Summary
NK cells of the innate immune system are equipped with a cytolytic machinery and produce cytokines which enable these cells to profoundly modify adaptive immune responses to foreign invaders, as well as to self-antigens. Here we discuss the recent advances in understanding how NK cells can proactively influence sequential pathogenic steps that are instrumental for the initiation and progression of autoimmune diseases in human and experimental disease models. We also discuss the possible use of NK cells as a surrogate marker for disease activity and responsiveness to immune therapy. Finally, we present results on NK cell-based therapies in inflammatory and autoimmune disorders with a focus on existing challenges and current promises for the development of more effective therapies.
Initiation and progression of autoimmune disease may require collaboration between the innate and adaptive immune systems
Autoimmune diseases derive from the failure of self/non-self discrimination for the immune system. Virtually most, if not all tissues, can become targeted for autoimmune destruction. The etiology of autoimmune disease remains elusive. In addition to susceptibility genes, several immune mechanisms have been proposed to explain the emergence and progression of autoimmunity. The elements that trigger immune responses toward self components and the mechanisms that control autoimmune disease progression are a matter of debate. Investigations conducted thus far on these aspects have focused on several aspects. First, foreign invaders (including bacteria, virus and parasites) elicit protective immunity in the secondary lymphoid tissues where the recruitment of NK cells, monocytes, dendritic cells, together with T and B cells, can induce local inflammation. However, unabated inflammation can sometimes cause bystander damage to host tissue. Additionally, molecular mimicry (shared genetic identity between foreign and self tissues that has been demonstrated in several experimental models) can lead to the spreading of immune responses to self antigens. Second, endogenous dead cells and tissues can expose self antigens and trigger autoimmunity. Third, infected cells lose self identity because of the expression of new antigens and can become targets of innate immune cells, and autoimmune disease exacerbation may associate with infectious episodes or defective immune regulation.
Effector functions of NK cells and their relevance to the adaptive inflammatory and autoimmune responses
Natural killer (NK) cells are large granular cells that constitute 5–10% of circulating lymphocytes in humans and 1–3% in mice, thus being third in lineage among lymphocytes, after T and B cells. NK cells are dispersed throughout lymphoid and non-lymphoid tissues, and can rapidly home to target organs under pathological situations (1). An important arm of the innate immune system, the NK cells lack T and B cell receptors and undergo activation without antigen presentation from antigen presenting cells (APCs). Certain viruses, mutant cells, common γ-chain cytokines and interferons produced during inflammatory responses can modulate the activity of NK cells through several activating and inhibitory receptors (Fig. 1). Upon activation, NK cells exhibit two types of functions: cytotoxicity and cytokine production. NK cell-mediated natural cytotoxicity against certain microbes and several cell types seems to be controlled by levels self MHC class I expression (Fig. 1). NK cells can produce large numbers of cytokines, sometimes at high concentrations, including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, immunoregulatory cytokines such as IL-5, IL-10, IL-13, IL-22, the growth factor GM-CSF, and the chemokines MIP-1α, MIP-1β, IL-8, and RANTES (2–4).
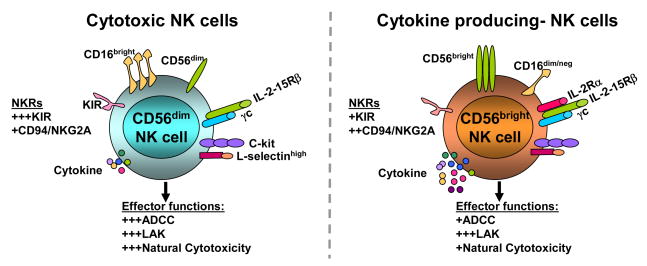
Fig. 1. Phenotype of human NK cells.
Human NK cells can be classified functionally as cytotoxic- and cytokine producing-NK cells. Cytotoxic NK cells are CD16highCD56dim. These cells express higher levels of KIR and lower levels of NKG2A, they bear receptors for IL-2/IL-15Rβ and γc, do not have α chains. In contrast, cytokine producing-NK cells are CD16dim/negCD56birght, they express lower levels of KIR and higher levels of NKG2A. In addition to IL-2/IL-15Rβ and γc, these NK cells also express IL-2 Rα (Fig 1).
