009) where we did not find activity differences in the left or right frontal cortex between groups for phonemic fluency. Rather, the more difficult (phonemic) task elicited right frontal activity (including the right IFG, BA 47) in both groups. Thus, increased right inferior frontal activity seems to be part of the normal brain response in younger adults and might be related to increased task difficulty and selection demands (Thompson-Schill et al., 1997; Persson et al., 2008).
The present study extended our analyses to patterns of task-induced negative activity. Consistent with previous studies on task-induced negative activity during semantic processing, non-language tasks and resting state fMRI or PET studies (see Buckner et al. 2008 for a comprehensive review) we showed clusters of negative activity mainly in anterior and posterior midline areas and temporo-parietal cortices bilaterally for both semantic and phonemic fluency tasks in the younger participants. Also in line with previous studies that compared older and younger adults (e.g., Persson et al., 2007; Park et al., 2010), we found reduced negative activity in the core areas of the so-called default network (i.e., anterior medial frontal cortex; posterior cingulate gyrus/precuneus) for older compared to younger subjects and no additional negative activity in the remaining parts of the network that was deactivated by the younger participants.
This was further supported by direct statistical comparison of the two groups. In particular, during both fluency tasks the older group had reduced negative activity in several clusters. Most importantly, the degree of negative activity in two of the four clusters during the semantic task that showed less negative activity in the older group (one overlapping with the core area of the default network in the precuneus, the other included the inferior parietal cortex) was negatively correlated with performance. Thus, in line with previous reports (Damoiseaux, et al., 2007; Persson et al., 2007; Park et al., 2010), older participants who had relatively more pronounced negative activity within the task-related negative activity network found in the younger adults actually performed better during the semantic fluency task. Essentially, the same pattern was found during the phonemic task. Here, four of the six differentially active negative clusters during the phonemic task were correlated with performance in the older group (i.e., the more negative activity, the more correct responses were generated). Noteworthy, is the fact that these clusters which were correlated with performance were located in the right hemisphere. Thus, stronger negative activity in the non-task dominant right hemisphere resulted in better performance across both fluency tasks.
However, we need to caution the reader that some of the correlations we report in the present manuscript would not have passed a more stringent level of significance (see for Figures 3–5 for clusters that survived after correcting the correlations for multiple comparisons). However, our main finding that older adults who recruit a network of neural resources similar to that recruited by younger adults perform better during the two tasks is supported by similar findings in (a) our own previous study that used the same paradigms (Meinzer et al., 2009), (b) a recent study on spatial working memory (Nagel et al., 2009), showing that a more youth like modulation of the BOLD signal was associated with higher levels of spatial working memory performance and, (c) a very recent quantitative meta-analysis on laterality patterns in old and young adults during functional imaging studies of perception, memory encoding, memory retrieval and executive functions (Spreng et al., in press).
Interestingly, the main difference between the two age groups during the phonemic task, compared to the semantic task, was an even greater difference in the degree in negative task-induced activity (i.e., larger clusters and a greater number of differentially activated clusters in the younger group; see Table 2). This was found despite (a) the absence of statistically significant differences in performance and (b) major differences in positive activity. The most likely explanation for this observation is that negative activity became more negative with increased task demands in younger adults, whereas it was less modulated in older adults. Indeed, when comparing the easier semantic fluency task with the more difficult phonemic task in younger adults, clusters of negative activity were larger and peak-values in clusters were higher (see Supplementary Table 2 for cluster sizes and z-values). Previously, it has been shown that older adults show less modulation of negative activity in response to increased task demands (Persson et al., 2007; Park et al., 2010). In the older group, when comparing the degree of negative activity between the two tasks, only one additional cluster in the medial frontal cortex showed more negative activity during the more difficult phonemic task, suggesting less modulation of negative activity.
