Abstract
Preventing the occurrence of cardiovascular disease (CVD) with nutritional interventions is a therapeutic strategy that may warrant greater research attention. The increased use of omega (ω)-3 fatty acids is a powerful example of one such nutritional strategy that may produce significant cardiovascular benefits. Marine food products have provided the traditional dietary sources of ω-3 fatty acids. Flaxseed is an alternative to marine products. It is one of the richest sources of the plant-based ω-3 fatty acid, alpha-linolenic acid (ALA). Based on the results of clinical trials, epidemiological investigations and experimental studies, ingestion of ALA has been suggested to have a positive impact on CVD. Because of its high ALA content, the use of flaxseed has been advocated to combat CVD. The purpose of the present review was to identify the known cardiovascular effects of flaxseed and ALA and, just as importantly, what is presently unknown.
Keywords: Cardiovascular disease, Fibre, Fish, Heart disease, Lignans, Nutrition, Polyunsaturated fatty acids
Abstract
La prévention de l’occurrence de maladies cardiovasculaires (MCV) au moyen d’interventions nutritionnelles est une stratégie thérapeutique qui pourrait susciter plus de recherches. L’utilisation accrue d’acides gras oméga (ω)-3 est un exemple évocateur de stratégie nutritionnelle qui peut procurer des bienfaits cardiovasculaires. Les produits alimentaires marins ont toujours constitué la source alimentaire d’acides gras ω-3. Les graines de lin peuvent toutefois remplacer les produits marins. C’est l’une des sources les plus riches d’acides gras ω-3 d’origine végétale, l’acide alpha-linolénique (ALA). Selon les résultats d’essais cliniques, d’études épidémiologiques et d’études expérimentales, il est postulé que l’ingestion d’ALA aurait des effets positifs sur les MCV. En raison du contenu élevé des graines de lin en ALA, on avance que son utilisation combat les MCV. La présente analyse visait à déterminer les effets cardiovasculaires connus des graines de lin et de l’ALA et, de manière tout aussi importante, ceux qu’on ne connaît pas encore.
Coronary artery disease (CAD) is a leading cause of death today and a major economic challenge for the health care system (1,2). There are now significant research data suggesting that CAD can be altered or prevented in large part by three major lifestyle changes. These three factors are nutritional modification, incorporating exercise into our daily lives and eliminating smoking (3). The implementation of only one of these three factors – nutritional modification – may generate significant effects on CAD. For example, increasing the consumption of omega (ω)-3 fatty acids may be a particularly powerful dietary strategy to combat CAD (4,5). Consumption of ω-3 polyunsaturated fatty acids (PUFAs) is usually in the form of marine oils from fish. Fish oil contains both docosahexaenoic acid (DHA, C22:6 ω-3) and eicosapentaenoic acid (EPA, C20:5 ω-3). There is strong scientific evidence from human trials that ω-3 fatty acids from fish or fish oil supplements (EPA and DHA) can significantly reduce risk factors for heart disease (such as reducing blood triglyceride [TG] levels) (6–8), reduce the risk of nonfatal and fatal myocardial infarctions, sudden death and all-cause mortality (9–11), and produce small reductions in blood pressure (12–14) and platelet aggregation, and the thrombotic complications associated with platelet aggregation (9–11). Despite the data supporting the contention that the long-chain ω-3 fatty acids EPA and DHA in fish oil have significant, beneficial cardiovascular potential, dietary endorsement has not been widespread. Concerns about fish taste, smell, toxin content, allergies and eructation following a fish meal are some of the factors that have limited their dietary use.
FLAXSEED AS A SOURCE OF ω-3 FATTY ACID
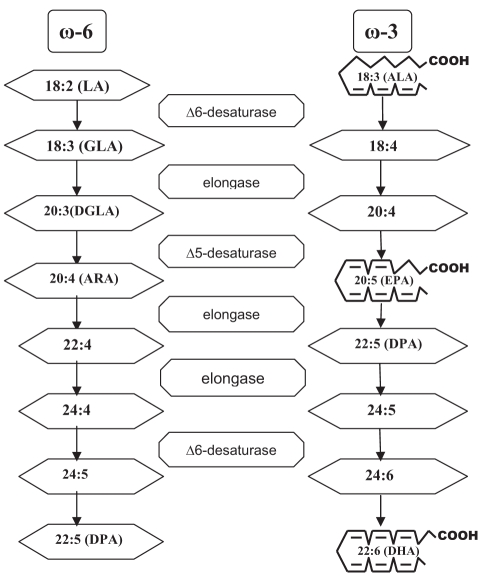
Recently, flaxseed (linseed) was identified as an alternative plant source of ω-3 fatty acids. The ω-3 fatty acids found in flaxseed differ from those in fish (Figure 1). Flaxseed is one of the richest plant sources of the ω-3 fatty acid alpha-linolenic acid (ALA, C18:3 ω-3) (15), but it is found in other foods as well (Table 1). The typical North American diet provides approximately 1.4 g of ALA per day, and 0.1 g to 0.2 g of EPA and DHA (16). ALA can be converted to long-chain ω-3 PUFA. In a cross-study meta-regression analysis (17) of plasma phospholipid ω-3 fatty acid concentrations after ALA supplementation, it was observed that ALA supplementation with up to 14 g/day resulted in dose-dependent but modest increases in plasma ALA concentrations. Some of the observed variability, especially at low ALA doses, was attributed to differences in the amount of n-6 PUFA linoleic acid (LA) concurrently administered in the diet. The dose response appeared linear (r2=0.79, P=0.008). There were small increases in EPA after ALA supplementation (r2=0.49, P=0.052); however, plasma phospholipid DHA concentrations did not detectably increase in this study.
Figure 1.
Interconversion of omega (ω)-6 and ω-3 fatty acids. Biochemical pathway. Δ Delta; ALA Alpha-linolenic acid; ARA Arachidonic acid; DGLA Dihomo-gamma-linolenic acid; DHA Docosahexaenoic acid; DPA Docosapentaenoic acid; EPA Eicosapentaenoic acid; GLA Gamma-linolenic acid; LA Linoleic acid
TABLE 1.