Both cytotoxic- and cytokine-producing NK cells can amplify innate immune responses by releasing inflammatory mediators and by lysing antigen presenting cells and infected or transformed cells. Subsequently, NK cells may promote or inhibit adaptive immune responses (see details in Fig. 3).
NK cells play an active role in the pathogenesis of autoimmune diseases because of their cytolytic activity, cytokine production, interaction with APCs and T and B cells.
NK cells actively participate in sequential events that lead to autoimmune disease
The natural cytotoxicity and the swift and bursting release of cytokines equips the NK cells with the ability to affect significantly the adaptive immune response to foreign invaders and self-antigens. Moreover, NK cells are active players in the mechanisms that influence the initiation and progression of autoimmunity (Fig. 2). First, NK cells orchestra the magnitude of inflammatory responses that can induce bystander self tissue damage, as demonstrated in a viral model of type 1 diabetes (T1D) (5). Second, NK cells profoundly affect immune responses by interacting with APCs such as dendritic cells and macrophage, or by directly interacting with autoreactive T and B cells (Fig 2, and Ref 6). Third, direct activation of NK cells due to reduced MHC class I molecules can associate, at least in groups of autoimmune patients, with mutations in the genes encoding for transport association with antigen processing (TAP) (7, 8). Those mutations can lead to reduced cell-surface expression of HLA class I molecules, that coupled with chronic infection and aberrant (increased) IFN production can promote the effector, pro-inflammatory activities of NK cells in the skin, kidney and other organs (7, 8).
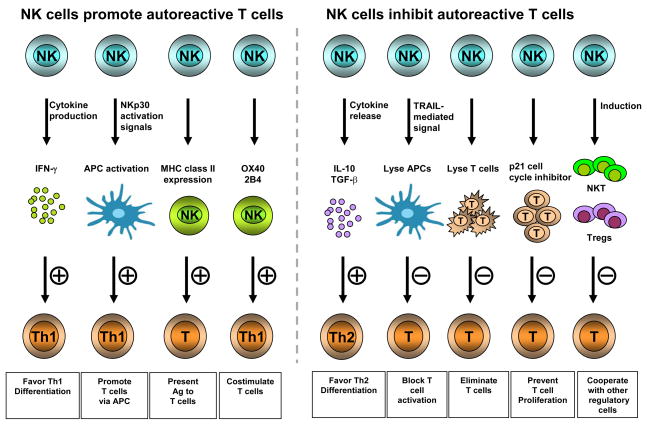
Fig. 2. Putative mechanisms by which NK cells promote or inhibit autoreactive T cells.
NK cells can produce factors that determine the differentiation of naïve T cells into cells with Th1- or Th2-cell phenotypes, thus impacting the development of autoimmune responses (35–38). NK cells and antigen-presenting cells (APCs) can reciprocally activate each other through cytokine production by both cell types and via co-stimulatory interactions (39–45). For example, NK cell-activated dendritic cells may synergize with NK cell-derived helper signals to instruct the generation of autoreactive Th1 cells (44). NK cells may also directly promote the generation of autoreactive T cells through the co-stimulatory molecules OX40 or 2B4 on APCs induced by NK cells (46, 47). Finally, some NK cells express antigen presentation machinery (such as MHC class II molecules) and can directly activate T cells (48, 49). On the other hand, NK cells produce regulatory cytokines or can directly kill APCs (39) and autoreactive T cells (49, 50); they may also act in concert with NKT cells and/or CD4+CD25+ regulatory T cells. These actions could lead to silencing of T cell responses and limit the inflammatory damage. The decision for promotion or inhibition is probably dependent on the stage of immunity and the specific organ system. The killing of target cells provides antigen that can fuel APC and drive T/B cell responses. The cytokine produced by NK cells may facilitate naïve T cells to differentiate into Th1, Th2 or Th-17. Once infection is contained, it is necessary to taper the immune response to avoid autoimmunity. NK cells may have evolved to assist the immune system to achieve this goal by killing APC and/or T cells, collaborating with Treg cells and NKT cells.
