Abstract
BACKGROUND
Acute hemodynamic collapse resulting in cardiogenic shock and impending end-organ failure is usually associated with certain death. The introduction of short-term mechanical circulatory support (MCS) devices offers potential therapy to these critically ill patients. The BVS 5000 device (ABIOMED Inc, USA) is widely used in the United States, but rarely in Canada, where device reimbursement remains a barrier.
OBJECTIVE
To present the Toronto General Hospital’s (Toronto, Ontario) initial five-year experience with this device to highlight the indications for use, common complications and overall success rates.
METHODS AND RESULTS
The institutional MCS database from 2001 to 2006 was reviewed, and 18 patients who received 30 devices in a variety of configurations were identified. The most common support configuration consisted of biventricular support (n=12), followed by isolated left ventricular support (n=4) and isolated right ventricular support in two recipients of an implantable long-term left ventricular assist device. Overall survival to device explant or transplant was 55% (n=10), of which five (50%) were successfully discharged from the hospital. The overall survival from device implant to hospital discharge was 28% (five of 18). The most common cause of death was multisystem organ failure.
CONCLUSIONS
MCS with the ABIOMED BVS 5000 can successfully resuscitate critically ill patients; however, earlier institution of this device would avoid irreversible end-organ injury, and lead to higher rates of device explant and hospital discharge. Short-term MCS devices should be available in all cardiac surgical centres in Canada to permit stabilization and evaluation of the acutely ill cardiac patient and subsequent management in a heart transplant facility.
Keywords: Acute cardiogenic shock, Transplantation, Ventricular assist device
Abstract
HISTORIQUE
Un collapsus hémodynamique aigu entraînant un choc cardiogène et une défaillance imminente des organes récepteurs s’associe généralement à une mort certaine. La mise en marché de dispositifs de soutien circulatoire mécanique (SCM) à court terme procure une thérapie potentielle à ces patients gravement malades. Le dispositif BVS 5000 (ABIOMED Inc., États-Unis) est largement utilisé aux États-Unis, mais rarement au Canada, où son remboursement demeure un obstacle.
OBJECTIF
Présenter la première expérience du Toronto General Hospital (Toronto, Ontario) avec ce dispositif depuis cinq ans afin de faire ressortir les indications d’utilisation, les complications courantes et le taux de succès global.
MÉTHODOLOGIE ET RÉSULTATS
Les chercheurs ont analysé la base de données de SCM de l’établissement entre 2001 et 2006 et ont dépisté 18 patients qui ont reçu 30 dispositifs dans diverses configurations. La principale configuration se composait d’un soutien biventriculaire (n=12), suivie d’un soutien ventriculaire gauche isolé (n=4) et d’un soutien ventriculaire droit isolé chez deux receveurs d’un dispositif d’assistance ventriculaire gauche à long terme. Le taux de survie global jusqu’à l’explantation ou à la transplantation du dispositif était de 55 % (n=10), dont cinq (50 %) ont obtenu leur congé de l’hôpital. La survie globale entre l’implantation du dispositif et le congé de l’hôpital était de 28 % (cinq cas sur 18). La défaillance multiviscérale était la principale cause de décès.
CONCLUSIONS
Le SCM au moyen du dispositif BVS 5000 d’ABIOMED peut assurer la réanimation de patients gravement malades. Toutefois, une utilisation plus rapide de ce dispositif permettrait d’éviter des lésions aux organes récepteurs et favoriserait un taux plus élevé d’explantations du dispositif et de congés de l’hôpital. Les dispositifs de SCM à court terme devraient être offerts dans tous les centres de chirurgie cardiaque du Canada afin de permettre la stabilisation et l’évaluation des patients cardiaques gravement malades et leur prise en charge subséquente dans un établissement de transplantation cardiaque.
Mechanical circulatory support (MCS) has an established role in the management of patients with cardiogenic shock refractory to conventional medical and interventional therapies (1–5). Mechanical devices can be used for short-term, intermediate-term or long-term circulatory support. Over the past five years, the Toronto General Hospital (Toronto, Ontario) has gained experience using the BVS 5000 (ABIOMED Inc, USA) device for short-term mechanical support in patients experiencing acute cardiogenic shock. The purpose of the present report was to describe our initial experience with the use of the ABIOMED BVS 5000 in a variety of cardiac conditions.