Selected sources of alpha-linolenic acid (ALA) and content of ALA in selected novel sources of omega-3 polyunsaturated fatty acids
| Source of ALA* | ALA content, g |
|---|---|
| Pumpkin seeds (1 tbsp) | 0.051 |
| Olive oil (1 tbsp) | 0.103 |
| Walnuts, black (1 tbsp) | 0.156 |
| Soybean oil (1 tbsp) | 1.231 |
| Rapeseed oil (1 tbsp) | 1.302 |
| Walnut oil (1 tbsp) | 1.414 |
| Flaxseeds (1 tbsp) | 2.350 |
| Walnuts, English (1 tbsp) | 2.574 |
| Flaxseed oil (1 tbsp) | 7.249 |
| Almonds (100 g) | 0.4 |
| Peanuts (100 g) | 0.003 |
| Beans, navy, sprouted (100 g) | 0.3 |
| Broccoli, raw (100 g) | 0.1 |
| Lettuce, red leaf (100 g) | 0.1 |
| Mustard (100 g) | 0.1 |
| Purslane (100 g) | 0.4 |
| Spinach (100 g) | 0.1 |
| Seaweed, spirulina, dried (100 g) | 0.8 |
| Beans, common, dry (100 g) | 0.6 |
| Chickpeas, dry (100 g) | 0.1 |
| Soybeans, dry (100 g) | 1.6 |
| Oats, germ (100 g) | 1.4 |
| Rice, bran (100 g) | 0.2 |
| Wheat, germ (100 g) | 0.7 |
| Avocados, California, raw (100 g) | 0.1 |
| Raspberries, raw (100 g) | 0.1 |
| Strawberries, raw (100 g) | 0.1 |
| Novel sources of ALA† | ALA content, g |
| Breads and pasta (100 g) | 0.1–1.6 |
| Cereals (and granola bars) (55 g) | 1.0–4.9 |
| Eggs (50 g or 1 egg) | 0.1–0.6 |
| Processed meats (100 g) | 0.5 |
| Salad dressing (14 g – 31 g) | 2.0–4.0 |
| Margarine spreads (10 g – 100 g) | 0.3–1.0 |
| Nutrition bars (50 g) | 0.1–2.2 |
Current dietary recommendations for adults suggest a daily intake of 2.22 g of ALA based on a 2000 kcal diet (18). Ingesting flaxseed can provide ALA to the circulation and tissues of the body. ALA levels are increased as early as two weeks after the initiation of flaxseed supplementation (19). The bioavailability of ALA is dependent on the type of flax ingested (ALA has greater bioavailability in oil than in milled seed, and has greater bioavailability in milled seed than in whole seed) (20). Crushing and milling of flaxseed substantially improve the bio-availability of enterolignans (21), likely due to the improved accessibility of the colon bacteria to crushed and ground flaxseed, the dose of flaxseed ingested (17) and the fat composition of the diet. For example, concurrent administration of LA in the diet will reduce ALA accumulation (17) because there is a competition among the enzymes involved in the elongation and desaturation of LA and ALA (Figure 1). A ratio of LA to ALA of 4:1 or lower has been shown to be optimal for the elongation of 11 g ALA to 1 g long-chain ω-3 PUFA (22). The age of the subject does not appear to influence ALA bio-availability or its conversion to DHA (23). The relative bioavailability of enterolignans from flaxseed does not differ in men versus women (21). Approximately 4 g of ALA appears to have biological effects similar to those of 0.3 g of long-chain ω-3 PUFA. Comparatively, EPA and DHA are more rapidly incorporated into plasma and membrane lipids, and produce more rapid effects than ALA. Therefore, the role of ALA in human nutrition may be more important in terms of long-term dietary intake (18).
EFFECTS OF FLAXSEED AND ALA INGESTION ON PRIMARY CARDIOVASCULAR END POINTS
Myocardial infarction, morbidity and mortality
If the two ω-3 PUFAs present in fish (EPA and DHA) are structurally different from the ALA found in flaxseed (24), their effects on CAD may be different as well. Unfortunately, data on the impact of the ALA found in flaxseed is not as well known or as mature as the fish data, and the health-related value of ALA or flaxseed has been debated (25–27).
Nine major studies (4,28–35) have reported that ALA levels are inversely correlated with primary cardiovascular events. The results are persuasive because most of these studies gathered data from large sample populations and/or over relatively long collection periods (multiple years) (Table 2).
TABLE 2.
Investigations reporting effects of alpha-linolenic acid (ALA) on primary cardiovascular end points
| Reference | Population (sample size) | Follow-up, years | Main results |
|---|---|---|---|
| Hu et al (28) | 76,283 women | 10 | Higher intake of ALA provided significant protection against fatal myocardial infarction |
| Albert et al (29) | 76,763 women | 18 | Inverse association between ALA and the risk of sudden cardiac death. Highest intakes of ALA obtained a 40% lower risk for sudden cardiac death |
| Erkkilä et al (4) | 415 | 5 | The content of ALA in the cholesteryl ester fraction, but not in phospholipids, tended to be associated with a reduced risk of death in patients with CAD |
| Baylin et al (30) | 964 | – | Inverse relationship between ALA in adipose tissue and nonfatal acute myocardial infarction |
| Oda et al (31) | 157 | – | Serum levels of ALA, EPA, DHA and total omega-3 polyunsaturated fatty acid were significantly lower in patients with acute myocardial infarction compared with the control group |
| Djoussé et al (32) | 2004 white men and women | – | ALA-rich diet is associated with a lower prevalence of calcified atherosclerotic plaque in the coronary arteries |
| Djoussé et al (33) | 4584 white men and women | – | A higher intake of ALA was inversely related to the prevalence OR of CAD by up to 40%. The reduction in the risk of CAD appeared to be independent of fish consumption |
| Ascherio et al (34) | 43,757 men | 6 | A 1% increase in ALA intake (as % of energy) resulted in a 40% lower risk of nonfatal CAD |
| Dolecek (35) | 12,866 men | 6–8 | Association between a high intake of ALA and a decreased risk of death from cardiovascular disease, CAD and all causes of death combined |
CAD Coronary artery disease; DHA Docosahexaenoic acid; EPA Eicosapentaenoic acid
More evidence that dietary ALA has significant cardioprotective efficacy has been demonstrated in secondary prevention trials. In the Lyon Diet Heart Study (36), ALA was associated with a decreased risk of recurrent fatal and nonfatal myocardial infarction, and a 73% reduction in risk of primary end points (cardiac mortality and morbidity) between the experimental and control groups. In a double-blinded, placebo-controlled study (37) conducted in India, 120 patients with suspected acute myocardial infarction were followed and supplemented with 2.9 g/day of ALA (enriched oil). After one year of follow-up, both cardiac death and nonfatal myocardial infarction were significantly lower in this group of patients compared with those on placebo.
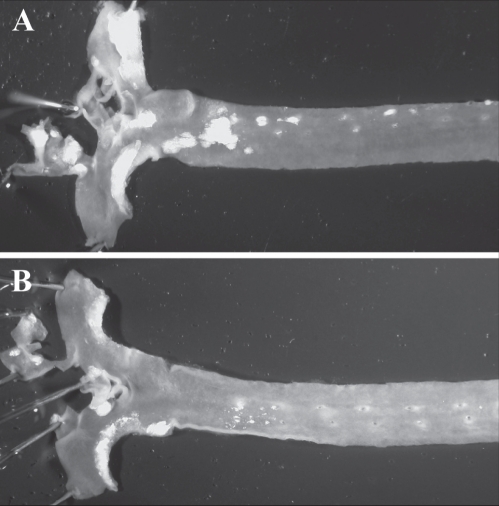
Work from animal studies may provide mechanistic insight. Atherosclerosis was significantly prevented by flaxseed supplementation in the hypercholesterolemic rabbit (38) and in the cholesterol-fed, low-density lipoprotein (LDL) receptor-deficient mouse (39) (Figure 2). Because there was a poor correlation between the progression of atherosclerotic lesions and the cholesterol-lowering effect of dietary flaxseed, the hypolipidemic effect of flaxseed is, at best, likely to be only one of the contributing factors to its antiatherogenic potential. Flaxseed (0.4 g/day) effectively inhibited the expression of inflammatory markers such as interleukin (IL)-6, mac-3, vascular cell adhesion molecule (VCAM)-1 and the proliferative marker proliferating cell nuclear antigen in aortic atherosclerotic tissue from LDL receptor-deficient mice (39). It was concluded that an important antiatherogenic role of ALA may involve a potent anti-inflammatory action. This is supported in clinical work. It is now well recognized that infectious disease and inflammation are important contributory factors to atherosclerotic CAD (40,41). Two independent studies of healthy subjects have shown that inflammatory markers, such as tumour necrosis factor-alpha (TNF-α), IL-1-beta, thromboxane B5 and prostaglandin E5, were significantly reduced after administration of an ALA-rich diet (13.7 g/day of ALA from flaxseed) (42), as were VCAM-1 and E-selectin after delivery of 2 g/day of ALA (43). ALA intake (8 g/day) from a flaxseed source decreases serum concentrations of serum amyloid A, IL-6 (44,45), soluble VCAM-1, soluble intercellular adhesion molecule-1, soluble E-selectin (46) as well as the production of TNF-α, IL-1-beta and prostaglandin E2 by peripheral blood mononuclear cells (42) over a period of four to 12 weeks with doses of ALA greater than 9 g/day.