Characteristics of NK cells in human autoimmunity
The phenotype and function of NK cells in human autoimmune conditions have revealed that in most, if not all cases, NK cells are reduced in number (Fig. 3) and have a compromised cytotoxic capacity, a process referred as to NK cell degeneration (6). NK cell degeneration can be observed and is not restricted to systemic lupus erythematosus (SLE), psoriasis, psoriatic arthritis, multiple sclerosis (MS), rheumatoid arthritis (RA), T1D, Sjögrens syndrome and myasthenia gravis (MG) (6). This phenomenon is reminiscent of the defective functions that have been documented in regulatory T-cell populations such as the CD4+CD25+ regulatory T cells and the CD1d-restricted NKT cells in human autoimmune diseases (9). This raises the possibility that these deficiencies might be related to the autoimmune process (9), although it is not yet known whether the degenerative characteristic could be a primary feature promoting autoimmunity or could rather be secondary to the disease pathogenesis. Of note, in experimental autoimmune MG (EAMG), IL-21 derived from autoreactive T cells promotes NK cell degeneration (10). Considering that NK cells play a disease-promoting role in EAMG (11), NK cell degeneration might serve as means by which autoreactive T cells can control autoimmunity (10). A better understanding of NK cell degeneration could lead to the clarification of certain aspects of disease pathogenesis and possibly rationalize new protocols for NK cell-based therapies (see following sections).
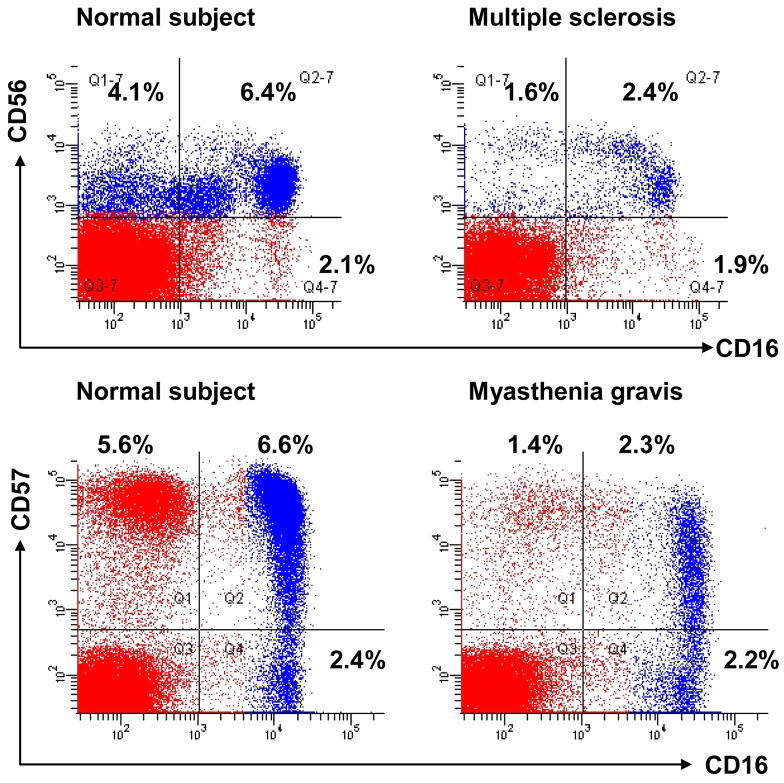
Fig. 3. NK cell degeneration in patients with autoimmune diseases.
Flow cytometry analysis of NK cell subpopulations in treatment naïve patients with multiple sclerosis and myasthenia gravis. The dot plot figures using peripheral blood mononuclear cells indicate that CD16, CD56 and CD57 positive NK cells are significantly reduced in patients compared with healthy subjects.
Diverse roles of NK cells in experimental and human autoimmunity
Studies on NK cells in human autoimmune disease have been mainly descriptive and mostly aimed at linking NK cell phenotypes with disease stages or use them as prognostic indicators of disease progression.
In experimental autoimmune diseases, the mechanisms of NK cell-mediated effects have been studied in more detail, yielding to conflicting results. Some studies have suggested that NK cells can promote autoimmune activity, while other studies have suggested that NK cells may play a regulatory role in autoimmunity by actively suppressing the genesis of autoreactive immune responses (6). Here we summarize the results from studies in animal models and in human disease.