In line with task difficulty accounting for some of our findings, left inferior frontal areas have been implicated with the controlled selection and retrieval of items from memory stores across a variety of tasks in younger adults, and increased task demands result in more pronounced activity (Thompson-Schill et al., 1997; Persson et al., 2008). Similar to our own study, left IFG activity has been found to be more widespread or reduced in older participants (Persson et al., 2008), potentially indicative of impaired left frontal selection processes. On the other hand, the right IFG has mainly been implicated with response inhibition (Aaron and Poldrack, 2006) and has been shown to be selectively up-regulated during non-linguistic (Simmonds, et al., 2008) and linguistic tasks (Yang et al., 2009; Ghogari and MacDonald, 2009) in more demanding contexts even in younger adults. In our own study, up-regulation of activity in right frontal control processes during the (easier) semantic task may thus reflect an ineffective means of compensating for impaired left-frontal selection processes in the older adults, resulting in reduced performance. During the more demanding phonemic task, both groups of participants showed a bilateral pattern of posterior IFG activity which was mirrored by decreased task performance compared to the semantic task. With regard to the cognitive mechanisms underlying the observed changes of activity in older adults: Verbal fluency tasks comprise several linguistic and non-linguistic cognitive processes: e.g., strategic search and retrieval from memory, response inhibition or working memory related processes. In particular, the frontal lobes have been implicated with word-retrieval processes and working memory during verbal fluency tasks (e.g., Costafreda et al., 2006). The present study, however, was not designed to elucidate the contribution of these different cognitive processes. This would have required different types of control tasks (e.g., Birn et al., 2010). In addition, even though the behavioral patterns during the two verbal fluency tasks in the present study are in line with previous behavioral studies, the paced design we used may have placed a greater emphasis on search and retrieval (executive/frontal functions). For example, Basho et al. (2007) directly compared the impact of paced and unpaced presentation of the stimuli during verbal fluency paradigms and found more pronounced activity during the paced paradigm in areas associated with sustained attention, motor planning and response inhibition. Only recently, new fMRI paradigms that allow to assess overt verbal fluency in a more standard (unpaced) way have been developed (Birn et al., 2010) and our results should be relicated using such paradigms.
With regard to the relationship between positive and negative activity patterns, it has been shown that prefrontal task-related positive activity is coupled with default mode activity (e.g., Greicius et al., 2003), thus raising the question of whether the loss of negative activity is associated with increased positive right frontal activity. One previous study (Persson et al., 2008) found that right increased frontal activity was correlated with decreased negative activity during a verb generation task; however, as they did not find an additional correlation with performance, the functional significance of these findings remains unclear. In our own study, we only found weak evidence for the coupling of the two phenomena, such that only during the semantic task two clusters (postcentral/inferior parietal gyrus and posterior cingulate/precuneus) were correlated with increased activity in the MFG in the older group. Activity in the right IFG was not correlated with the degree of negative activity loss and no correlations were found for the phonemic task. Thus, at least for the verbal fluency tasks we used, the two phenomena do not seem to be closely linked.
Indeed, recent studies reported that ß-amyloid deposition in healthy older adults overlaps with core areas of the default network, and the degree of amyloid deposition is correlated with the loss of negative activity even in non-demented older adults (Sperling et al., 2009; Hedden et al., 2009). Thus, decreased negative activity might be a physiological marker of early brain pathology in older adults, but may be relatively independent from structural impairment in prefrontal areas or from the impact of task difficulty. Thus, even though both phenomena may co-occur (as indicated by the weak association during the semantic task), most likely they represent different types of processes that are not causally related and may have different effects on performance. This is further supported by the fact that we found the loss of negative activity to be correlated with performance during the phonemic task, in the absence of increased frontal activity.
A note of caution needs to be made with respect to the interpretation of our results. Differences in functional activity between tasks or groups of participants mirror the behavioral performance during these tasks or participants. Thus, the pattern of activity cannot be interpreted as causing behavioral impairment, but rather as being the result of the behavioral performance in the scanner. Based on the present data we cannot make assumptions on the underlying causes of these changes, which would require additional information about grey and white matter integrity in our sample or the degree of amyloid deposition, which was not available in this study.