Figure 2.
Atherosclerotic plaque formation in representative aortas taken from low-density lipoprotein receptor-deficient mice fed for 24 weeks with a diet supplemented with 2% cholesterol (A) or a diet supplemented with 2% cholesterol and 10% flaxseed (B). Note the significantly reduced plaque formation when flaxseed is included in the diet. Data from reference 39
In a cross-sectional study, Djoussé et al (47) found that a higher intake of dietary ALA (highest tertiles 0.89 g/day) was inversely associated with heart rate-adjusted QT and JT intervals in a dose-response manner in both men and women. The authors suggested that dietary ALA might be associated with a reduced risk of abnormally prolonged repolarization. This is supported by animal work. Rabbits fed flaxseed exhibited a shorter QT interval than the controls, whereas the longest QT intervals were measured in the cholesterol-fed group (48). QT prolongation is associated with arrhythmias (49,50). The addition of flaxseed to the cholesterol-supplemented diet significantly shortened the QT interval in these hearts. Shortening of the QT interval was associated with a marked reduction in ventricular fibrillation in the flax-fed and the cholesterol plus flax-fed groups. Flaxseed, therefore, appears to exert its protective effect by shortening the QT interval and the action potential duration of the heart (48). It was suggested that alterations in Na+/Ca2+ exchange and action potential characteristics contributed to the antiarrhythmic action of flaxseed (48,51). The ALA content of flaxseed was also suggested to be the primary component within flaxseed that provided the antiarrhythmic action (48). This antiarrhythmic action may explain the lower incidence of sudden death in subjects ingesting ALA (29).
Stroke
Data collected from 1575 participants in the National Heart, Lung, and Blood Institute (NHLBI) Family Heart Study (52) showed that an ALA-rich diet (highest quartile 1.19 g/day) was associated with lower prevalence odds of carotid plaques and with thinner segment-specific carotid intimal/media thickness. Seidelin et al (53) also reported that patients with cerebral infarction had lower levels of ALA in subcutaneous adipose tissue than matched controls. In a case-control study (54) of 96 middle-aged men with incident stroke, Simon et al (54) found that a significant 0.06% increase in phospholipid ALA content was associated with a 28% decrease in the risk of stroke. Only ALA found in the cholesterol ester fraction was associated with a decreased risk of stroke in multivariate models. A 0.13% increase in the serum level of ALA was associated with a 37% decrease in the risk of stroke. PUFAs (particularly ALA and DHA) may be potent protectors against cerebral ischemia (55,56), possibly via the activation of the TREK-1 potassium channel (57).
Unfortunately, trials that test the beneficial effects of flaxseed in primary or secondary prevention of stroke in humans have not been conducted. Based on the data provided above, we argue that this is now warranted.
EFFECTS OF FLAXSEED AND ALA INGESTION ON SECONDARY CARDIOVASCULAR END POINTS AND CARDIOVASCULAR RISK FACTORS
The discussion above demonstrates that ALA can have significant effects on primary cardiovascular end points. Flaxseed, through its high content of ALA, may have similar effects but these data are not yet available in humans. What is impressive about these results is the consistency of the positive effects and the unusually large sample size that has been used in many of these investigations. To gain further confidence about the cardiovascular effects of flaxseed and ALA, and to gain some insight into how these substances work in our bodies, many experimental studies have examined secondary end points and risk factors.
High blood cholesterol
Most studies have reported modest effects of flaxseed on blood total cholesterol (TC), LDL cholesterol or high-density-lipoprotein (HDL) cholesterol (44,58–68). Harper et al (65) found that ALA from flaxseed (3 g/day) tends to increase the concentrations of the large, less atherogenic LDL1 and LDL2 subfractions. The smaller diameter and more dense LDL particles have a greater proclivity for oxidation and an enhanced ability to penetrate the intima compared with the larger, less dense LDL particles. Concentrations of these LDL particles were significant, independent predictors of new CAD events in patients undergoing treatment with gemfibrozil (66). Recently, Patade et al (67) found that mild to moderate hypercholesterolemic Native American postmenopausal women who consumed flaxseed (30 g/day) for three months exhibited a reduction of TC by 7% and LDL cholesterol by 10% without changes in HDL cholesterol or TG concentrations. Zhang et al (68) also found significant reductions in TC (22%) and LDL cholesterol levels (24%) in hypercholesterolemic subjects after an eight-week dietary supplementation with 600 mg/day of secoisolariciresinol diglucoside (SDG), a lignan derived from flaxseed. These results suggest that specific subgroups of patients could obtain more beneficial hypolipemic effects from flaxseed or its components. In general, flaxseed-enriched diets have been reported to induce anywhere from 0% to 18% decreases in LDL and 0% to 11% decreases in TC (69). With only one exception that reported a 16% decrease in HDL concentrations in men (61), most studies reported no changes in HDL levels in response to dietary flax-seed (59,63,64,70,71).
TGs
The NHLBI Family Heart Study (72), which included 4440 subjects, showed that dietary ALA (highest quintile 1.24 g/day) is associated with lower plasma TG concentrations. Zhao et al (46) demonstrated an 18% decrease in blood TG levels when patients ingested 17.5 g of ALA/day over a six-week intervention study. However, the data are not consistent. An increase (59), a decrease (47,60) or no effect on circulating TG levels (44,73) have been reported in response to a flaxseed diet. The age of the subject may be a confounding factor. Younger subjects responded with a decrease in blood TG concentrations after flaxseed (6 g ALA/day) ingestion, whereas older subjects did not (23).
Lipoprotein (a)
An elevated concentration of plasma lipoprotein (a) (Lp[a]) is a risk factor for CAD, cerebrovascular disease, atherosclerosis, thrombosis and stroke (74). In a recent double-blinded, randomized, controlled clinical trial, Bloedon et al (61) demonstrated that 40 g/day of ground flaxseed reduced Lp(a) by 14% after 10 weeks of supplementation. Similarly, Arjmandi et al (70) reported that 38 g/day of whole flaxseed lowered Lp(a) by 7.4% in postmenopausal women after six weeks of treatment.