Type 1 diabetes
T1D is a multifactorial disease in which multiple genes interact with environmental factors to lead to autoimmunity against islet autoantigens with subsequent immune-mediated destruction of insulin-producing pancreatic β cells. T1D is believed to be primarily a T cell-mediated disease that requires both CD4+ and CD8+ T cells. Additionally, there is evidence supporting the involvement of other immune cells in the β-cell destruction, including B cells, macrophages, dendritic cells and NK cells.
Studies on the role of NK cells is animal models such as the Bio Breeding (BB) rat and the nonobese diabetic (NOD) mouse have provided evidence that NK cells may either promote or prevent islet cell destruction (12). These contrasting results may reflect a diverse role of NK cells in different models and a possible different activity depending o the stage of the disease (13–15).
Using pancreatic samples removed post-mortem from 29 patients, Willcox and colleagues attempted to detect the presence of NK cells and other lymphocytes in situ with immunochemical staining. NK cells were rarely detected, even in heavily inflamed islets (16). However, these results do not preclude the possibility that NK cells can be present in the pancreas before overt T1D or that NK cells could influence recruitment of other lymphocyte populations during initial development of insulitis.
In the peripheral blood of T1D patients, both a numeric reduction of NK cell numbers and their functional abnormalities have been reported (12). However, there is no consensus as to whether these changes are transitory or stable.
In trying to correlate NK cell activity and stage of T1D, a recent study of newly diagnosed and long-standing T1D patients found a reduction of NK cell frequency in recent onset patients but not in patients with long-standing disease (17). On the other hand, NK cell defects (as an altered production of IFN-γ and expression of NKp46 and NKp30) are evident in long-standing patients, particularly in those with higher levels of glycosylated hemoglobin levels. Thus, these studies neither allow to draw conclusions on the timing of NK cell abnormalities (primary or secondary to the diabetic metabolic activity or to other defects in T1D) (18) nor to relate the findings to the processes leading to β-cell destruction.
Rheumatoid arthritis and systemic lupus erythematosus
RA is a chronic systemic autoimmune disease that is mainly characterized by joint inflammation and immune-mediated destruction. SLE is another systemic autoimmune condition that is characterized by rashes, arthritis, kidney and central nervous system (CNS) involvement, and associates with antibodies that are specific for double-stranded DNA and several other nuclear antigens.
The impact of NK cells in several models of RA and, to a less degree SLE, has been investigated by using depletion antibodies such as the anti-NK1.1 mAb. Disease is enhanced when NK1.1+ cells are removed, suggesting a possible protective role for NK cells (19).
Moreover, in both RA and SLE, defective NK cell activities including cytokine production and cytotoxicity, have been reported (20, 21). Also, a group of patients with juvenile rheumatoid arthritis exhibited macrophage activation syndrome, which is associated with decreased NK cell function and the absence of circulating CD56bright NK cells (20). The inverse relationship between NK cells and macrophages has suggested the possibility that NK cells could actively curb macrophage activity (9, 22), also in view of the fact that CD56bright NK cells accumulate within inflammatory joint lesions (23). Additionally, NK cells may engage with CD14+ monocytes to amplify inflammatory responses (23) and provide a local milieu for monocytes in the inflamed joints to differentiate into DC (24). Collectively, these studies reveal that NK cells actively participate in the initiation of joint pathology and perhaps determine the intensity of local inflammation by engaging other lymphocyte populations.
Myasthenia gravis
MG is an autoimmune disease of the neuromuscular junctions. In nearly 85% of patients, muscular weakness derives from activity at the postsynaptic membrane of the neuromuscular junction of IgG autoantibody produced by autoreactive B cells against the nicotinic acetylcholine receptor (AChR).
The role of NK cells has been studied In the animal models of MG, EAMG. Depletion of NK cells at disease initiation blunts the production of AChR autoantibodies and ameliorates EAMG (11). However, it is not clear whether such effects are a result of interrupted interactions between NK cells and autoreactive T cells, or between NK cells and autoantibody-producing B cells, or both.
MG patients clearly have reduced numbers of circulating NK cells. However, compared to patients with MS, CD57+, but not CD56+ NK cells, appear reduced in MG (Fig. 3).