Many have speculated that changes in activity seen in advanced age are compensatory for age-related structural deterioration (e.g., see Park and Reuter-Lorenz, 2009 for review). In fact, activity seen in advanced age resembles that of (ostensibly) compensatory activity after stroke (for review see Crosson et al., 2007). Although changes in negative activity have not been assessed in stroke patients with acquired language disorders (aphasia), our own results and those of other groups (Wingfield and Grossman, 2006; Wierenga et al., 2008; Tyler et al., 2010, Peelle et al., 2010) show striking similarities to patterns of language network reorganization found in aphasia patients for increased positive activity. For example, the literature on language comprehension in aphasia patients has demonstrated effective compensation by right fronto-temporal areas during language comprehension tasks for single words, while it seems to be less able to participate in complex tasks at the level of sentence comprehension (e.g., Crinion et al., 2005). Moreover, even though right frontal areas may contribute to improved word-retrieval (e.g., Crosson et al., 2009), increased right frontal activity during language production tasks is usually associated with larger lesions in the left hemisphere and less favorable outcome (for review see Heiss and Thiel, 2006).
In summary, we assessed task-related positive and negative task-related activity in older and younger adults. Even though expressed in different ways in the two fluency tasks, a consistent pattern emerged: Older adults in general performed better when they had a pattern of brain activity similar to the younger group. This is not limited to right frontal positive activity, but also for the degree of negative activity. Thus, the functional integrity and efficient recruitment of the network used by younger adults seems to be necessary during highly demanding language production tasks. Furthermore, compensatory right frontal up-regulation, or the loss of right sided negative activity, might be more detrimental to performance than during other types of linguistic or non-linguistic tasks. The underlying causes of these changes in task-related patterns of functional activity need to be addressed in future studies.
Supplementary Material
Acknowledgments
This work was supported by grants from the German Science Foundation to MM (DFG, ME 3161/2-1); the US Department of Veterans Affairs Rehabilitation Research and Development service to BC (Research Career Scientist Award B3470S) and TC (Career Development Award B6699W); the National Institute on Deafness and other Communication Disorders to BC (R01 DC007387); and the National Institute of Health for SH (T32DC008768). We thank Atchar Sudhyadhom for assistance with fMRI sequence programming and Billy and Douglas Nyland for editorial assistance.
Footnotes
Disclosure Statement: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner R. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basho S, Palmer ED, Rubio MA, Wulfeck B, Muller RA. Effects of generation mode in fMRI adaptations of semantic fluency: Paced production and overt speech. Neuropsychologia. 2007;45:1697–1706. doi: 10.1016/j.neuropsychologia.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Srteer RA, Ball R, Ranieri W. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, Martin A. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49:1099–1107. doi: 10.1016/j.neuroimage.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Aging and Language Production. Curr Dir Psychol Sci. 2004;13:21–24. doi: 10.1111/j.0963-7214.2004.01301006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Language and aging. In: Salthouse TA, editor. The handbook and aging and cognition. Psychology Press; New York: 2008. pp. 373–443. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Crinion J, Price CJ. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain. 2005;128:2858–2871. doi: 10.1093/brain/awh659. [DOI] [PubMed] [Google Scholar]
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang YL, Moore AB, Raymer AM, Briggs RW, Sherod MG, Wierenga CE, White KD. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev. 2007;17:157–77. doi: 10.1007/s11065-007-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Moore AB, McGregor KM, Chang YL, Benjamin M, Gopinath K, Sherod ME, Wierenga CE, Peck KK, Briggs RW, Rothi LJ, White KD. Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain Lang. 2009;111:73–85. doi: 10.1016/j.bandl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Ford A, McGregor K, Meinzer M, Cheshkov S, Li X, Walker-Batson D, Briggs RW. Functional imaging and related techniques: An introduction for rehabilitation researchers. J Rehabil Res Dev. 2010;47:7–33. doi: 10.1682/jrrd.2010.02.