High blood pressure
In the National NHLBI Family Heart Study (75), Djoussé et al found that dietary ALA (highest quartile 1.09 g/day) was associated with a lower prevalence of hypertension and lower systolic blood pressure in 4594 subjects. Others (76,77) have confirmed that dietary ALA is associated with lower blood pressure values. ALA may lower blood pressure by acting as a precursor for eicosanoids, which can generate the production of prostaglandins and leukotrienes that may reduce vascular tone (78).
Studies on high blood pressure (HBP) using flaxseed as a source of ALA are inconclusive. Paschos et al (73) have reported that 12 weeks of dietary flaxseed supplementation (8 g/day of ALA) resulted in a significant decrease in systolic and diastolic blood pressure in dyslipidemic patients. Conversely, Stuglin and Prasad (62) found no changes in blood pressure in a shorter four-week intervention using 32.7 g/day of total flaxseed. However, their study examined healthy men and may indicate that pathological conditions are required to detect significant changes in HBP.
The mechanism for any change in blood pressure is unclear. However, shorter studies using healthy individuals, obese subjects or dyslipidemic patients may provide some insight. Administration of 8 g of ALA/day to dyslipidemic men or 20 g/day of flax oil containing ALA to obese adults with markers of insulin resistance has resulted in significant decreases in systolic, diastolic and mean arterial blood pressure (58,73). Systemic arterial compliance was also improved significantly (58). This would agree well with experimental work in which Dupasquier et al (38) found that a flaxseed-supplemented, ALA-rich diet (12.5 g of flaxseed/day; ALA comprises 70% of total fatty acids) preserved vascular relaxation from the deleterious effects that an atherogenic, high-cholesterol diet can induce. These results would suggest that ALA may directly induce beneficial vascular effects.
Tobacco smoking
Previous studies have shown that smoking could inhibit the interconversion of short-chain PUFAs (ALA, linolenic acid ) to long-chain PUFAs (EPA, DHA) (79,80). The data are limited by their focus on cigarette-smoking mothers, and the link among smoking, ALA metabolism and an increased risk for CAD in a more generalized population needs further research attention.
C-reactive protein
Patients with elevated basal levels of C-reactive protein (CRP) are at an increased risk for diabetes, hypertension and cardiovascular disease (CVD) (81). CRP levels of 0.3 mg/dL or greater are associated with a higher risk of death in patients with acute coronary syndromes (82). The intake of an ALA-rich diet (6.5% of energy/day from ALA) has been associated with a large 75% decrease in CRP levels in blood samples from hypercholesterolemic men and women (46). The authors suggested that the inhibition of vascular inflammation may result in a decrease in CVD risk. In another independent study, ALA levels in a plasma cholesterol fraction have also been negatively correlated with CRP concentrations (83). Hallund et al (84) found that SDG isolated from flaxseed (500 mg/day) reduced CRP concentration by approximately 15% in healthy postmenopausal women when they were compared with a placebo group during a six-week intervention period. These results, however, contrast with those of Dodin et al (85), who reported no changes in CRP in a similar population after 12 months of daily supplementation with 40 g of flaxseed.
Effects in obese subjects
Because of its high fat content, it is possible that flaxseed supplementation will induce weight gain. However, dietary flaxseed intervention studies (86,87) have not found any evidence of weight gain or changes in body mass index after supplementation. On the contrary, Nestel et al (58) reported that in obese human subjects, 20 g/day of ALA from flaxseed oil significantly increased arterial compliance and decreased LDL oxidation when it was compared with an oleic acid and saturated fat intervention. ALA was thought to be the component within flaxseed oil that was responsible for this effect. In overweight adolescents, a significant association among plasma fatty acid composition, metabolic syndrome and low-grade inflammation has been identified (83). In this study, ALA levels in plasma cholesterol esters were also inversely related to CRP. From these findings, it was suggested that a high intake of long-chain PUFAs, especially ω-3 PUFAs, may protect obese subjects against metabolic syndrome and low-grade inflammation in early adolescence. Consistent with this hypothesis, Faintuch et al (87) found that morbidly obese patients decreased their white blood cell count, CRP levels, serum amyloid A and fibronectin after two weeks of 5 g/day ALA supplementation from flaxseed. Conversely, however, Nelson et al (86) did not find an alteration in inflammatory markers after an eight-week intervention with ALA in healthy, abdominally obese adults.
In experimental animal trials, SDG significantly reduced high-fat diet-induced visceral and liver fat accumulation, hyperlipidemia, hypercholesterolemia, hyperinsulinemia and hyperleptinemia (88). The mechanism proposed for these actions was the regulation of adipogenesis-related gene expression through an increase in peroxi-some proliferator-activated receptor-gamma-mediated DNA binding activity induced by flaxseed lignans. Studies on differences in the conversion of ALA to EPA and DHA in obese or metabolic syndrome subjects have not been conducted.
Diabetes mellitus
Djoussé et al (89) studied 3993 nondiabetic subjects and found that a higher consumption of ALA was associated with higher plasma insulin, but not glucose levels. The authors suggested that plant-based ω-3 fatty acids might influence insulin secretion in vivo, and improve glucose use and efficiency. Studies in animal models of diabetes mellitus have shown that SDG from flaxseed can prevent the development of type 1 diabetes by approximately 71% (90) and type 2 diabetes by 80% (91). Pan et al (92) reported more modest but statistically significant improvements in glycemic control in type 2 diabetic patients treated for 12 weeks with 360 mg/day of flaxseed-derived lignan supplement. Das (93) has proposed that a defect in the activity of delta-6 and delta-5 desaturases (Figure 1) may be a factor that predisposes individuals to the development of insulin resistance syndrome because long-chain PUFAs can increase cell membrane fluidity, enhance the number of insulin receptors and the affinity of insulin to its receptors; suppress TNF-α, IL-6, macrophage migration inhibitory factor and leptin synthesis; increase the number of glucose transporter type 4 receptors; serve as endogenous ligands of peroxisome proliferator-activated receptors; modify lipolysis; and regulate the balance between pro- and antioxidants.
Ingestion of flaxseed or ALA may help in preventing or treating a variety of diabetic complications. For example, in 1062 adults older than 40 years of age with self-reported diagnosed diabetes, Tao et al (94) found that dietary intake of ALA (highest quintile greater than 2.11 g/day) was positively associated with lower odds of peripheral neuropathy. In an animal model, Velasquez et al (95) reported that flaxseed meals reduced proteinuria and ameliorated nephropathy in type 2 diabetes mellitus. In type 2 diabetic patients, 5 g/day of flaxseed oil consumption has been associated with a significant reduction of plasmin alpha-2-plasmin inhibitor complex level, plasminogen activator inhibitor-1 activity and thrombin antithrombin III complex level after two weeks of intervention (96). Because diabetic patients are more likely to develop thrombotic events, these findings have important clinical implications.