Multiple sclerosis
MS is an inflammatory disease characterized by cellular influx, demyelination and axonal damage of the central nervous system (CNS). The initiation of the disease seems to stem from the interplay between cells of the innate and adaptive immune systems, including NK cells.
The CNS has specific features of inflammation and autoimmune responses: 1) abundance of CNS antigens in relation to the periphery; 2) active participation of local APCs, microglia, astrocytes and peripherally migrated DCs and macrophages; 3) migrated myelin-reactive T cells undergo reactivation, further differentiation, expansion and perhaps antigenic determinant spreading.
NK cells can readily home to the CNS during inflammation and other pathological conditions (1). The ability of NK cells to kill various transformed and/or virus-infected cells raises the important question as to whether direct NK-cell cytolytic effects can contribute to the pathogenesis of inflammatory, degenerative and autoimmune disorders of the CNS. It has been demonstrated that NK cells can mediate damage to neurons, oligodendrocytes and microglia (12) yet the biological relevance of these findings has to be defined in vivo.
Contrasting the potentially detrimental effects of NK cells on the CNS, emerging evidence in MS and its animal model, experimental autoimmune encephalomyelitis (EAE), has supported the possibility of regulatory functions of NK cells during CNS inflammation (11).
EAE is induced in C57BL/6 mice with myelin oligodendrocyte glycoprotein (MOG) peptide, which activates T cells and other lymphocytes in the periphery. MOG-activated cells subsequently home to the CNS where they cause pronounced inflammation and demyelination, followed by a monophasic neurological deficiency that resembles a form of MS in humans known as acute disseminated encephalomyelitis. Studies of EAE in B6 mice (25), or blockade of NK cell homing to the CNS via germ-line deletion of the chemokine fracktalkine receptor CX3CR1 resulted in fatal CNS inflammation and demyelination (26). In the latter model, reduction of CNS-resident NK cells alone was sufficient to induce severe EAE, suggesting that CNS-resident NK cells are more important than their peripheral counterparts in controlling severity of the disease.
Similar to autoimmune diseases, in MS patients naïve to treatment the NK cells are present in reduced numbers and have a compromised cytotoxic function (9). Such deficiency appears to be restricted to CD56+ NK cells; whereas CD57+ and CD16+ NK cells appear unaffected (Figure 1). There are no data available on whether cytokine production by NK cells is altered in MS patients.
Interestingly, the relapsing-remitting (RR) course of MS provides an opportunity to investigate whether the disease course associates with NK cell changes. NK cells in the remission of MS show elevation of IL-5 and augmented expression of CD95 and a decreased expression of IL-12Rβ2 (27), which are phenotypes reminiscent of NK cells. Notably, in a longitudinal study of placebo-treated RRMS patients, the cycles of NK cell functional activity had a periodicity of about 10 weeks, and a lower activity appeared associated with the appearance of active lesions or onset of clinical attacks, supporting the possibility of a potentially protective role of NK cells in ongoing MS (28).
Other studies have monitored NK cell activities during immunomodulatory therapies. Glatiramer acetate (GA or Copaxone) is an FDA-approved drug for RRMS. GA is a myelin basic protein analogue consisting of four amino acid (Glu, Ala, Lys, Tyr). A recent study demonstrated that GA enhanced the cytolysis of human NK cells against autologous and allogeneic dendritic cells but, in contrast, it did not alter the percentage of NK cells expressing NKG2D, NKp30 or NKp44 (29). GA also inhibited the release of IFN-γ but increased the release of TNF-α by NK cells (29).
Kastrukoff et al studied the relationship between NK cell activity (killing of K562 target cells) and disease activity (measured as new MRI lesions) in RRMS patients receiving IFN-β therapy on alternative days. Interestingly, it was found that RRMS patients with elevated NK cell activity may not only be at greater risk for the development of active lesions but may also be likely to better respond to IFN-β (30). The treatment of RRMS subjects with IFN-β1a over 12 months significantly increased both the percentages of CD56bright NK cells and Foxp3 mRNA expression compared to baseline values (31).
These studies indicate that NK cell activity may not only reflect at some extent the course of MS but that it may also serve as a surrogate marker for responsiveness to immunomodulatory therapies.