METHODS
The hospital’s five-year experience with the ABIOMED BVS 5000 system was retrospectively reviewed. The institutional research ethics board granted approval to extract clinical data from patients’ charts. In addition, periprocedural data were captured prospectively in the institutional MCS database. The BVS 5000 circulatory support system became available at the Toronto General Hospital in 2001. Between 2001 and 2006, 18 patients were supported with this device. The indications for support were varied and are listed in Table 1. In general, patients presented with hemodynamic collapse precluding timely assessment of transplant eligibility. Early in the hospital’s experience, the ABIOMED system was the only available device capable of providing right ventricular support and was the system of choice in patients with evidence of biventricular failure.
TABLE 1.
Cardiac conditions supported using the BVS 5000 (ABIOMED Inc, USa) (n=18) at the Toronto General Hospital (Toronto, Ontario)
| Precardiotomy shock | n | Postcardiotomy shock | n |
|---|---|---|---|
| Acute viral myocarditis | 4 | S/P CABG | 3 |
| Postpartum cardiomyopathy | 2 | S/P implantable LVAD | 2 |
| Endocardial fibroelastosis | 1 | S/P heart transplant | 6 |
| Total | 7 | Total | 11 |
CABG Coronary artery bypass grafting; LVAD Left ventricular assist device; S/P Status post
Device
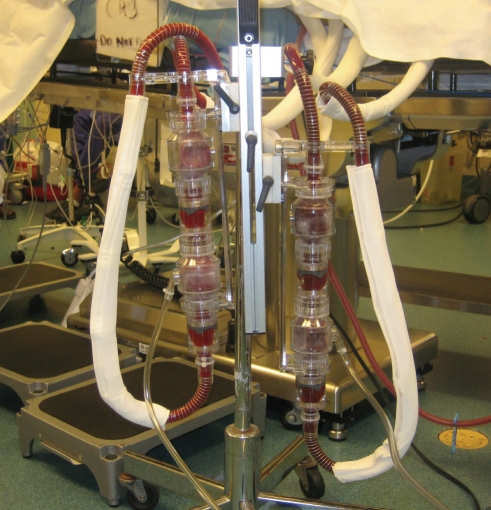
The BVS 5000 is a pneumatically driven, pulsatile, extracorporeal, asynchronous ventricular assist device capable of providing cardiac outputs of 5 L/min to 6 L/min. The blood pump consists of a single-use, dual-chamber device comprised of an atrial (filling) chamber and a ventricular (pumping) chamber (Figures 1 and 2). The blood pump fills passively by gravity and no vacuum is required. Patients are managed with a standard or high-flow console. Relative to the preload and afterload, the console attempts to generate maximum cardiac outputs of 5 L/min to 6 L/min with a target stroke volume of 80 mL. The device consists of a disposable component (US$15,000) and the reusable consoles (US$35,000 to US$50,000).
Figure 1.
The BVS 5000 paracorporeal circulatory support system (ABIOMED Inc, USA)
Figure 2.
BVS 5000 console (ABIOMED Inc, USA) at a patient’s bedside
Surgical technique
The devices were implanted using a standard median sternotomy approach and normothermic cardiopulmonary bypass. Standard cannulation techniques were used for left ventricular assist device (LVAD) implantation, using the left ventricular apical cannulation for inflow and the ascending aorta for outflow. For right ventricular assist device (RVAD) implantation, right atrial cannulation was used for inflow, and the pulmonary artery or right ventricular outflow tract was used for outflow.
A 10 mm or 12 mm Hemashield graft (Boston Scientific Corporation, USA) was used for arterial outflow. A 36 Fr or 42 Fr angled atrial cannula was used for both LVAD and RVAD inflow. A 36 Fr angled atrial cannula was used rarely for RVAD outflow via the right ventricular outflow tract using a modified technique previously described for insertion of a Thoratec RVAD (Thoratec Corporation, USA) (6).
Patients were weaned from cardiopulmonary bypass and then LVAD support was actuated. Following decannulation from the bypass circuit, RVAD support was instituted when needed. Protamine sulphate was administered to completely reverse heparin anticoagulation.