Coagulation and coagulation factors
Despite these findings in diabetic patients, flaxseed ingestion does not influence platelet function in healthy individuals. One might expect flaxseed and its content of ALA to induce an inhibition of platelet aggregation because fish oils (and EPA and DHA) are well-known inhibitors of platelet aggregation. However, many studies have not detected any changes in platelet aggregation after supplementing the diet with flaxseed for one to three months (20,97,98). Freese and Mutanen (99) reported no differences in collagen-induced platelet aggregation and thromboxane production, aggregation to the thromboxane A2 mimic I-BOP, urinary excretion of 11-dehydrothromboxane B2 and beta-thromboglobulin, bleeding time, plasma fibrinogen concentration, antithrombin III activity, factor VII coagulant activity, or activity of plasminogen activator inhibitor 1 in healthy subjects receiving 5.9 g/day of ALA from flaxseed oil or 5.2 g/day of EPA plus DHA supplementation for four weeks. In both groups, LA intake was kept constant. In a long-term trial (six months), no differences in factors VIIa, VIIc, VIIag, XIIa, XIIag, fibrinogen concentrations, plasminogen activator inhibitor-1 or tissue plasminogen activator activity were found when subjects supplemented with PUFA of plant or marine origin were compared with the control group (98). The ratio of ω-3 PUFAs to ω-6 PUFAs in dietary fat seems to play a major role in modulating hemostatic function (100). Only two studies have shown some changes in platelet aggregation as a function of flaxseed ingestion (101); in one of these, the changes were isolated and modest at best (23). More extensive dose-response studies are needed to assess the association between ALA and any changes in bleeding times before definitive conclusions can be drawn (102).
Estrogen
Flaxseed is a rich source of lignans. Lignans are phytoestrogens that have been shown to exert hormonal effects (103). Studies conducted in postmenopausal women have reported that flaxseed supplementation (38 g/day of whole flaxseed) can lower serum LDL cholesterol, Lp(a) (70), serum TC, non-HDL cholesterol, TGs, apolipoprotein A1 and apolipoprotein B (71). Dietary flaxseed (40 g/day of crushed flax-seed) has effects that are similar to hormone replacement therapy for decreasing menopausal symptoms (63) as well as ‘hot flashes’ in post-menopausal women not taking estrogen therapy (104). Recently, data collected from 56,007 French women followed over eight years showed that breast cancer risk was not related to any dietary PUFA overall and was inversely associated with ALA intake from fruit and vegetables but positively related to ALA intake from nut mixes and processed food (16). More research in this area is warranted because recent results showed that conjugated equine estrogens provided no overall protection against myocardial infarction or coronary death in generally healthy postmenopausal women during a seven-year period of use (105) and that the increased risk of breast cancer associated with the use of estrogen plus progestin markedly declined soon after discontinuation of combined hormone therapy (106).
Stress
Psychosocial factors can contribute to CAD (107). Serum ω-3 fatty acids have been associated with variations in mood, personality and behaviour in hypercholesterolemic subjects (108). Spence et al (109) reported that the rise in blood pressure during mental stress, a strong predictor of atherosclerosis progression, is ameliorated by flaxseed. The study also found a decrease in plasma cortisol values in the group treated with flaxseed. This study was conducted in high-risk post-menopausal women.
WHAT WE DO NOT KNOW ABOUT THE EFFECTS OF FLAXSEED AND ALA INGESTION ON CVD
It is important to identify not only what we currently know about dietary flaxseed and ALA but also what is not known. Secondary prevention trials using flaxseed as a source of ALA have not been conducted. No studies have been conducted on the effects of ALA or flaxseed on left ventricular hypertrophy. This could be important because ALA supplementation can reduce HBP, a state that can lead to left ventricular hypertrophy. The influence of ALA supplementation on ventricular remodelling after myocardial infarction is also unknown. There are no epidemiological or clinical randomized studies using flaxseed as a preventive intervention in a healthy population or in subjects without heart disease but who are identified as at risk for CVD disease. Because of the effects of ALA on the coronary risk factors described above, it is logical to predict that a rich source of ALA like flaxseed should have strong beneficial effects in the primary and secondary prevention of CVD. However, without data to test this hypothesis, it remains speculative.
CONCLUSIONS
In view of these data, it is possible to advocate a potential pleiotropic effect of ALA beyond the more conventional cholesterol-lowering actions of most cardiovascular drugs. Most importantly, the body of ALA research now argues persuasively for the initiation of careful, randomized, controlled trials of dietary flaxseed and/or ALA in a patient population with symptoms of atherosclerotic heart disease.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Canadian Institutes of Health Research, and through the St Boniface Hospital and Research Foundation. Dr Rodriguez-Leyva was a Visiting Scientist of the Heart and Stroke Foundation of Canada. Chantal Bassett was a Trainee of the Heart and Stroke Foundation of Canada. Richelle McCullough was a Trainee of the Institute of Cardiovascular Sciences.
REFERENCES
- 1.World Health Organization. World Health Statistics. 2008. [Accessed on February 9, 2009]. < http://www.who.int/whosis/whostat/>.
- 2.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics – 2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Erkkilä AT, Lehto S, Pyorala K, Uusitupa MI. n-3 Fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am J Clin Nutr. 2003;78:65–71. doi: 10.1093/ajcn/78.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Lamotte M, Annemans L, Kawalec P, Zoellners Y. A multi-country health-economic evaluation of highly concentrated n-3 polyunsaturated fatty acids in the secondary prevention after myocardial infarction. Herz. 2006;31(Suppl 3):74–82. [PubMed] [Google Scholar]
- 6.Harris WS. n-3 fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65(Suppl 5):1645S–54S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 7.Sanders TA, Oakley FR, Miller GJ, et al. Influence of n-6 versus n-3 polyunsaturated fatty acids in diets low in saturated fatty acids on plasma lipoproteins and hemostatic factors. Arterioscler Thromb Vasc Biol. 1997;17:3449–60. doi: 10.1161/01.atv.17.12.3449. [DOI] [PubMed] [Google Scholar]
- 8.Grimsgaard S, Bonaa KH, Hansen JB, et al. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997;66:649–59. doi: 10.1093/ajcn/66.3.649. [DOI] [PubMed] [Google Scholar]