Overall, NK cells could regulate inflammation and influence self-tolerance at multiple steps. The above findings caution against a single role for NK cells in different autoimmune diseases or even during the different stages of the same autoimmune disease. The possible mechanisms operated by NK cells on autoreactive helper T cells, mainly based on studies in experimental autoimmune diseases, are summarized in Fig. 2.
NK cell-based therapy in autoimmune diseases
If a defective NK cell function can lead to the emergence and progression of autoimmune diseases, approaches that restore and boost the activity of NK cells should have therapeutic value. This idea has been tested by administering daclizumab, a humanized mAb directed against the IL-2Rα chain, resulting in reduced brain inflammation in MS. Therapy with daclizumab associated with a gradual decline in circulating CD4+ and CD8+ T cells and a significant expansion of CD56bright NK cells in vivo, and this effect correlated with treatment responses (32). Similarly, the administration of a humanized IL-2R blocking antibody induced a 4–20 fold expansion of CD56bright regulatory NK cells which secreted a large quantity of IL-10, with beneficial effects on the remission of uveitis (33, 34).
Conclusion
It is conceivable that our ability to harness NK cells for therapeutic purposes may depend on a better understanding of the biology of these cells and their relationship to autoimmunity. Specifically, the identification of new NK receptors and ligands may offer more tools to manipulate the balance between the inhibitory and activating signals for NK cells. Also the discovery of molecules that affect the sensitivity of target cells to NK cell-mediated activity can allow better manipulation of NK cell-based immune intervention. Progresses in these areas will likely result in clinical advances that could possibly improve the management of certain autoimmune conditions.
Acknowledgments
We thank the present and past members of our laboratories for their contribution in this field; Dr. C. Dayao for his assistance in the preparation of this review; Drs. L. Van Kaer, H.-G. Ljunggren, D. Huang, R. Ransohoff, T. Vollmer and D. Campagnolo for discussion and collaborations. Our laboratories are supported by the MDA, NMSS, ABRC, BNF, NIH and NSFC.
References
- 1.Shi F, Ransohoff R, editors. Nature Killer Cells in the Central Nervous System. 2009. in Press. [Google Scholar]
- 2.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008–4010. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nature immunology. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 5.Flodstrom M, Maday A, Balakrishna D, Cleary MM, Yoshimura A, Sarvetnick N. Target cell defense prevents the development of diabetes after viral infection. Nature immunology. 2002;3:373–382. doi: 10.1038/ni771. [DOI] [PubMed] [Google Scholar]
- 6.Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nature reviews. 2006;6:751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- 7.Zimmer J, Bausinger H, de la Salle H. Autoimmunity mediated by innate immune effector cells. Trends in immunology. 2001;22:300–301. doi: 10.1016/s1471-4906(01)01923-8. [DOI] [PubMed] [Google Scholar]
- 8.Moins-Teisserenc HT, Gadola SD, Cella M, Dunbar PR, Exley A, Blake N, Baykal C, Lambert J, Bigliardi P, Willemsen M, Jones M, Buechner S, Colonna M, Gross WL, Cerundolo V. Association of a syndrome resembling Wegener’s granulomatosis with low surface expression of HLA class-I molecules. Lancet. 1999;354:1598–1603. doi: 10.1016/s0140-6736(99)04206-3. [DOI] [PubMed] [Google Scholar]
- 9.La Cava A, Van Kaer L, Fu Dong S. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends in immunology. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Van Kaer L, La Cava A, Price M, Campagnolo DI, Collins M, Young DA, Vollmer TL, Shi FD. Autoreactive T cells mediate NK cell degeneration in autoimmune disease. J Immunol. 2006;176:5247–5254. doi: 10.4049/jimmunol.176.9.5247. [DOI] [PubMed] [Google Scholar]
- 11.Shi FD, Wang HB, Li H, Hong S, Taniguchi M, Link H, Van Kaer L, Ljunggren HG. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nature immunology. 2000;1:245–251. doi: 10.1038/79792. [DOI] [PubMed] [Google Scholar]
- 12.Dotta F, Fondelli C, Falorni A. Can NK cells be a therapeutic target in human type 1 diabetes? European journal of immunology. 2008;38:2961–2963. doi: 10.1002/eji.200838851. [DOI] [PubMed] [Google Scholar]
- 13.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poirot L, Benoist C, Mathis D. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8102–8107. doi: 10.1073/pnas.0402065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clinical and experimental immunology. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodacki M, Svoren B, Butty V, Besse W, Laffel L, Benoist C, Mathis D. Altered natural killer cells in type 1 diabetic patients. Diabetes. 2007;56:177–185. doi: 10.2337/db06-0493. [DOI] [PubMed] [Google Scholar]