Anticoagulation
Patients were not anticoagulated for the first 24 h after surgery; however, heparin therapy was used thereafter for the duration of mechanical support. Bedside activated clotting time was performed as a routine. The activated clotting times were maintained between 180 s and 200 s.
Antibiotics
The antibiotic regimen included standard Gram-positive coverage using cefazolin or vancomycin. Antibiotic coverage was broadened if there were signs of infection postoperatively.
Weaning
An attempt at weaning was made if there was stable cardiac rhythm, minimal inotropic drug support and evidence of extracardiac organ recovery (eg, kidneys, liver and lungs). Flows were gradually decreased while simultaneously monitoring the cardiac function using transesophageal echocardiography. If patients were unable to wean and recovery was not achieved within the first week of support, the decision to convert to long-term mechanical assistance versus device withdrawal was based on transplant candidacy. In two instances in which the BVS 5000 was replaced with an AB5000 device (ABIOMED Inc), the blood pump exchanges were performed bedside in the intensive care unit. The AB5000 device is capable of long-term support (more than 60 days) as a bridge to transplant or recovery.
RESULTS
Between 2001 and 2006, the Toronto General Hospital has supported 18 patients with the ABIOMED BVS 5000 device. There were seven male and 11 female patients with a mean (± SD) age of 40±18 years (range 24 to 61 years). The mode of circulatory support (univentricular or biventricular) was largely a function of the specific indication for support (Table 2).
TABLE 2.
Support mode according to indication
| Acute fulminant myocarditis | Postpartum CMP | EFE | S/P CABG | S/P implantable LVAD | S/P OHT | |
|---|---|---|---|---|---|---|
| LVAD (n=4) | 1 | – | – | 2 | – | 1 |
| RVAD (n=2) | – | – | – | – | 2 | – |
| BiVAD (n=12) | 3 | 2 | 1 | 1 | – | 5 |
BiVAD Biventricular assist device; CABG Coronary artery bypass grafting; CMP Cardiomyopathy; EFE Endocardial fibroelastosis; LVAD Left ventricular assist device; OHT Orthotopic heart transplant; RVAD Right ventricular assist device; S/P Status post
Devices were inserted for postcardiotomy shock in 11 of 18 cases (61%) and precardiotomy shock in seven of 18 cases (39%). The mean duration of support was six days (range one to 10 days).
Overall, 10 patients (56%) were successfully bridged to explant or to heart transplant (Table 3), of which five were eventually discharged from the hospital. Overall survival to hospital discharge was 28%.
TABLE 3.
Clinical outcome according to diagnosis
| Indication | n | Weaned or bridged to AB5000* or transplant | Expired on device | Discharged |
|---|---|---|---|---|
| Acute fulminant myocarditis | 4 | 3 (75) | 1 (25) | 3 (75) |
| Postpartum CMP | 2 | 2 (100) | 0 (0) | 0 (0) |
| EFE | 1 | 0 (0) | 1 (100) | 0 (0) |
| S/P CABG | 3 | 1 (33) | 2 (66) | 0 (0) |
| S/P LVAD | 2 | 1 (50) | 1 (50) | 0 (0) |
| S/P OHT | 6 | 3 (50) | 3 (50) | 2 (33) |
Data presented as n (%) unless otherwise indicated.
ABIOMED Inc, USA. CABG Coronary artery bypass graft; CMP Cardiomyopathy; EFE Endocardial fibroelastosis; LVAD Left ventricular assist device; OHT Orthotopic heart transplant; S/P Status post
Thirteen patients received BVS 5000 devices as a bridge to recovery, two as a bridge to bridge (BVS 5000 conversion to AB5000) and three as a bridge to transplantation. Of the 13 bridge-to-recovery patients, five (39%) were successfully weaned from support and three (24%) were successfully discharged. Of the two patients who subsequently died after weaning from the device, one developed severe right venticular failure after LVAD explant while the second developed sudden ventricular fibrillation 6 h after LVAD explant despite minimal inotropic support and hemodynamic stability at the time. Of the two bridge-to-bridge patients, one patient was successfully transplanted and eventually discharged from the hospital, while one patient died of pump thrombus in the AB5000 device. Of the three bridge-to-transplant patients, one was discharged home after transplantation. One patient died of profound vasoplegia following transplantation and a second died of massive pulmonary embolus following protamine administration. The causes of death in the entire group were cardiac (n=6, 53%), multisystem organ failure (n=3, 23%), sepsis (n=1, 7%) and intracardiac thrombosis (n=2, 15%).