- 9.Bucher HC, Hengstler P, Schindler C, et al. N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 10.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 11.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:e20–e30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 12.Howe PR. Dietary fats and hypertension. Focus on fish oil. Ann N Y Acad Sci. 1997;827:339–52. doi: 10.1111/j.1749-6632.1997.tb51846.x. [DOI] [PubMed] [Google Scholar]
- 13.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–33. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 14.Appel LJ, Miller ER, III, Seidler AJ, et al. Does supplementation of diet with ‘fish oil’ reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med. 1993;153:1429–38. [PubMed] [Google Scholar]
- 15.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006;83:1526S–35S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 16.Thiébaut AC, Chajès V, Gerber M, et al. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer. 2009;124:924–31. doi: 10.1002/ijc.23980. [DOI] [PubMed] [Google Scholar]
- 17.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(Suppl):1467S–76S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 18.Simopoulos AP. Human requirement for N-3 polyunsaturated fatty acids. Poult Sci. 2000;79:961–70. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- 19.Francois CA, Connor SL, Bolewicz LC, Connor WE. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003;77:226–33. doi: 10.1093/ajcn/77.1.226. [DOI] [PubMed] [Google Scholar]
- 20.Austria JA, Richard MN, Chahine MN, et al. Bioavailability of alpha-linolenic acid in subjects after ingestion of three different forms of flaxseed. J Am Coll Nutr. 2008;27:214–21. doi: 10.1080/07315724.2008.10719693. [DOI] [PubMed] [Google Scholar]
- 21.Kuijsten A, Arts IC, van’t Veer P, et al. The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J Nutr. 2005;135:2812–6. doi: 10.1093/jn/135.12.2812. [DOI] [PubMed] [Google Scholar]
- 22.Indu M, Ghafoorunissa N-3 fatty acids in Indian diets – comparison of the effects of precursor (alpha-linolenic acid) vs product (long chain n-3 polyunsaturated fatty acids) Nutr Res. 1992;12:569–82. [Google Scholar]
- 23.Patenaude A, Rodriguez-Leyva D, Edel AL, et al. Bioavailability of alpha linolenic acid from flaxseed diets as a function of the age of the subject. Eur J Clin Nutr. 2009;63:1123–9. doi: 10.1038/ejcn.2009.41. [DOI] [PubMed] [Google Scholar]
- 24.Harper CR, Edwards MJ, DeFilipis AP, Jacobson TA. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr. 2006;136:83–7. doi: 10.1093/jn/136.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Burdge GC, Calder PC. Dietary α-linolenic acid and health-related outcomes: A metabolic perspective. Nutr Res Rev. 2006;19:26–52. doi: 10.1079/NRR2005113. [DOI] [PubMed] [Google Scholar]
- 26.Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71(Suppl 1):179–88. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 27.Whelan J, Rust C. Innovative dietary sources of n-3 fatty acids. Annu Rev Nutr. 2006;26:75–103. doi: 10.1146/annurev.nutr.25.050304.092605. [DOI] [PubMed] [Google Scholar]
- 28.Hu FB, Stampfer MJ, Manson JE, et al. Dietary intake of α-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. 1999;69:890–7. doi: 10.1093/ajcn/69.5.890. [DOI] [PubMed] [Google Scholar]
- 29.Albert CM, Oh K, Whang W, et al. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112:3232–8. doi: 10.1161/CIRCULATIONAHA.105.572008. [DOI] [PubMed] [Google Scholar]
- 30.Baylin A, Kabagambe EK, Ascherio A, et al. Adipose tissue α-linolenic acid and nonfatal acute myocardial infarction in Costa Rica. Circulation. 2003;107:1586–91. doi: 10.1161/01.CIR.0000058165.81208.C6. [DOI] [PubMed] [Google Scholar]
- 31.Oda E, Hatada K, Katoh K, et al. A case-control pilot study on n-3 polyunsaturated fatty acid as a negative risk factor for myocardial infarction. Int Heart J. 2005;46:583–91. doi: 10.1536/ihj.46.583. [DOI] [PubMed] [Google Scholar]
- 32.Djoussé L, Arnett DK, Carr JJ, et al. Dietary linolenic acid is inversely associated with calcified atherosclerotic plaque in the coronary arteries. The NHLBI Family Heart Study. Circulation. 2005;111:2921–6. doi: 10.1161/CIRCULATIONAHA.104.489534. [DOI] [PubMed] [Google Scholar]
- 33.Djoussé L, Pankow JS, Eckfeldt JH, et al. Relation between dietary linolenic acid and coronary artery disease in the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr. 2001;74:612–9. doi: 10.1093/ajcn/74.5.612. [DOI] [PubMed] [Google Scholar]
- 34.Ascherio A, Rimm EB, Giovannucci EL, et al. Dietary fat and risk of coronary heart disease in men: Cohort follow up study in the United States. BMJ. 1996;313:84–90. doi: 10.1136/bmj.313.7049.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200:177–82. doi: 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]
- 36.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–85. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 37.Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction; the Indian experiment of infarct survival – 4. Cardiovasc Drugs Ther. 1997;11:485–91. doi: 10.1023/a:1007757724505. [DOI] [PubMed] [Google Scholar]
- 38.Dupasquier CM, Weber AM, Ander BP, et al. Effects of dietary flaxseed on vascular contractile function and atherosclerosis during prolonged hypercholesterolemia in rabbits. Am J Physiol Heart Circ Physiol. 2006;291:H2987–96. doi: 10.1152/ajpheart.01179.2005. [DOI] [PubMed] [Google Scholar]
- 39.Dupasquier CM, Dibrov E, Kostenuk AL, et al. Dietary flaxseed inhibits atherosclerosis in the LDL receptor deficient mouse in part through anti-proliferative and anti-inflammatory actions. Am J Physiol Heart Circ Physiol. 2007;293:H2394–402. doi: 10.1152/ajpheart.01104.2006. [DOI] [PubMed] [Google Scholar]
- 40.Hu H, Pierce GN, Zhong G. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J Clin Invest. 1999;103:747–53. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirono S, Dibrov E, Hurtado C, et al. Chlamydia pneumoniae stimulates proliferation of vascular smooth muscle cells through induction of endogenous heat shock protein 60. Circ Res. 2003;93:710–6. doi: 10.1161/01.RES.0000095720.46043.F2. [DOI] [PubMed] [Google Scholar]
- 42.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–22. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 43.Thies F, Miles EA, Nebe-von-Caron G, et al. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids. 2001;36:1183–93. doi: 10.1007/s11745-001-0831-4. [DOI] [PubMed] [Google Scholar]
- 44.Rallidis LS, Paschos G, Liakos GK, et al. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167:237–42. doi: 10.1016/s0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 45.Rallidis LS, Paschos G, Papaioannou ML, et al. The effect of diet enriched with alpha-linolenic acid on soluble cellular adhesion molecules in dyslipidaemic patients. Atherosclerosis. 2004;174:127–32. doi: 10.1016/j.atherosclerosis.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–7. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 47.Djoussé L, Rautaharju PM, Hopkins PN, et al. Dietary linolenic acid and adjusted QT and JT intervals in the NHLBI Family Heart Study. J Am Coll Cardiol. 2005;17:45, 1716–22. doi: 10.1016/j.jacc.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 48.Ander BP, Weber AR, Rampersad PP, Gilchrist JS, Pierce GN, Lukas A. Dietary flaxseed protects against ventricular fibrillation induced by ischemia-reperfusion in normal and hypercholesterolemic rabbits. J Nutr. 2004;134:3250–6. doi: 10.1093/jn/134.12.3250. [DOI] [PubMed] [Google Scholar]