- 18.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N. Multiple immuno-regulatory defects in type-1 diabetes. The Journal of clinical investigation. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson N, Bremell T, Tarkowski A, Carlsten H. Protective role of NK1.1+ cells in experimental Staphylococcus aureus arthritis. Clinical and experimental immunology. 1999;117:63–69. doi: 10.1046/j.1365-2249.1999.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villanueva J, Lee S, Giannini EH, Graham TB, Passo MH, Filipovich A, Grom AA. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis research & therapy. 2005;7:R30–37. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clinical and experimental immunology. 2005;141:165–173. doi: 10.1111/j.1365-2249.2005.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedvetzki S, Sowinski S, Eagle RA, Harris J, Vely F, Pende D, Trowsdale J, Vivier E, Gordon S, Davis DM. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 23.Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–6426. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 24.Zhang AL, Colmenero P, Purath U, Teixeira de Matos C, Hueber W, Klareskog L, Tarner IH, Engleman EG, Soderstrom K. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007;110:2484–2493. doi: 10.1182/blood-2007-02-076364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. The Journal of experimental medicine. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, Littman DR, Ransohoff RM. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. Faseb J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K, Miyake S, Kondo T, Terao K, Hatakenaka M, Hashimoto S, Yamamura T. Natural killer type 2 bias in remission of multiple sclerosis. The Journal of clinical investigation. 2001;107:R23–29. doi: 10.1172/JCI11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastrukoff LF, Morgan NG, Zecchini D, White R, Petkau AJ, Satoh J, Paty DW. A role for natural killer cells in the immunopathogenesis of multiple sclerosis. Journal of neuroimmunology. 1998;86:123–133. doi: 10.1016/s0165-5728(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 29.Sand KL, Knudsen E, Rolin J, Al-Falahi Y, Maghazachi AA. Modulation of natural killer cell cytotoxicity and cytokine release by the drug glatiramer acetate. Cell Mol Life Sci. 2009;66:1446–1456. doi: 10.1007/s00018-009-8726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastrukoff LF, Morgan NG, Zecchini D, White R, Petkau AJ, Satoh J, Paty DW. Natural killer cells in relapsing-remitting MS: effect of treatment with interferon beta-1B. Neurology. 1999;52:351–359. doi: 10.1212/wnl.52.2.351. [DOI] [PubMed] [Google Scholar]
- 31.Vandenbark AA, Huan J, Agotsch M, La Tocha D, Goelz S, Offner H, Lanker S, Bourdette D. Interferon-beta-1a treatment increases CD56(bright) natural killer cells and CD4+CD25+ Foxp3 expression in subjects with multiple sclerosis. Journal of neuroimmunology. 2009;215:125–128. doi: 10.1016/j.jneuroim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–5191. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 34.Bielekova B, Howard T, Packer AN, Richert N, Blevins G, Ohayon J, Waldmann TA, McFarland HF, Martin R. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Archives of neurology. 2009;66:483–489. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 36.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nature reviews. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nature immunology. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 39.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nature immunology. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 42.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nature immunology. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 43.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cellular immunology. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. The Journal of clinical investigation. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 46.Romagnani S. Induction of TH1 and TH2 responses: a key role for the ‘natural’ immune response? Immunol Today. 1992;13:379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nature immunology. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 48.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nature immunology. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 49.Chambers BJ, Salcedo M, Ljunggren HG. Triggering of natural killer cells by the costimulatory molecule CD80 (B7–1) Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- 50.Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2005;163:24–30. doi: 10.1016/j.jneuroim.2005.02.011. [DOI] [PubMed] [Google Scholar]