The most common complication was excessive bleeding (76% requiring more than four units of packed red blood cells). The usual source was the aortic anastomosis site. However, relatively few patients needed to return to the operating room for bleeding, with an overall reoperation rate of 23%, which is lower than generally reported in the literature (1–5). The majority (70%) needed prolonged ventilation (more than 24 h). This was mainly related to the initial reluctance to extubate patients while on support. Overall, only 11% of the patients were extubated while still on support. Other complications included sepsis in four patients (22%), stroke in one (6%), liver dysfunction in five (28%) and acute renal failure requiring dialysis in five (28%).
DISCUSSION
Mechanical support for the failing heart has interested and challenged cardiac specialists for decades. There have been significant advances in the treatment of chronic heart failure, primarily involving cardiac assist devices for long-term mechanical support. However, support of the acutely decompensated patient remains challenging, with mortality rates ranging from 50% to 80% depending on the etiology of heart failure and the mode of therapy used. Although extracorporeal membrane oxygenation (ECMO) is a valid alternative for the rapid institution of hemodynamic support, it is a very labour-intensive therapy associated with significant morbidity and mortality. Furthermore, even with ‘extended-use’ membranes, the clinical results in adult patients supported for more than seven days are dismal. Over the past several years, MCS using short-term devices has become a successful and acceptable approach to this difficult problem. More recently, the capability of bedside conversion from a short-term to intermediate-term device has greatly simplified the management of these critically ill patients. The introduction of the AB5000 device has enabled us to support patients for more than 90 days without the need for a major second surgical procedure – a feat not possible with traditional ECMO circuits.
In the current series, we present our experience with the BVS 5000 for short-term support as a bridge to recovery, bridge to bridge, and bridge to transplant. In patients with acute cardiogenic shock, the BVS 5000 is our preferred choice for short-term stabilization. This device has several advantages and has, therefore, gained worldwide popularity for treating cardiogenic shock. It is safe and easy to implant. The device is simple to operate, and automatically adjusts for changes in patient preload and afterload, thus requiring minimal operator intervention. It has been easier for nurses to manage and has required no added personnel for device management – unlike ECMO support, which requires an attending perfusionist. The device provides pulsatile flow, which has been documented to improve clinical outcome (7,8), myocardial blood flow (9) and splanchnic perfusion (10), to reduce systemic vascular resistance and to attenuate catecholamine response (11). In addition, the device functions independent of cardiac rhythm and can provide support during potentially fatal arrhythmias (12,13). Furthermore, in situations where native cardiac recovery is anticipated, the centrally cannulated ABIOMED device produces more complete ventricular unloading, maximizing the possibility of recovery.
At the Toronto General Hospital, we have implanted 30 BVS 5000 devices in 18 patients over five years for a variety of conditions (Table 1) requiring ventricular support. Biventricular assist device support was initiated in 67% of patients and LVAD support was initiated in 22%. Isolated RVAD support was required for only two (11%) patients who experienced severe right heart failure after insertion of a HeartMate LVAD device (Thoratec Corporation). The use of the ABIOMED BVS 5000 device has generally been limited to short-term stabilization of these patients. The BVS 5000 is not the preferred device for long-term use given its association with a high incidence of multisystem organ failure (40%) and sepsis in addition to the limited mobility afforded to supported patients. Furthermore, the system (and the related AB5000 intermediate-term device) requires systemic anticoagulation, which further exacerbates the most common morbidity – bleeding requiring blood product transfusion. Although our institution has limited experience with mobilizing these patients, the introduction of the AB5000 console will theoretically allow hospital discharge while on biventricular support.