- 49.Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85:321–41. doi: 10.1016/s0025-7125(05)70318-7. [DOI] [PubMed] [Google Scholar]
- 50.Antzelevitch C, Shimizu W, Yan GX, et al. Cellular basis for QT dispersion. J Electrocardiol. 1998;30(Suppl):168–75. doi: 10.1016/s0022-0736(98)80070-8. [DOI] [PubMed] [Google Scholar]
- 51.Ander BP, Hurtado C, Raposo CS, et al. Differential sensitivities of the NCX1.1 and NCX1.3 isoforms of the Na+-Ca2+ exchanger to α-linolenic acid. Cardiovasc Res. 2007;73:395–403. doi: 10.1016/j.cardiores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Djoussé L, Folsom AR, Province MA, Hunt SC, Ellison RC. Dietary linolenic acid and carotid atherosclerosis: The NHLBI Family Heart Study. Am J Clin Nutr. 2003;77:819–25. doi: 10.1093/ajcn/77.4.819. [DOI] [PubMed] [Google Scholar]
- 53.Seidelin KN, Jensen B, Haugaard SB, Reith J, Olsen TS. Ischemic stroke and n-3 fatty acids. J Stroke Cereb Dis. 1997;6:405–9. doi: 10.1016/s1052-3057(97)80042-0. [DOI] [PubMed] [Google Scholar]
- 54.Simon JA, Fong J, Bernert JT, Jr, Browner WS. Serum fatty acids and the risk of stroke. Stroke. 1995;26:778–82. doi: 10.1161/01.str.26.5.778. [DOI] [PubMed] [Google Scholar]
- 55.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–93. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heurteaux C, Laigle C, Blondeau N, Jarretou G, Lazdunski M. Alphalinolenic acid and riluzole treatment confer cerebral protection and improve survival after focal brain ischemia. Neuroscience. 2006;137:241–51. doi: 10.1016/j.neuroscience.2005.08.083. [DOI] [PubMed] [Google Scholar]
- 57.Heurteaux C, Guy N, Laigle C, et al. TREK-1, a K(–)channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–95. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nestel PJ, Pomeroy SE, Sasahara T, et al. Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. Arterioscler Thromb Vasc Biol. 1997;17:1163–70. doi: 10.1161/01.atv.17.6.1163. [DOI] [PubMed] [Google Scholar]
- 59.Cunnane SC, Hamadeh MJ, Liede AC, et al. Nutritional attributes of traditional flaxseed in healthy young adults. Am J Clin Nutr. 1995;61:62–8. doi: 10.1093/ajcn/61.1.62. [DOI] [PubMed] [Google Scholar]
- 60.Singer P, Berger I, Wirth M, Godicke W, Jaeger W, Voigt S. Slow desaturation and elongation of linoleic and alpha-linolenic acids as a rationale of eicosapentaenoic acid-rich diet to lower blood pressure and serum lipids in normal, hypertensive and hyperlipemic subjects. Prostaglandins Leukot Med. 1986;24:173–93. doi: 10.1016/0262-1746(86)90125-3. [DOI] [PubMed] [Google Scholar]
- 61.Bloedon LT, Balikai S, Chittams J, et al. Flaxseed and cardiovascular risk factors: Results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr. 2008;27:65–74. doi: 10.1080/07315724.2008.10719676. [DOI] [PubMed] [Google Scholar]
- 62.Stuglin C, Prasad K. Effect of flaxseed consumption on blood pressure, serum lipids, hemopoietic system and liver and kidney enzymes in healthy humans. J Cardiovasc Pharmacol Ther. 2005;10:23–7. doi: 10.1177/107424840501000103. [DOI] [PubMed] [Google Scholar]
- 63.Lemay A, Dodin S, Kadri N, Jacques H, Forest JC. Flaxseed dietary supplement versus hormone replacement therapy in hypercholesterolemic menopausal women. Obstet Gynecol. 2002;100:495–504. doi: 10.1016/s0029-7844(02)02123-3. [DOI] [PubMed] [Google Scholar]
- 64.Clark WF, Kortas C, Heidenheim AP, et al. Flaxseed in lupus nephritis: A two-year nonplacebo-controlled crossover study. J Am Coll Nutr. 2001;20:143–8. doi: 10.1080/07315724.2001.10719026. [DOI] [PubMed] [Google Scholar]
- 65.Harper CR, Edwards MC, Jacobson TA. Flaxseed oil supplementation does not affect plasma lipoprotein concentration or particle size in human subjects. J Nutr. 2006;136:2844–8. doi: 10.1093/jn/136.11.2844. [DOI] [PubMed] [Google Scholar]
- 66.Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by Gemfibrozil therapy in the Veterans affairs high-density lipoprotein intervention trial. Circulation. 2006;113:1556–63. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 67.Patade A, Devareddy L, Lucas EA, Korlagunta K, Daggy BP, Arjmandi BH. Flaxseed reduces total and LDL cholesterol concentrations in Native American postmenopausal women. J Womens Health (Larchmt) 2008;17:355–66. doi: 10.1089/jwh.2007.0359. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Wang X, Liu Y, et al. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br J Nutr. 2008;99:1301–9. doi: 10.1017/S0007114507871649. [DOI] [PubMed] [Google Scholar]
- 69.Bloedon LT, Szapary PO. Flaxseed and cardiovascular risk. Nutr Rev. 2004;62:18–27. doi: 10.1111/j.1753-4887.2004.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 70.Arjmandi BH, Khan DA, Juma S. Whole flaxseed consumption lowers serum LDL-cholesterol and lipoprotein(a) concentrations in postmenopausal women. Nutr Res. 1998;18:1203–14. [Google Scholar]
- 71.Lucas EA, Wild RD, Hammond LJ, et al. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J Clin Endocrinol Metab. 2002;87:1527–32. doi: 10.1210/jcem.87.4.8374. [DOI] [PubMed] [Google Scholar]
- 72.Djoussé L, Hunt SC, Arnett DK, et al. Dietary linolenic acid is inversely associated with plasma triacylglycerol: The NHLBI Family Heart Study. Am J Clin Nutr. 2003;78:1098–102. doi: 10.1093/ajcn/78.6.1098. [DOI] [PubMed] [Google Scholar]
- 73.Paschos GK, Magkos F, Panagiotakos DB, Votteas V, Zampelas A. Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur J Clin Nutr. 2007;61:1201–6. doi: 10.1038/sj.ejcn.1602631. [DOI] [PubMed] [Google Scholar]
- 74.Wilde C. Hidden Causes of Heart Attack and Stroke: Inflammation Cardiology’s New Frontier. Studio City: Abigon Press; 2003. pp. 184–5. [Google Scholar]
- 75.Djoussé L, Arnett DK, Pankow JS, Hopkins PN, Province MA, Ellison RC. Dietary linolenic acid is associated with a lower prevalence of hypertension in the NHLBI Family Heart Study. Hypertension. 2005;45:368–73. doi: 10.1161/01.HYP.0000154679.41568.e6. [DOI] [PubMed] [Google Scholar]
- 76.Bemelmans WJ, Muskiet FA, Feskens EJ, et al. Associations of alpha-linolenic acid and linoleic acid with risk factors for coronary heart disease. Eur J Clin Nutr. 2000;54:865–71. doi: 10.1038/sj.ejcn.1601102. [DOI] [PubMed] [Google Scholar]
- 77.Takeuchi H, Sakurai C, Noda R, et al. Antihypertensive effect and safety of dietary alpha-linolenic acid in subjects with high-normal blood pressure and mild hypertension. J Oleo Sci. 2007;56:347–60. doi: 10.5650/jos.56.347. [DOI] [PubMed] [Google Scholar]
- 78.Salonen JT, Salonen R, Ihanainen M, et al. Blood pressure, dietary fats, and antioxidants. Am J Clin Nutr. 1988;48:1226–32. doi: 10.1093/ajcn/48.5.1226. [DOI] [PubMed] [Google Scholar]