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the "default network" in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober B. California Verbal Learning Test: Manual. Pearson; San Antonio: 2000. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Functions System: an update. J Int Neuropsychol Soc. 2004;10:301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State" A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab N, Gabrieli JD, Glover GH. Assessing the influence of scanner background noise on auditory processing. II. An fMRI study comparing auditory processing in the absence and presence of recorded scanner noise using a sparse design. Hum Brain Mapp. 2007;28:721–732. doi: 10.1002/hbm.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW. The neural basis of cognitive control: response selection and inhibition. Brain Cogn. 2009;71:72–83. doi: 10.1016/j.bandc.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JA, Churchill N, McIntosh AR. A Multivariate Analysis of Age-Related Differences in Default Mode and Task-Positive Networks across Multiple Cognitive Domains. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW. “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp. 1999;7:213–223. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta-analysis. Neuropsychologia. 2004;42:1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeown WJ, Shanks MF, Forbes-McKay KE, Venneri A. Patterns of brain activity during a semantic task differentiate normal aging from early Alzheimer's disease. Psychiatry Res. 2009;173:218–227. doi: 10.1016/j.pscychresns.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, Gonzalez-Rothi LJ, Crosson B. Neural signatures of semantic and phonemic fluency in young and old adults. J Cogn Neurosci. 2009;21:2007–2018. doi: 10.1162/jocn.2009.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, Rockstroh B. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39:2038–2046. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Obleser J, Flaisch T, Eulitz C, Rockstroh B. Recovery from aphasia as a function of language therapy in an early bilingual patient demonstrated by fMRI. Neuropsychologia. 2007;45:1247–56. doi: 10.1016/j.neuropsychologia.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Obleser J, Assadollahi R, Djundja D, Barthel G, Rockstroh B. Brain regions essential for improved lexical access in an aged aphasic patient: A case report. BMC Neurol. 2006;6:28. doi: 10.1186/1471-2377-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Backman L, Lindenberger U, Heekeren HR. Performance level modulates adult age differences in brain activation during spatial working memory. Proc Natl Acad Sci. 2009;106:22552–22557. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults' comprehension of spoken sentences: Age differences in resource allocation and connectivity. Cereb Cortex. 2010;21:418–423. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Altered deactivation in individuals with genetic risk for Alzheimer's disease. Neuropsychologia. 2008;46:1679–1687. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effect of practice on the functional anatomy of task performance. Proc Natl Acad Sci. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Stamatakis EA, Tam PP, Tyler LK. Word retrieval failures in old age: the relationship between structure and function. J Cogn Neurosci. 2010;22:1530–1540. doi: 10.1162/jocn.2009.21321. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörös P, Bose A, Sokoloff LG, Graham SJ, Stuss DT. Age-related changes in the functional neuroanatomy of overt speech production. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnsson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady SL. Reliable differences in brain activity between young and old adults: A quantitative meta-anaysis across multiple cognitive domains. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2010.01.009. in press. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving Syntactic Processing across the Adult Life Span: The Modulation of the Frontotemporal Language System in the Context of Age-Related Atrophy. Cereb Cortex. 2010;20:352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Memory Scale-Revised. Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJ, Conway T, Cato MA, Briggs R, Crosson B. Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiol Aging. 2008:436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wiig EH, Secord W. Test of Language Competence: Expanded. Psychological Corporation; NY: 1989. [Google Scholar]
- Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006;96:2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- Yang FG, Edens J, Simpson C, Krawczyk DC. Differences in task demands influence the hemispheric lateralization and neural correlates of metaphor. Brain Lang. 2009;111:114–124. doi: 10.1016/j.bandl.2009.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.