With regard to the indications for use, better results were observed in acute fulminant myocarditis and post-transplant patients. In the myocarditis group, there were three (75%) survivors: one was successfully weaned and two were bridged to transplant (one directly and the second after the device was exchanged for an AB5000 longer term device). In the latter patient, the AB5000 device was exchanged twice due to thrombus formation in the device. We see this as one of the biggest advantages of the ABIOMED devices because they can be exchanged at the bedside safely – the significance of which cannot be overemphasized in these critically ill patients. Given the current knowledge of acute myocarditis, overall success rates of 50% to 70% with MCS are attainable either by cardiac recovery or transplantation (14,15). In the post-transplant group, three of six (50%) patients were weaned and two (33%) were successfully discharged home. Various investigators have reported encouraging results in this group, with wean rates ranging between 47% and 100%, and discharge rates between 20% and 71% (16–20). The available knowledge emphasizes the recoverability of ventricular function in the transplanted heart during primary graft failure or severe acute rejection with cardiogenic shock. In these circumstances, early application of the BVS 5000 can successfully support the failing donor heart, allowing long-term survival. As with all forms of MCS, earlier institution generally results in higher rates of successful support to either recovery or transplantation. However, the decision to proceed with ventricular assist device insertion can often be difficult, and the risks of significant bleeding and ongoing coagulopathy must be weighed against the risk of impending end-organ damage.
Our overall results demonstrated 55% survival on support and a 28% discharge from hospital rate. The outcomes from several single-institution studies reported similar wean and discharge rates (Table 4). It is clear that increasing experience, particularly with the decision to implant the device at an earlier stage, results in superior outcomes.
TABLE 4.
Single-institution studies reporting the outcomes of BVS 5000 (ABIOMED Inc, USa) ventricular assist device support
The success of an ABIOMED program depends on several factors. Proper patient selection and timing of insertion are the two most important determinants of outcome. Dekkers et al (21) showed that time to implantation of less than 1 h yielded the highest wean and discharge rates, with patients implanted more than 5 h following clinical diagnosis yielding no survivors. The ABIOMED registry has shown that wean and discharge rates can be improved by adhering to the principle of early insertion. It is important to realize that the use of two or more inotropic drugs at maximal dosages is associated with an unacceptable mortality rate if hemodynamics are not rapidly improved. Early referral before the occurrence of multiple system organ failure is pivotal to the success of MCS. Importantly, there is still a large difference between the wean rate and the discharge rate. This primarily stems from our lack of insight into markers that will predict meaningful and persistent recovery of the injured myocardium, and this forms the basis of future research. Until then, one way of bridging this gap is conversion to long-term devices or heart transplantation.
CONCLUSION
Acute cardiogenic shock continues to present a medical and surgical challenge demanding rapid recognition and resuscitation. The ABIOMED BVS 5000 device is a safe and effective device for short-term support in this extremely high-risk patient population who otherwise face certain death. The simplicity with which it can be exchanged with the long-term ABIOMED AB5000 further adds to its versatility. Our results support the use of short-term MCS devices in all cardiac surgical centres in Canada.
REFERENCES
- 1.Samuels LE, Holmes EC, Thomas MP, et al. Management of acute cardiac failure with mechanical assist: Experience with the Abiomed BVS 5000. Ann Thorac Surg. 2001;71:S67–S85. doi: 10.1016/s0003-4975(00)02644-8. [DOI] [PubMed] [Google Scholar]
- 2.Helman DN, Morales DL, Edwards NM, et al. Left ventricular assist device bridge-to-transplant network improves survival after failed cardiotomy. Ann Thorac Surg. 1999;68:1187–94. doi: 10.1016/s0003-4975(99)00911-x. [DOI] [PubMed] [Google Scholar]