- 79.Rise P, Marangoni F, Galli C. Regulation of PUFA metabolism: Pharmacological and toxicological aspects. Prostaglandins Leukot Essent Fatty Acids. 2002;67:85–9. doi: 10.1054/plef.2002.0403. [DOI] [PubMed] [Google Scholar]
- 80.Agostoni C, Riva E, Giovannini M, et al. Maternal smoking habits are associated with differences in infants’ long-chain polyunsaturated fatty acids in whole blood: A case-control study. Arch Dis Child. 2008;93:414–8. doi: 10.1136/adc.2007.129817. [DOI] [PubMed] [Google Scholar]
- 81.Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas I, Stefanadis C. Five-year incidence of cardiovascular disease and its predictors in Greece: The ATTICA study. Vasc Med. 2008;13:113–21. doi: 10.1177/1358863x07087731. [DOI] [PubMed] [Google Scholar]
- 82.Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–24. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 83.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82:1178–84. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 84.Hallund J, Tetens I, Bügel S, Tholstrup T, Bruun JM. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. Nutr Metab Cardiovasc Dis. 2008;18:497–502. doi: 10.1016/j.numecd.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Dodin S, Cunnane SC, Mâsse B, et al. Flaxseed on cardiovascular disease markers in healthy menopausal women: A randomized, double-blind, placebo-controlled trial. Nutrition. 2008;24:23–30. doi: 10.1016/j.nut.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 86.Nelson TL, Stevens JR, Hickey MS. Inflammatory markers are not altered by an eight week dietary α-linolenic acid intervention in healthy abdominally obese adult males and females. Cytokine. 2007;38:101–6. doi: 10.1016/j.cyto.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 87.Faintuch J, Horie LM, Barbeiro HV, et al. Systemic inflammation in morbidly obese subjects: Response to oral supplementation with alpha-linolenic acid. Obes Surg. 2007;17:341–7. doi: 10.1007/s11695-007-9062-x. [DOI] [PubMed] [Google Scholar]
- 88.Fukumitsu S, Aida K, Ueno N, Ozawa S, Takahashi Y, Kobori M. Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br J Nutr. 2008;6:1–8. doi: 10.1017/S0007114508911570. [DOI] [PubMed] [Google Scholar]
- 89.Djoussé L, Hunt SC, Tang W, Eckfeldt JH, Province MA, Ellison RC. Dietary linolenic acid and fasting glucose and insulin: The National Heart, Lung, and Blood Institute Family Heart Study. Obesity. 2006;14:295–300. doi: 10.1038/oby.2006.38. [DOI] [PubMed] [Google Scholar]
- 90.Prasad K. Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: Effect of secoisolariciresinol diglucoside (SDG) Mol Cell Biochem. 2000;209:89–96. doi: 10.1023/a:1007079802459. [DOI] [PubMed] [Google Scholar]
- 91.Prasad K. Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J Lab Clin Med. 2001;138:32–9. doi: 10.1067/mlc.2001.115717. [DOI] [PubMed] [Google Scholar]
- 92.Pan A, Sun J, Chen Y, et al. Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: A randomized, double-blind, cross-over trial. PLoS ONE. 2007;2:e1–148. doi: 10.1371/journal.pone.0001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Das UN. A defect in the activity of Δ6 and Δ5 desaturases may be a factor predisposing to the development of insulin resistance syndrome. Prostaglandins Leukot Essent Fatty Acids. 2005;72:343–50. doi: 10.1016/j.plefa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Tao M, McDowell MA, Saydah SH, Eberhardt MS. Relationship of polyunsaturated fatty acid intake to peripheral neuropathy among adults with diabetes in the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 2008;31:93–5. doi: 10.2337/dc07-0931. [DOI] [PubMed] [Google Scholar]
- 95.Velasquez MT, Bhathena SJ, Ranich T, et al. Dietary flaxseed meal reduces proteinuria and ameliorates nephropathy in an animal model of type II diabetes mellitus. Kidney Int. 2003;64:2100–7. doi: 10.1046/j.1523-1755.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 96.Tohgi N. Effect of alpha-linolenic acid-containing linseed oil on coagulation in type 2 diabetes. Diabetes Care. 2004;27:2563–4. doi: 10.2337/diacare.27.10.2563. [DOI] [PubMed] [Google Scholar]
- 97.Kelly DS, Nelson JG, Love JE, et al. Dietary alpha-linolenic acid alters tissue fatty acid composition, but not blood lipids, lipoproteins or coagulation status in humans. Lipids. 1993;28:533–7. doi: 10.1007/BF02536085. [DOI] [PubMed] [Google Scholar]
- 98.Finnegan YE, Howarth D, Minihane AM, et al. Plant and marine derived (n-3) polyunsaturated fatty acids do not affect blood coagulation and fibrinolytic factors in moderately hyperlipidemic humans. J Nutr. 2003;133:2210–3. doi: 10.1093/jn/133.7.2210. [DOI] [PubMed] [Google Scholar]
- 99.Freese R, Mutanen M. Linolenic acid and marine long-chain n-3 fatty acids differ only slightly in their effects on hemostatic factors in healthy subjects. Am J Clin Nutr. 1997;66:59l–8. doi: 10.1093/ajcn/66.3.591. [DOI] [PubMed] [Google Scholar]
- 100.Knapp HR. Dietary fatty acids in human thrombosis and hemostasis. Am J Clin Nutr. 1997;65:1687S–98S. doi: 10.1093/ajcn/65.5.1687S. [DOI] [PubMed] [Google Scholar]
- 101.Allman MA, Pena NM, Pang D. Supplementation with flaxseed oil versus sunflower oil in healthy young men consuming a low fat diet: Effects on platelet composition and function. Eur J Clin Nutr. 1995;49:169–78. [PubMed] [Google Scholar]
- 102.Akabas SR, Deckelbaum RJ. Summary of a workshop on n-3 fatty acids: Current status of recommendations and future directions. Am J Clin Nutr. 2006;83(Suppl):1536S–8S. doi: 10.1093/ajcn/83.6.1536S. [DOI] [PubMed] [Google Scholar]
- 103.Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer. 2001;39:58–65. doi: 10.1207/S15327914nc391_8. [DOI] [PubMed] [Google Scholar]
- 104.Pruthi S, Thompson SL, Novotny PJ, et al. Pilot evaluation of flaxseed for the management of hot flashes. J Soc Integr Oncol. 2007;5:106–12. doi: 10.2310/7200.2007.007. [DOI] [PubMed] [Google Scholar]
- 105.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: The Women’s Health Initiative. Arch Intern Med. 2006;166:357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 106.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573–87. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams RB. Blood pressure reactivity to psychological stress: A new risk factor for coronary disease? Hypertension. 2006;47:329–30. doi: 10.1161/01.HYP.0000200688.37802.01. [DOI] [PubMed] [Google Scholar]
- 108.Conklin SM, Harris JI, Manuck SB, Yao JK, Hibbeln JR, Muldoon MF. Serum ω-3 fatty acids are associated with variation in mood, personality and behavior in hypercholesterolemic community volunteers. Psychiatry Res. 2007;152:1–10. doi: 10.1016/j.psychres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 109.Spence JD, Thornton T, Muir AD, Westcott ND. The effect of flax seed cultivars with differing content of α-linolenic acid and lignans on responses to mental stress. J Am Coll Nutr. 2003;22:494–501. doi: 10.1080/07315724.2003.10719327. [DOI] [PubMed] [Google Scholar]