- 3.Korfer R, El-Banayosy A, Arusoglu L, et al. Temporary pulsatile ventricular assist devices and biventricular assist devices. Ann Thorac Surg. 1999;68:678–83. doi: 10.1016/s0003-4975(99)00541-x. [DOI] [PubMed] [Google Scholar]
- 4.Jett GK. Abiomed BVS 5000. Experience and potential advantages. Ann Thorac Surg. 1996;61:301–13. doi: 10.1016/0003-4975(95)01009-2. [DOI] [PubMed] [Google Scholar]
- 5.Morgan JA, Stewart AS, Lee BJ, Oz MC, Naka Y. Role of the Abiomed BVS 5000 device for short-term support and bridge to transplantation. ASAIO J. 2004;50:360–3. doi: 10.1097/01.mat.0000130680.63196.7b. [DOI] [PubMed] [Google Scholar]
- 6.Rao V, Oz MC, Edward NM, Naka Y. A new off-pump technique for thoratec right ventricular assist device insertion. Ann Thorac Surg. 2001;71:1719–20. doi: 10.1016/s0003-4975(01)02427-4. [DOI] [PubMed] [Google Scholar]
- 7.Taylor KM, Bain WH, Davidson KG, Turner MA. Comparative clinical study of pulsatile and non-pulsatile perfusion in 350 consecutive patients. Thorax. 1982;37:324–30. doi: 10.1136/thx.37.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minami K, El-Banayosy A, Posival H, et al. Improvement of survival in patients with cardiogenic shock by using non-pulsatile and pulsatile ventricular assist device. Int J Artif Organs. 1992;15:715–21. [PubMed] [Google Scholar]
- 9.Ciardullo RC, Schaff HV, Flaherty JT, Donahoos JS, Gott VL. Comparison of regional myocardial blood flow and metabolism distal to a critical coronary stenosis in a fibrillating heart during alternative periods of pulsatile and non-pulsatile perfusion. J Thorac Cardiovasc Surg. 1978;75:193–205. [PubMed] [Google Scholar]
- 10.Gaer JAR, Shaw ADS, Wild R, et al. Effect of cardiopulmonary bypass on gastrointestinal perfusion and function. Ann Thorac Surg. 1994;57:371–5. doi: 10.1016/0003-4975(94)90999-7. [DOI] [PubMed] [Google Scholar]
- 11.Minami K, Korner MM, Vyska K, Kleeseik K, Knoble K, Korfer R. Effect of pulsatile perfusion on plasma catecholamine levels and hemodynamics during and after cardiac operations with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1990;99:82–91. [PubMed] [Google Scholar]
- 12.Kulick DM, Bolman RM, III, Salerno CT, Bank AJ, Park SJ. Management of recurrent ventricular tachycardia with ventricular assist device placement. Ann Thorac Surg. 1998;66:571–3. doi: 10.1016/s0003-4975(98)00512-8. [DOI] [PubMed] [Google Scholar]
- 13.Thomas NJ, Harvey AT. Bridge to recovery with the Abiomed BVS 5000 device in a patient with intractable ventricular tachycardia. J Thorac Cardiovasc Surg. 1999;117:831–2. doi: 10.1016/S0022-5223(99)70310-7. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy RE, Boehmer JP, Hurban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (non-fulminant) myocarditis. N Engl J Med. 2000;342:690–5. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 15.Karliner JS. Fulminant myocarditis. N Engl J Med. 2000;342:734–5. doi: 10.1056/NEJM200003093421011. [DOI] [PubMed] [Google Scholar]
- 16.Minev PV, El-banayosy A, Minami K, Kortke H, Kizner L, Korfer R. Differential indications for mechanical circulatory support following heart transplantation. Intensive Care Med. 2001;27:1321–7. doi: 10.1007/s001340101006. [DOI] [PubMed] [Google Scholar]
- 17.Arafa O, Fiane AE, Svennevig JL, Gerian OR. Mechanical circulatory support of heart transplant patients. Transplant Proc. 2001;33:1603–4. doi: 10.1016/s0041-1345(00)02609-9. [DOI] [PubMed] [Google Scholar]
- 18.Petrowski JA, Patel VS, Russell SD, Milano CA. BVS 5000 support after cardiac transplantation. J Thorac Cardiovasc Surg. 2003;126:442–7. doi: 10.1016/s0022-5223(02)73613-1. [DOI] [PubMed] [Google Scholar]
- 19.Minami K, Posival H, El-Banayosy A, et al. Mechanical ventricular support using pulsatile Abiomed BVS 5000 and centrifugal Biomedicus pump in postcardiotomy shock. Int J Artif Organs. 1994;17:492–8. [PubMed] [Google Scholar]
- 20.Couper GS, Dekkers RJ, Adams DH. The logistics and cost-effectiveness of circulatory support: Advantages of the Abiomed BVS 5000. Ann Thorac Surg. 1999;68:646–9. doi: 10.1016/s0003-4975(99)00584-6. [DOI] [PubMed] [Google Scholar]
- 21.Dekkers RJ, Fitzgerald DJ, Couper GS. Five-year clinical experience with Abiomed BVS 5000 as a ventricular assist device for cardiac failure. Perfusion. 2001;16:13–8. doi: 10.1177/026765910101600103. [DOI] [PubMed] [Google Scholar]